Dietary Patterns among Smokers and Non-Smokers: Findings from the National Health and Nutritional Examination Survey (NHANES) 2017–2018
Abstract
1. Introduction
2. Materials and Methods
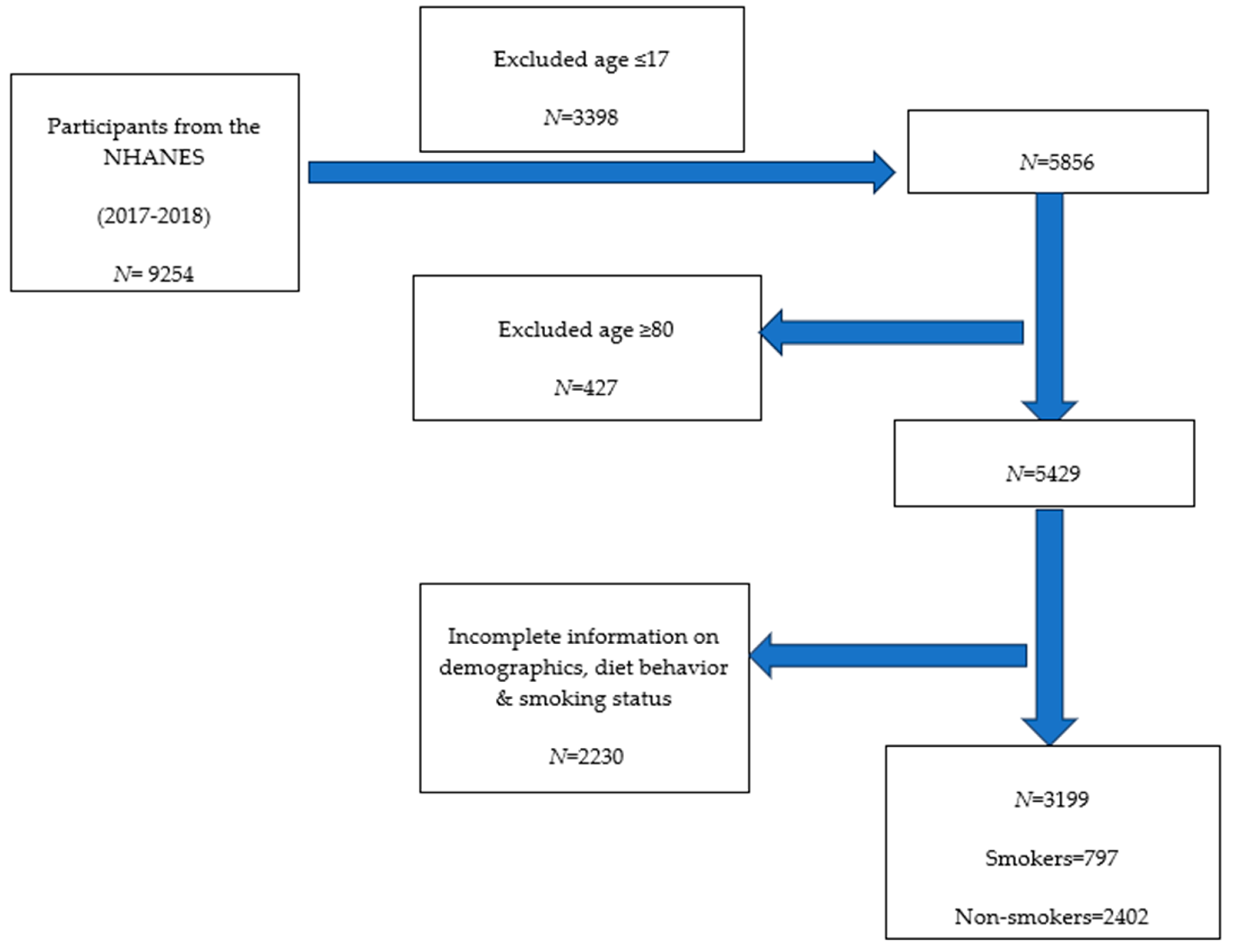
2.1. The NHANES 2017–2018 Cohort
2.2. Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC Tobacco Free Burden of Tobacco Use in the U.S. Available online: https://www.cdc.gov/tobacco/campaign/tips/resources/data/cigarette-smoking-in-united-states.html (accessed on 9 March 2024).
- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; US Department of Health and Human Services: Washington, DC, USA, 2014. [Google Scholar]
- Cornelius, M.E. Tobacco Product Use Among Adults—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 397–405. [Google Scholar] [CrossRef]
- CDC Tobacco Free Diseases/Conditions Featured in the Tips Campaign. Available online: https://www.cdc.gov/tobacco/campaign/tips/diseases/index.html (accessed on 9 March 2024).
- Hatsukami, D.K.; Luo, X.; Jensen, J.A.; Al’Absi, M.; Allen, S.S.; Carmella, S.G.; Chen, M.; Cinciripini, P.M.; Denlinger-Apte, R.; Drobes, D.J. Effect of Immediate vs Gradual Reduction in Nicotine Content of Cigarettes on Biomarkers of Smoke Exposure: A Randomized Clinical Trial. JAMA 2018, 320, 880–891. [Google Scholar] [CrossRef]
- Denlinger-Apte, R.L.; Kotlyar, M.; Koopmeiners, J.S.; Tidey, J.W.; Luo, X.; Benowitz, N.L.; Jensen, J.A.; Ikuemonisan, J.O.; Pacek, L.R.; Smith, T.T. Effects of Very Low Nicotine Content Cigarettes on Smoking Behavior and Biomarkers of Exposure in Menthol and Non-Menthol Smokers. Nicotine Tob. Res. 2019, 21, S63–S72. [Google Scholar] [CrossRef]
- Hatsukami, D.K.; Kotlyar, M.; Hertsgaard, L.A.; Zhang, Y.; Carmella, S.G.; Jensen, J.A.; Allen, S.S.; Shields, P.G.; Murphy, S.E.; Stepanov, I.; et al. Reduced Nicotine Content Cigarettes: Effects on Toxicant Exposure, Dependence and Cessation. Addiction 2010, 105, 343–355. [Google Scholar] [CrossRef]
- Donny Eric, C.; Denlinger Rachel, L.; Tidey Jennifer, W.; Koopmeiners Joseph, S.; Benowitz Neal, L.; Vandrey Ryan, G.; Al’absi, M.; Carmella Steven, G.; Cinciripini Paul, M.; Dermody Sarah, S.; et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N. Engl. J. Med. 2015, 373, 1340–1349. [Google Scholar] [CrossRef]
- Lin, W.; Krebs, N.M.; Zhu, J.; Foulds, J.; Horn, K.; Muscat, J.E. Comparison between Gradual Reduced Nicotine Content and Usual Nicotine Content Groups on Subjective Cigarette Ratings in a Randomized Double-Blind Trial. Int. J. Environ. Res. Public Health 2020, 17, 7047. [Google Scholar] [CrossRef]
- Mercincavage, M.; Souprountchouk, V.; Tang, K.Z.; Dumont, R.L.; Wileyto, E.P.; Carmella, S.G.; Hecht, S.S.; Strasser, A.A. A Randomized Controlled Trial of Progressively Reduced Nicotine Content Cigarettes on Smoking Behaviors, Biomarkers of Exposure, and Subjective Ratings. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1125–1133. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Dains, K.M.; Hall, S.M.; Stewart, S.; Wilson, M.; Dempsey, D.; Jacob III, P. Smoking Behavior and Exposure to Tobacco Toxicants during 6 Months of Smoking Progressively Reduced Nicotine Content Cigarettes. Cancer Epidemiol. Biomark. Prev. 2012, 21, 761–769. [Google Scholar] [CrossRef]
- Hammond, D.; O’Connor, R.J. Reduced Nicotine Cigarettes: Smoking Behavior and Biomarkers of Exposure among Smokers Not Intending to Quit. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2032–2040. [Google Scholar] [CrossRef]
- Foulds, J.; Veldheer, S.; Pachas, G.; Hrabovsky, S.; Hameed, A.; Allen, S.I.; Cather, C.; Azzouz, N.; Yingst, J.; Hammett, E. The Effects of Reduced Nicotine Content Cigarettes on Biomarkers of Nicotine and Toxicant Exposure, Smoking Behavior and Psychiatric Symptoms in Smokers with Mood or Anxiety Disorders: A Double-Blind Randomized Trial. PLoS ONE 2022, 17, e0275522. [Google Scholar] [CrossRef]
- Krebs, N.M.; Zhu, J.; Wasserman, E.; Kuprewicz, R.; Martinez, D.J.; Veldheer, S.; Livelsberger, C.; Modesto, J.; Reinhart, L.; Trushin, N.; et al. Switching to Progressively Reduced Nicotine Content Cigarettes in Smokers with Low Socioeconomic Status: A Double-Blind Randomized Clinical Trial. Nicotine Tob. Res. 2021, 23, 992–1001. [Google Scholar] [CrossRef]
- Delnevo, C.D.; Ganz, O.; Goodwin, R.D. Banning Menthol Cigarettes: A Social Justice Issue Long Overdue. Nicotine Tob. Res. 2020, 22, 1673–1675. [Google Scholar] [CrossRef]
- Villanti, A.C.; Mowery, P.D.; Delnevo, C.D.; Niaura, R.S.; Abrams, D.B.; Giovino, G.A. Changes in the Prevalence and Correlates of Menthol Cigarette Use in the USA, 2004–2014. Tob. Control 2016, 25, ii14–ii20. [Google Scholar] [CrossRef]
- Le, T.T.; Mendez, D. An Estimation of the Harm of Menthol Cigarettes in the United States from 1980 to 2018. Tob. Control 2022, 31, 564–568. [Google Scholar] [CrossRef]
- Wickham, R.J. The Biological Impact of Menthol on Tobacco Dependence. Nicotine Tob. Res. 2020, 22, 1676–1684. [Google Scholar] [CrossRef]
- Lin, W.; Hobkirk, A.L.; Zhu, J.; Krebs, N.M.; Hayes, J.E.; Richie Jr, J.P.; Liao, J.; Horn, K.; Foulds, J.; Muscat, J.E. Effect of Menthol on Nicotine Reduction: Pooled Results from Two Double-Blind Randomized Controlled Trials. Brain Res. Bull. 2022, 189, 131–138. [Google Scholar] [CrossRef]
- Levy, D.T.; Blackman, K.; Tauras, J.; Chaloupka, F.J.; Villanti, A.C.; Niaura, R.S.; Vallone, D.M.; Abrams, D.B. Quit Attempts and Quit Rates Among Menthol and Nonmenthol Smokers in the United States. Am. J. Public Health 2011, 101, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.S.; Fiore, M.C.; Baker, T.B. Smoking Cessation in Smokers Who Smoke Menthol and Non-Menthol Cigarettes. Addiction 2014, 109, 2107–2117. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-Processed Foods: What They Are and How to Identify Them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Baraldi, L.G.; Steele, E.M.; Canella, D.S.; Monteiro, C.A. Consumption of Ultra-Processed Foods and Associated Sociodemographic Factors in the USA between 2007 and 2012: Evidence from a Nationally Representative Cross-Sectional Study. BMJ Open 2018, 8, e020574. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J. The Hidden Dangers of Fast and Processed Food. Am. J. Lifestyle Med. 2018, 12, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Steele, E.M.; Li, Y.; Karageorgou, D.; Micha, R.; Monteiro, C.A.; Mozaffarian, D. Consumption of Ultraprocessed Foods and Diet Quality Among U.S. Children and Adults. Am. J. Prev. Med. 2022, 62, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Gutor, S.; Dikalova, A.E. Pathological Mechanisms of Cigarette Smoking, Dietary, and Sedentary Lifestyle Risks in Vascular Dysfunction: Mitochondria as a Common Target of Risk Factors. Pflug. Arch.-Eur. J. Physiol. 2023, 475, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Rauber, F.; Louzada, M.L.d.C.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-Processed Food Intake and Risk of Cardiovascular Disease: Prospective Cohort Study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Debras, C.; Druesne-Pecollo, N.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes Among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern. Med. 2020, 180, 283–291. [Google Scholar] [CrossRef]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of Ultra-Processed Foods and Cancer Risk: Results from NutriNet-Santé Prospective Cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [PubMed]
- Garrett, B.E. Socioeconomic Differences in Cigarette Smoking Among Sociodemographic Groups. Prev. Chronic Dis. 2019, 16, 180553. [Google Scholar] [CrossRef]
- Alotaibi, S.A.; Alsuliman, M.A.; Durgampudi, P.K. Smoking Tobacco Prevalence among College Students in the Kingdom of Saudi Arabia: Systematic Review and Meta-Analysis. Tob. Induc. Dis. 2019, 17, 35. [Google Scholar] [CrossRef]
- Sogari, G.; Velez-Argumedo, C.; Gómez, M.I.; Mora, C. College Students and Eating Habits: A Study Using An Ecological Model for Healthy Behavior. Nutrients 2018, 10, 1823. [Google Scholar] [CrossRef]
- Alasqah, I.; Mahmud, I.; East, L.; Usher, K. A Systematic Review of the Prevalence and Risk Factors of Smoking among Saudi Adolescents. Saudi Med. J. 2019, 40, 867–878. [Google Scholar] [CrossRef]
- Marbin, J.; Balk, S.J.; Gribben, V.; Groner, J.; SECTION ON TOBACCO CONTROL; Walley, S.C.; Boykan, R.; Jenssen, B.P.; Mih, B.; Alfieri, N.L.; et al. Health Disparities in Tobacco Use and Exposure: A Structural Competency Approach. Pediatrics 2021, 147, e2020040253. [Google Scholar] [CrossRef]
- United States: National Archives and Records Administration: Office of the Federal Register; United States: Department of Health and Human Services; United States: Food and Drug Administration. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. Part II: Proposed Rules. Fed. Register. 2018, 83, 11633–11843. [Google Scholar]
- Tobacco Product Standard for Menthol in Cigarettes. Available online: https://www.federalregister.gov/documents/2022/05/04/2022-08994/tobacco-product-standard-for-menthol-in-cigarettes (accessed on 25 May 2024).
- USDA MyPlate Protein Foods Group–One of the Five Food Groups. Available online: https://www.myplate.gov/eat-healthy/protein-foods (accessed on 25 May 2024).
- USFDA. Using the Nutrition Facts Label and MyPlate to Make Healthier Choices. 2024. Available online: https://www.fda.gov/food/nutrition-facts-label/using-nutrition-facts-label-and-myplate-make-healthier-choices (accessed on 25 May 2024).
- NHANES-About the National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 10 March 2024).
- NHANES Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 10 March 2024).
- Cai, J.; Bidulescu, A. The Association between E-Cigarette Use or Dual Use of e-Cigarette and Combustible Cigarette and Prediabetes, Diabetes, or Insulin Resistance: Findings from the National Health and Nutrition Examination Survey (NHANES). Drug Alcohol. Depend. 2023, 251, 110948. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A. Current Cigarette Smoking among Adults—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. Association Between Time to First Cigarette Use and Urine Biomarkers of Tobacco Exposure in Adult Smokers. Lung 2024, 202, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. The Association between Body Mass Index and Glycohemoglobin (HbA1c) in the US Population’s Diabetes Status. Int. J. Environ. Res. Public Health 2024, 21, 517. [Google Scholar] [CrossRef]
- Lin, W.; Zhu, J.; Hayes, J.E.; Richie, J.P.; Muscat, J.E. Comparison of Carcinogen Biomarkers in Smokers of Menthol and Nonmenthol Cigarettes: The 2015–2016 National Health and Nutrition Examination Survey Special Sample. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. Disparities in Healthcare and HBV Vaccination by Smoking Status: Findings from the National Health and Nutrition Examination Survey (NHANES) 2017–2018. Healthcare 2024, 12, 41. [Google Scholar] [CrossRef]
- Deierlein, A.L.; Litvak, J.; Liu, C.; Stein, C.R. Diet Quality, Diet-Related Factors and Disability Status among Male Adults of Reproductive Age in the USA. Public Health Nutr. 2023, 26, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- NHANES Tutorials-Sample Code Module. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/samplecode.aspx (accessed on 11 March 2024).
- Cai, J.; Bidulescu, A. Associations between E-Cigarette Use or Dual Use of e-Cigarette and Combustible Cigarette and Metabolic Syndrome: Results from the National Health and Nutrition Examination Survey (NHANES). Ann. Epidemiol. 2023, 85, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. Comparison of Urine Heavy Metals in Exclusive Menthol and Non-Menthol Cigarette Users by Race/Ethnicity: The 2015–2016 National Health and Nutrition Examination Survey Special Sample. Tob. Prev. Cessat. 2023, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. Understanding the Mediation Effects of Cigarettes per Day on Time to First Cigarette and Carcinogen Biomarkers: National Health and Nutrition Examination Survey 2015–2016. Tob. Induc. Dis. 2024, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. Minimally Processed Foods Are More Satiating and Less Hyperglycemic than Ultra-Processed Foods: A Preliminary Study with 98 Ready-to-Eat Foods. Food Funct. 2016, 7, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Poti, J.M.; Braga, B.; Qin, B. Ultra-Processed Food Intake and Obesity: What Really Matters for Health—Processing or Nutrient Content? Curr. Obes. Rep. 2017, 6, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, L.; Kesse-Guyot, E.; Allès, B.; Touvier, M.; Srour, B.; Hercberg, S.; Buscail, C.; Julia, C. Association Between Ultraprocessed Food Consumption and Risk of Mortality Among Middle-Aged Adults in France. JAMA Intern. Med. 2019, 179, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.-C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA Food Classification and the Trouble with Ultra-Processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-S.; Shim, S.-Y.; Cha, H.-J.; Kim, J.; Kim, H.C. Socioeconomic Characteristics and Trends in the Consumption of Ultra-Processed Foods in Korea from 2010 to 2018. Nutrients 2021, 13, 1120. [Google Scholar] [CrossRef]
- Moodie, R.; Stuckler, D.; Monteiro, C.; Sheron, N.; Neal, B.; Thamarangsi, T.; Lincoln, P.; Casswell, S. Profits and Pandemics: Prevention of Harmful Effects of Tobacco, Alcohol, and Ultra-Processed Food and Drink Industries. Lancet 2013, 381, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Moubarac, J.-C.; Batal, M.; Louzada, M.L.; Steele, E.M.; Monteiro, C.A. Consumption of Ultra-Processed Foods Predicts Diet Quality in Canada. Appetite 2017, 108, 512–520. [Google Scholar] [CrossRef] [PubMed]
- da Costa Louzada, M.L.; Ricardo, C.Z.; Steele, E.M.; Levy, R.B.; Cannon, G.; Monteiro, C.A. The Share of Ultra-Processed Foods Determines the Overall Nutritional Quality of Diets in Brazil. Public Health Nutr. 2018, 21, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-Processed Food Consumption among US Adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Martínez Steele, E.; Du, M.; Pomeranz, J.L.; O’Connor, L.E.; Herrick, K.A.; Luo, H.; Zhang, X.; Mozaffarian, D.; Zhang, F.F. Trends in Consumption of Ultra processed Foods Among US Youths Aged 2-19 Years, 1999-2018. JAMA 2021, 326, 519–530. [Google Scholar] [CrossRef]
- USFDA. Preliminary Scientific Evaluation of the Possible Public Health Effects of Menthol versus Nonmenthol Cigarettes. Available online: https://www.fda.gov/media/86497 (accessed on 22 May 2024).
- Gardiner, P.S. The African Americanization of Menthol Cigarette Use in the United States. Nicotine Tob. Res. 2004, 6, S55–S65. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Le, T.T.T. Consequences of a Match Made in Hell: The Harm Caused by Menthol Smoking to the African American Population over 1980–2018. Tob. Control 2022, 31, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Celebucki, C.C.; Wayne, G.F.; Connolly, G.N.; Pankow, J.F.; Chang, E.I. Characterization of Measured Menthol in 48 U.S. Cigarette Sub-Brands. Nicotine Tob. Res. 2005, 7, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Taylor, K.M.; Lisko, J.G.; Tran, H.; Watson, C.H.; Holman, M.R. Menthol Content in U.S. Marketed Cigarettes. Nicotine Tob. Res. 2016, 18, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lucas, J.H.; Pang, C.; Zhao, R.; Rahman, I. Tobacco and Menthol Flavored Nicotine-Free Electronic Cigarettes Induced Inflammation and Dysregulated Repair in Lung Fibroblast and Epithelium. Respir. Res. 2024, 25, 23. [Google Scholar] [CrossRef]
- Fryar, C.D.; Carroll, M.D.; Afful, J. Prevalence of Overweight, Obesity, and Severe Obesity among Adults Aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats. 2020. Available online: https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm (accessed on 24 August 2021).
- Chan, J.M.; Rimm, E.B.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Obesity, Fat Distribution, and Weight Gain as Risk Factors for Clinical Diabetes in Men. Diabetes Care 1994, 17, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Colditz, G.A.; Willett, W.C.; Rotnitzky, A.; Manson, J.E. Weight Gain as a Risk Factor for Clinical Diabetes Mellitus in Women. Ann. Intern. Med. 1995, 122, 481–486. [Google Scholar] [CrossRef]
- Ford, E.S.; Williamson, D.F.; Liu, S. Weight Change and Diabetes Incidence: Findings from a National Cohort of US Adults. Am. J. Epidemiol. 1997, 146, 214–222. [Google Scholar] [CrossRef]
- Diabetes Quick Facts|Basics|Diabetes|CDC. Available online: https://www.cdc.gov/diabetes/basics/quick-facts.html (accessed on 8 March 2024).
- American Diabetes Association. Good to Know: Race and Type 2 Diabetes. Clin. Diabetes 2020, 38, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.E.; Margetts, B.M. Relationship between Diet and Smoking--Is the Diet of Smokers Different? J. Epidemiol. Community Health 1991, 45, 270–272. [Google Scholar] [CrossRef]
- Norouzzadeh, M.; Teymoori, F.; Farhadnejad, H.; Moslehi, N.; Mirmiran, P.; Rahideh, S.T.; Azizi, F. Cigarette Smoking and Cardiovascular Disease Incidence and All-Cause Mortality: The Modifying Role of Diet Quality. BMC Public. Health 2024, 24, 1021. [Google Scholar] [CrossRef]
- Tagtow, A.; Raghavan, R. Assessing the Reach of MyPlate Using National Health and Nutrition Examination Survey Data. J. Acad. Nutr. Diet. 2017, 117, 181–183. [Google Scholar] [CrossRef]
- Epstein, S.B.; Jean-Pierre, K.; Lynn, S.; Kant, A.K. Media Coverage and Awareness of the 2010 Dietary Guidelines for Americans and MyPlate. Am. J. Health Promot. 2013, 28, e30–e39. [Google Scholar] [CrossRef]
- Chrisman, M.; Diaz Rios, L.K. Evaluating MyPlate After 8 Years: A Perspective. J. Nutr. Educ. Behav. 2019, 51, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Kimokoti, R.W.; Newby, P.K. Dietary Patterns, Smoking, and Cardiovascular Diseases: A Complex Association. Curr. Nutr. Rep. 2013, 2, 113–125. [Google Scholar] [CrossRef]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.L.; Ezzati, M. The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A Comparative Risk Assessment of Burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S.; Katan, M.B. Diet, Nutrition and the Prevention of Hypertension and Cardiovascular Diseases. Public. Health Nutr. 2004, 7, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Muscat, J.E. Knowledge and Beliefs Regarding Harm from Specific Tobacco Products: Findings from the H.I.N.T. Survey. Am. J. Health Promot. 2021, 089011712110261. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Martinez, S.A.; Ding, K.; Beebe, L.A. Knowledge and Perceptions of Tobacco-Related Harm Associated with Intention to Quit among Cigarette Smokers, e-Cigarette Users, and Dual Users: Findings from the US Population Assessment of Tobacco and Health (PATH) Wave 1. Subst. Use Misuse 2021, 56, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. Knowledge of the Health Consequences of Heavy Alcohol Consumption among Individuals with Different Substance Use Statuses: A Cross-Sectional Analysis of 2019 HINT Survey. Chronic Illn. 2023, 8, 17423953231213853. [Google Scholar] [CrossRef]
- Goyal, N.; Hennessy, M.; Lehman, E.; Lin, W.; Agudo, A.; Ahrens, W.; Boccia, S.; Brennan, P.; Brenner, H.; Cadoni, G.; et al. Risk Factors for Head and Neck Cancer in More and Less Developed Countries: Analysis from the INHANCE Consortium. Oral Dis. 2023, 29, 1565–1578. [Google Scholar] [CrossRef]
| Smokers N = 797 (23.8%) | Non-Smokers N = 2402 (76.2%) | p-Value | |
|---|---|---|---|
| Gender | <0.001 | ||
| Male | 469 (54.4) | 940 (40.8) | |
| Female | 328 (45.6) | 1462 (59.2) | |
| Race/ethnicity * | 0.058 | ||
| Mexican American | 73 (7.0) | 346 (9.4) | |
| Other Hispanic | 40 (4.1) | 247 (7.4) | |
| NH-White | 354 (66.6) | 656 (59.5) | |
| NH-Black | 216 (12.3) | 563 (12.3) | |
| Others | 114 (10.0) | 590 (11.5) | |
| Education | <0.001 | ||
| <High school diploma | 430 (54.3) | 875 (30.7) | |
| ≥High school diploma | 367 (45.7) | 1527 (69.3) | |
| Annual household income | <0.001 | ||
| USD 0 to USD 54,999 | 602 (67.4) | 1241 (39.3) | |
| ≥USD 55,000 | 195 (32.6) | 1161 (60.7) | |
| BMI (kg/m2) | 0.084 | ||
| Underweight (Below 18.5) | 29 (3.2) | 29 (1.5) | |
| Healthy Weight (18.5–24.9) | 229 (29.3) | 609 (25.5) | |
| Overweight (25.0–29.9) | 231 (30.0) | 771 (30.6) | |
| Obesity (30.0 and above) | 308 (37.5) | 993 (42.5) | |
| Heard of My Plate | |||
| Yes | 134 (21.0) | 582 (29.7) | 0.001 |
| No | 663 (79.0) | 1820 (70.3) | |
| Age, years | 43.2 (0.9) | 45.8 (0.7) | 0.02 |
| BMI (kg/m2) | 28.9 (0.4) | 29.7 (0.3) | <0.001 |
| Income to poverty ratio | 2.2 (0.08) | 3.3 (0.06) | 0.02 |
| Model | Non-Smokers | Smokers Coefficient # | 95% CI | p-Value |
|---|---|---|---|---|
| (1) Number of meals not home-prepared | ||||
| Crude | Ref. | 0.9 | 0.8, 1.0 | 0.11 |
| Adjusted * | Ref. | 0.8 | 0.7, 1.1 | 0.18 |
| (2) Number of ready-to-eat foods in the past 30 days | ||||
| Crude | Ref. | 1.2 | 0.9, 1.6 | 0.17 |
| Adjusted * | Ref. | 1.2 | 0.7, 2.1 | 0.3 |
| (3) Number of frozen meals/pizzas in the past 30 days | ||||
| Crude | Ref. | 1.9 | 1.4, 2.6 | <0.001 |
| Adjusted * | Ref. | 1.7 | 1.8, 2.8 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Alfheeaid, H.A.; Alasqah, I.; Alqarawi, N.; Alotaibi, S.A.; Alribdi, F.F.; Almutairi, S.; Lima, M.J.; Teixeira-Lemos, E.; Raposo, A. Dietary Patterns among Smokers and Non-Smokers: Findings from the National Health and Nutritional Examination Survey (NHANES) 2017–2018. Nutrients 2024, 16, 2035. https://doi.org/10.3390/nu16132035
Lin W, Alfheeaid HA, Alasqah I, Alqarawi N, Alotaibi SA, Alribdi FF, Almutairi S, Lima MJ, Teixeira-Lemos E, Raposo A. Dietary Patterns among Smokers and Non-Smokers: Findings from the National Health and Nutritional Examination Survey (NHANES) 2017–2018. Nutrients. 2024; 16(13):2035. https://doi.org/10.3390/nu16132035
Chicago/Turabian StyleLin, Wenxue, Hani A. Alfheeaid, Ibrahim Alasqah, Nada Alqarawi, Saad Abdullah Alotaibi, Fatmah Fahad Alribdi, Sulaiman Almutairi, Maria João Lima, Edite Teixeira-Lemos, and António Raposo. 2024. "Dietary Patterns among Smokers and Non-Smokers: Findings from the National Health and Nutritional Examination Survey (NHANES) 2017–2018" Nutrients 16, no. 13: 2035. https://doi.org/10.3390/nu16132035
APA StyleLin, W., Alfheeaid, H. A., Alasqah, I., Alqarawi, N., Alotaibi, S. A., Alribdi, F. F., Almutairi, S., Lima, M. J., Teixeira-Lemos, E., & Raposo, A. (2024). Dietary Patterns among Smokers and Non-Smokers: Findings from the National Health and Nutritional Examination Survey (NHANES) 2017–2018. Nutrients, 16(13), 2035. https://doi.org/10.3390/nu16132035











