Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Study Selection
2.4. Quality Assessment
2.5. Risk-of-Bias Assessment
2.6. Data Extraction
3. Results
3.1. Study Selection
3.2. Quality Assessment
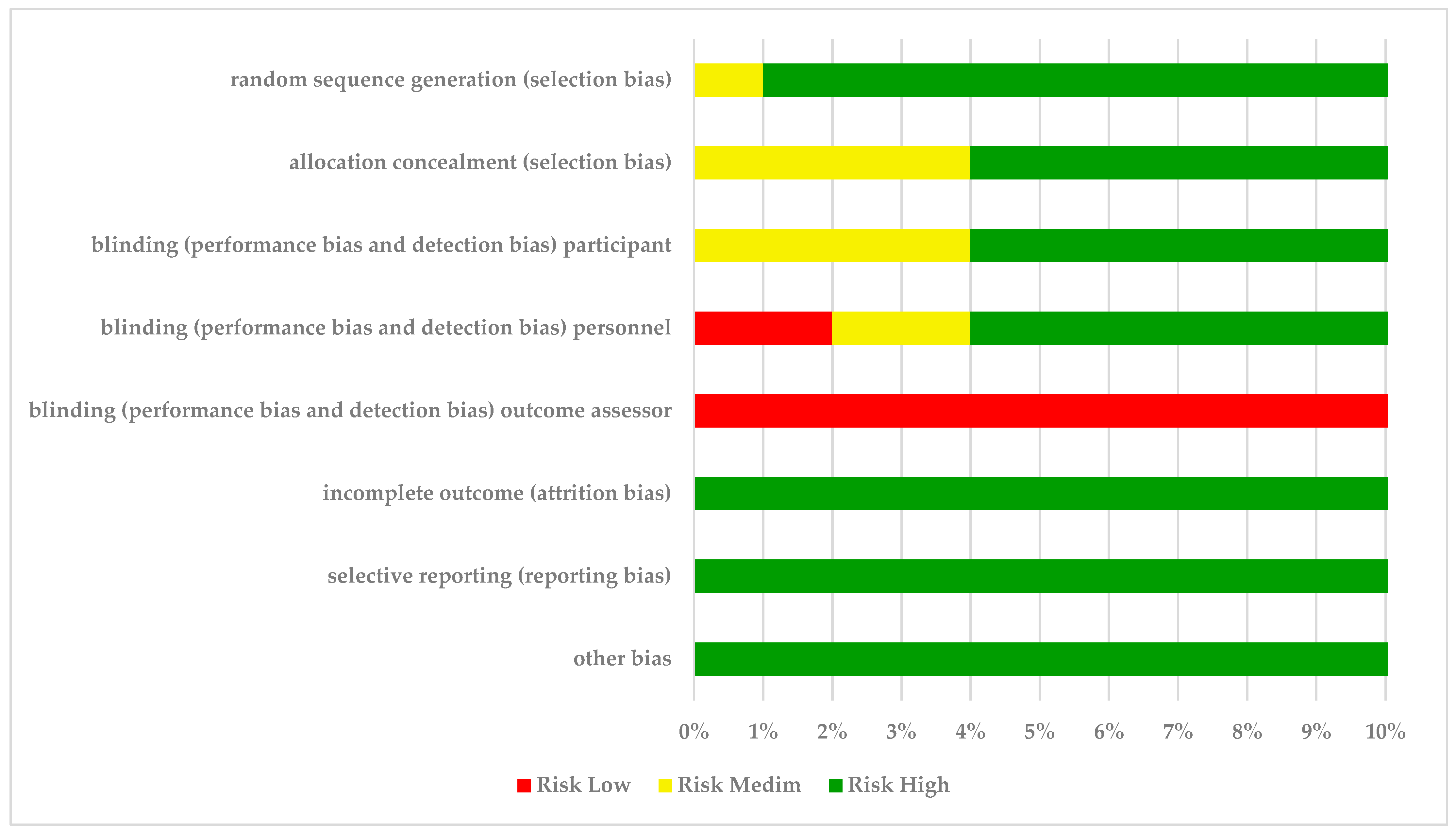
3.3. Risk-of-Bias Assessment
3.4. Outcome Evaluation
3.4.1. Characteristics of the Sample
3.4.2. Omega-3 Supplementation
3.4.3. Inflammatory Markers
3.4.4. Muscle Damage
3.4.5. Oxidant Response
3.4.6. Sports Performance
4. Discussion
4.1. Omega-3 Supplementation
4.2. Inflammatory Markers
4.3. Muscle Damage
4.4. Oxidant Response
4.5. Sports Performance
5. Limitations and Strengths
6. Practical Applications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Search Strategy
| Database | Keywords | Hits |
| PubMed | (omega-3 OR omega-3 supplementation OR Polyunsaturated fatty acids) AND (“muscle recovery”), AND (athletic performance OR improved athletic performance) AND (exercise-induced muscle damage OR muscle soreness OR muscle damage) AND (eccentric exercise) AND (inflammation OR oxidative stress) AND (benefits). Filters: Full text, Trial, in the last 10 years | 678 |
| Scopus | (“omega-3” [Title/Abstract] OR “omega-3 supplementation” [Title/Abstract] OR “Polyunsaturated fatty acids” [Title/Abstract]) AND (“muscle recovery” [Title/Abstract]), AND (“athletic performance” [Title/Abstract] OR “improved athletic performance” [Title/Abstract]) AND (“exercise-induced muscle damage” [Title/Abstract] OR “muscle soreness” [Title/Abstract] OR “muscle damage” [Title/Abstract]) AND (“eccentric exercise” [Title/Abstract]) AND (“inflammation” [Title/Abstract] OR “oxidative stress” [Title/Abstract]) AND (“benefits” [Title/Abstract]). In Title Abstract Keyword in All Text—with Publication Year from 2013 to 2024. Filters: Full text, Trial, in the last 10 years | 51 |
| Web of Science | ((omega-3 OR omega-3 supplementation OR Polyunsaturated fatty acids (topic)) AND (“muscle recovery”), AND ((athletic performance OR improved athletic performance (topic)) AND ((exercise-induced muscle damage OR muscle soreness OR muscle damage(topic)) AND ((eccentric exercise (topic)) AND ((inflammation OR oxidative stress (topic)) AND ((benefits (topic)). Anywhere Publication 2013-2024, Filters: Full text, Trial, in the last 10 years | 768 |
References
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Calvo, J.S.; Martínez, A.C.; García, A.C.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; Orazio, N.D. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 17–19. [Google Scholar] [CrossRef]
- Škrgat, S.; Korošec, P.; Kern, I.; Šilar, M.; Šelb, J.; Fležar, M.; Marčun, R. Systemic and Airway Oxidative Stress in Competitive Swimmers. Respir. Med. 2018, 137, 129–133. [Google Scholar] [CrossRef]
- Reid, M.B. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med. Sci. Sports Exerc. 2001, 33, 371–376. [Google Scholar] [CrossRef]
- Miguel, A.M.C.S.; Roche, E.; Herranz-López, M.; Miguel, M.C.S.; Mielgo-Ayuso, J.; Fernández-Lázaro, D. Impact of Melatonin Supplementation on Sports Performance and Circulating Biomarkers in Highly Trained Athletes: A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 1011. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Fernández-Lázaro, D. Nutrition and Muscle Recovery. Nutrients 2021, 13, 294. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction, and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Zaboli, G.; Igl, W.; Johansson, A.C.V.; Ameur, A.; Enroth, S.; Rivas, M.A.; Daly, M.J.; Schmitz, G.; Hicks, A.A.; Meitinger, T.; et al. Genetic Adaptation of Fatty-Acid Metabolism: A Human-Specific Haplotype Increasing the Biosynthesis of Long-Chain Omega-3 and Omega-6 Fatty Acids. Am. J. Hum. Genet. 2012, 90, 809–820. [Google Scholar] [CrossRef]
- Black, K.E.; Witard, O.C.; Baker, D.; Healey, P.; Lewis, V.; Tavares, F.; Christensen, S.; Pease, T.; Smith, B. Adding Omega-3 Fatty Acids to a Protein-Based Supplement during Pre-Season Training Results in Reduced Muscle Soreness and the Better Maintenance of Explosive Power in Professional Rugby Union Players. Eur. J. Sport. Sci. 2018, 18, 1357–1367. [Google Scholar] [CrossRef]
- Mickleborough, T.D.; Lindley, M.R.; Ionescu, A.A.; Fly, A.D. Protective Effect of Fish Oil Supplementation on Exercise-Induced Bronchoconstriction in Asthma. Chest 2006, 129, 39–49. [Google Scholar] [CrossRef]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-Related Effects of Eicosapentaenoic Acid on Innate Immune Function in Healthy Humans: A Comparison of Young and Older Men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [CrossRef]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish Oil-Derived n-3 PUFA Therapy Increases Muscle Mass and Function in Healthy Older Adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Gann, J.J.; Huber, S.R.; Andre, T.L.; La Bounty, P.M.; Bowden, R.G.; Gordon, P.M.; Grandjean, P.W. Effects of Fish Oil Supplementation on Postresistance Exercise Muscle Soreness. J. Diet. Suppl. 2017, 14, 89–100. [Google Scholar] [CrossRef]
- Lembke, P.; Capodice, J.; Hebert, K.; Swenson, T. Influence of Omega-3 (N3) Index on Performance and Wellbeing in Young Adults after Heavy Eccentric Exercise. J. Sports Sci. Med. 2014, 3, 151–156. [Google Scholar]
- Tsuchiya, Y.; Yanagimoto, K.; Nakazato, K.; Hayamizu, K.; Ochi, E. Eicosapentaenoic and Docosahexaenoic Acids-Rich Fish Oil Supplementation Attenuates Strength Loss and Limited Joint Range of Motion after Eccentric Contractions: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Eur. J. Appl. Physiol. 2016, 116, 1179–1188. [Google Scholar] [CrossRef]
- Camandola, S.; Leonarduzzi, G.; Musso, T.; Varesio, L.; Carini, R.; Scavazza, A.; Chiarpotto, E.; Baeuerle, P.A.; Poli, G. Nuclear Factor KB Is Activated by Arachidonic Acid but Not by Eicosapentaenoic Acid. Biochem. Biophys. Res. Commun. 1996, 229, 643–647. [Google Scholar] [CrossRef]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear Factor-KappaB: Its Role in Health and Disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef]
- Ochi, E.; Tsuchiya, Y.; Yanagimoto, K. Effect of eicosapentaenoic acids-rich fish oil supplementation on motor nerve function after eccentric contractions. J. Int. Soc. Sports Nutr. 2017, 14, 23. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. Omega-3 Fatty Acids Supplementation Attenuates Inflammatory Markers after Eccentric Exercise in Untrained Men. Clin. J. Sport Med. 2011, 21, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Gladman, S.J.; Huang, W.; Lim, S.-N.; Dyall, S.C.; Boddy, S.; Kang, J.X.; Knight, M.M.; Priestley, J.V.; Michael-Titus, A.T. Improved Outcome after Peripheral Nerve Injury in Mice with Increased Levels of Endogenous ω-3 Polyunsaturated Fatty Acids. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Patten, G.S.; Abeywardena, M.Y.; McMurchie, E.J.; Jahangiri, A. Dietary Fish Oil Increases Acetylcholine- and Eicosanoid-Induced Contractility of Isolated Rat Ileum. J. Nutr. 2002, 132, 2506–2513. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. The Effects of Ingestion of Omega-3 Fatty Acids on Perceived Pain and External Symptoms of Delayed Onset Muscle Soreness in Untrained Men. Clin. J. Sport Med. 2009, 19, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Tipton, K.D.; Klein, S.; Wolfe, R.R. An Abundant Supply of Amino Acids Enhances the Metabolic Effect of Exercise on Muscle Protein. Am. J. Physiol. 1997, 273, E122–E129. [Google Scholar] [CrossRef] [PubMed]
- Mcglory, C.; Wardle, S.L.; Macnaughton, L.S.; Witard, O.C.; Scott, F.; Dick, J.; Bell, J.G.; Phillips, S.M.; Galloway, S.D.R.; Hamilton, D.L.; et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol. Rep. 2016, 4, e12715. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.H.; Radonic, P.W.; Wolever, T.M.S.; Wells, G.D.; Lewis, E.J.H.; Radonic, P.W.; Wolever, T.M.S.; Lewis, E.J.H.; Radonic, P.W.; Wolever, T.M.S.; et al. 21 days of mammalian omega-3 fatty acid supplementation improves aspects of neuromuscular function and performance in male athletes compared to olive oil placebo. J. Int. Soc. Sports Nutr. 2015, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S.; et al. Implementing the 27 PRISMA 2020 Statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: The PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance. Br. J. Sports Med. 2022, 56, 175–195. [Google Scholar] [CrossRef] [PubMed]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Law, M.; Stewart, D.; Letts, L.; Pollock, N.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998. [Google Scholar]
- Moseley, A.M.; Elkins, M.R.; Van derWees, P.J.; Pinheiro, M.B. Using research to guide practice: The Physiotherapy Evidence Database (PEDro). Braz. J. Phys. Ther. 2020, 24, 384–391. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Eldridge, S.; Li, T. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Updated February 2021; Cochran: Pittsburg, PA, USA, 2021; Available online: https://training.cochrane.org/handbook (accessed on 26 May 2024).
- Tomczyk, M.; Bidzan-Wiącek, M.; Kortas, J.A.; Kochanowicz, M.; Jost, Z.; Fisk, H.L.; Calder, P.C.; Antosiewicz, J. Omega-3 fatty acid supplementation affects tryptophan metabolism during a 12-week endurance training in amateur runners: A randomized controlled trial. Sci. Rep. 2024, 14, 4102. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Ueda, H.; Yanagimoto, K.; Kato, A.; Ochi, E. 4-Week Eicosapentaenoic Acid-Rich Fish Oil Supplementation Partially Protects Muscular Damage Following Eccentric Contractions. J. Int. Soc. Sports Nutr. 2021, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Vandusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Kerksick, C.M.; Mangine, G.T.; Holmes, A.J.; Lee, M.; Endito, M.R.; et al. Impact of Varying Dosages of Fish Oil on Recovery and Soreness Following Eccentric Exercise. Nutrients 2020, 12, 2246. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gandía, V.; Torregrosa-García, A.; Luque-Rubia, A.J.; Abellán-Ruiz, M.S.; Victoria-Montesinos, D.; López-Román, F.J. Re-esterified DHA improves ventilatory threshold 2 in competitive amateur cyclists. J. Int. Soc. Sports Nutr. 2020, 17, 51. [Google Scholar] [CrossRef]
- Barquilha, G.; Miguel, C.; Dos, M.; Caçula, K.G.; Polotow, T.G.; Vasconcellos, C.V.; Fernandes, A.; Rodrigues, L.E.; Lambertucci, R.H.; Duarte, T.; et al. Fish Oil Supplementation Improves the Repeated-Bout Effect Strength Training. Nutrients 2023, 15, 1708. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.S.; Din, U.; Tarum, J.; Selby, A.; Quinlan, J.; Bass, J.J.; Gharahdaghi, N.; Boereboom, C.; Abdulla, H.; Franchi, M.V.; et al. Omega-3 supplementation during unilateral resistance exercise training in older women: A within subject and double-blind placebo-controlled trial. Clin. Nutr. ESPEN 2021, 46, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Heileson, J.L.; Machek, S.B.; Harris, D.R.; Tomek, S.; de Souza, L.C.; Kieffer, A.J.; Barringer, N.D.; Gallucci, A.; Forsse, J.S.; Funderburk, L.L.K. The effect of fish oil supplementation on resistance training-induced adaptations. J. Int. Soc. Sports Nutr. 2023, 20, 2174704. [Google Scholar] [CrossRef]
- Jakeman, J.R.; Lambrick, D.M.; Wooley, B.; Babraj, J.A.; Faulkner, J.A. Effect of an Acute Dose of Omega-3 Fish Oil Following Exercise-Induced Muscle Damage. Eur. J. Appl. Physiol. 2017, 117, 575–582. [Google Scholar] [CrossRef]
- Lee, S.-R.; Directo, D.; Khamoui, A. V Fish Oil Administration Combined with Resistance Exercise Training Improves Strength, Resting Metabolic Rate, and Inflammation in Older Adults. Aging Clin. Exp. Res. 2022, 34, 3073–3081. [Google Scholar] [CrossRef]
- Mullins, V.A.; Graham, S.; Cummings, D.; Wood, A.; Ovando, V.; Skulas-Ray, A.C.; Polian, D.; Wang, Y.; Hernandez, G.D.; Lopez, C.M.; et al. Effects of Fish Oil on Biomarkers of Axonal Injury and Inflammation in American Football Players: A Placebo-Controlled Randomized Controlled Trial. Nutrients 2022, 14, 2139. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Meaney, M.P.; Dew, D.A. No Positive Influence of Ingesting Chia Seed Oil on Human Running Performance. Nutrients 2015, 7, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Y.; McMurray, D.N.; Chapkin, R.S. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur. J. Pharmacol. 2016, 785, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Campo, D.J.; Ávila-Gandía, V.; López-Román, F.J.; Miñarro, J.; Contreras, C.; Soto-Méndez, F.; Domingo Pedrol, J.C.; Luque-Rubia, A.J. Supplementation of Re-Esterified Docosahexaenoic and Eicosapentaenoic Acids Reduce Inflammatory and Muscle Damage Markers after Exercise in Endurance Athletes: A Randomized, Controlled Crossover Trial. Nutrients 2020, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; He, Q.; Shi, L.; Wu, Y. Impact of Antarctic krill oil supplementation on skeletal muscle injury recovery after resistance exercise. Eur. J. Nutr. 2023, 62, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, A.; López-Grueso, R.; Martínez-Sanz, J.M.; Mielgo-Ayuso, J. Basic biochemical, hematological and hormonal parameters for monitoring the health and nutritional status in athletes. Rev. Esp. Nutr. Hum. Diet. 2014, 18, 155. [Google Scholar] [CrossRef]
- Kyriakidou, Y.; Wood, C.; Ferrier, C.; Dolci, A.; Elliott, B. The Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Exercise-Induced Muscle Damage. J. Int. Soc. Sports Nutr. 2021, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Nakamura, Y.; Gosslau, A.M.; Li, S. Are there serious adverse effects of omega-3 polyunsaturated fatty acid supplements? J. Food Bioact. 2019, 7, 1–6. [Google Scholar] [CrossRef]
- Jeromson, S.; Gallagher, I.J.; Galloway, S.D.R.; Hamilton, D.L. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef] [PubMed]
- Tachtsis, B.; Camera, D.; Lacham-Kaplan, O. Potential Roles of N-3 PUFAs during Skeletal Muscle Growth and Regeneration. Nutrients 2018, 10, 309. [Google Scholar] [CrossRef]
- DiLorenzo, F.M.; Drager, C.J.; Rankin, J.W. Docosahexaenoic Acid Affects Markers of Inflammation and Muscle Damage after Eccentric Exercise. J. Strength Cond. Res. 2014, 28, 2768–2774. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Canals-Garzón, C.; Guisado-Barrilao, R.; Martínez-García, D.; Chirosa-Ríos, I.J.; Jerez-Mayorga, D.; Guisado-Requena, I.M. Effect of Antioxidant Supplementation on Markers of Oxidative Stress and Muscle Damage after Strength Exercise: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 1803. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Larson, D.E.; Fisher-Wellman, K.H.; Galpin, A.J.; Schilling, B.K. Effect of Eicosapentaenoic and Docosahexaenoic Acid on Resting and Exercise-Induced Inflammatory and Oxidative Stress Biomarkers: A Randomized, Placebo Controlled, Cross-over Study. Lipids Health Dis. 2009, 8, 36. [Google Scholar] [CrossRef]
- Gray, P.; Chappell, A.; Jenkinson, A.M.; Thies, F.; Gray, S.R. Fish Oil Supplementation Reduces Markers of Oxidative Stress but Not Muscle Soreness after Eccentric Exercise. Int. J. Sport. Nutr. Exerc. Metab. 2014, 24, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Da Boit, M.; Mastalurova, I.; Brazaite, G.; McGovern, N.; Thompson, K.; Gray, S.R. The Effect of Krill Oil Supplementation on Exercise Performance and Markers of Immune Function. PLoS ONE 2015, 10, e0139174. [Google Scholar] [CrossRef]
- Pereira Panza, V.S.; Diefenthaeler, F.; da Silva, E.L. Benefits of Dietary Phytochemical Supplementation on Eccentric Exercise-Induced Muscle Damage: Is Including Antioxidants Enough? Nutrition 2015, 31, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Philpott, J.D.; Donnelly, C.; Walshe, I.H.; MacKinley, E.E.; Dick, J.; Galloway, S.D.R.; Tipton, K.D.; Witard, O.C. Adding Fish Oil to Whey Protein, Leucine, and Carbohydrate Over a Six-Week Supplementation Period Attenuates Muscle Soreness Following Eccentric Exercise in Competitive Soccer Players. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 26–36. [Google Scholar] [CrossRef]
- Chalchat, E.; Gaston, A.-F.; Charlot, K.; Peñailillo, L.; Valdés, O.; Tardo-Dino, P.-E.; Nosaka, K.; Martin, V.; Garcia-Vicencio, S.; Siracusa, J. Appropriateness of Indirect Markers of Muscle Damage Following Lower Limbs Eccentric-Biased Exercises: A Systematic Review with Meta-Analysis. PLoS ONE 2022, 17, e0271233. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, J.; Zhu, W. Omega-3 Polyunsaturated Fatty Acid Supplementation for Reducing Muscle Soreness after Eccentric Exercise: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2020, 2020, 8062017. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant Supplements and Endurance Exercise: Current Evidence and Mechanistic Insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Fayh, A.P.T.; Borges, K.; Cunha, G.S.; Krause, M.; Rocha, R.; de Bittencourt, P.I.H.J.; Moreira, J.C.F.; Friedman, R.; da Silva Rossato, J.; Fernandes, J.R.; et al. Effects of N-3 Fatty Acids and Exercise on Oxidative Stress Parameters in Type 2 Diabetic: A Randomized Clinical Trial. J. Int. Soc. Sports Nutr. 2018, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, D.; Verri, M.; Giolitto, A.; Doria, E.; Ghitti, M.; Dossena, M. Effect of 8-Week n-3 Fatty-Acid Supplementation on Oxidative Stress and Inflammation in Middle- and Long-Distance Running Athletes: A Pilot Study. J. Int. Soc. Sports Nutr. 2020, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, F.; Storsve, A.B.; Burri, L.; Ding, Y.; Banquells, M.; Riera, J.; Björk, P.; Ferrer-Roca, V.; Domingo, J.C. Krill-Oil-Dependent Increases in HS-Omega-3 Index, Plasma Choline and Antioxidant Capacity in Well-Conditioned Power Training Athletes. Nutrients 2021, 13, 4237. [Google Scholar] [CrossRef] [PubMed]
- Berge, R.K.; Ramsvik, M.S.; Bohov, P.; Svardal, A.; Nordrehaug, J.E.; Rostrup, E.; Bruheim, I.; Bjørndal, B. Krill Oil Reduces Plasma Triacylglycerol Level and Improves Related Lipoprotein Particle Concentration, Fatty Acid Composition and Redox Status in Healthy Young Adults—A Pilot Study. Lipids Health Dis. 2015, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.G.; Santos, V.C.; Levada-Pires, A.C.; Jacintho, T.M.; Gorjão, R.; Pithon-Curi, T.C.; Cury-Boaventura, M.F. Effects of DHA-Rich Fish Oil Supplementation on the Lipid Profile, Markers of Muscle Damage, and Neutrophil Function in Wheelchair Basketball Athletes before and after Acute Exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 596–604. [Google Scholar] [CrossRef]
- Atashak, S.; Sharafi, H.; Azarbayjani, M.A. Effect of Omega-3 Supplementation On The Blood Levels Of Oxidative Stress. Muscle Damage Inflamm. Markers After Acute Resist. 2013, 45, 22–29. [Google Scholar]
- De Salazar, L.; Contreras, C.; Torregrosa-García, A.; Luque-Rubia, A.J.; Ávila-Gandía, V.; Domingo, J.C.; López-Román, F.J. Oxidative Stress in Endurance Cycling Is Reduced Dose-Dependently after One Month of Re-Esterified DHA Supplementation. Antioxidants 2020, 18, 1145. [Google Scholar] [CrossRef] [PubMed]
- McKinley-Barnard, S.K.; Andre, T.L.; Gann, J.J.; Hwang, P.S.; Willoughby, D.S. Effectiveness of Fish Oil Supplementation in Attenuating Exercise-Induced Muscle Damage in Women During Midfollicular and Midluteal Menstrual Phases. J. Strength Cond. Res. 2018, 32, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Luostarinen, R.; Saldeen, T. Dietary Fish Oil Decreases Superoxide Generation by Human Neutrophils: Relation to Cyclooxygenase Pathway and Lysosomal Enzyme Release. Prostaglandins Leukot. Essent. Fatty Acids 1996, 55, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Levine, P.H.; Weiner, B.H.; Johnson, M.H.; Doyle, E.M.; Ellis, P.A.; Hoogasian, J.J. Dietary N-3 Fatty Acid Supplementation Reduces Superoxide Production and Chemiluminescence in a Monocyte-Enriched Preparation of Leukocytes. Am. J. Clin. Nutr. 1990, 51, 804–808. [Google Scholar] [CrossRef]
- Lenn, J.; Uhl, T.; Mattacola, C.; Boissonneault, G.; Yates, J.; Ibrahim, W.; Bruckner, G. The Effects of Fish Oil and Isoflavones on Delayed Onset Muscle Soreness. Med. Sci. Sports Exerc. 2002, 34, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.B.; Butterfield, D.A. Measurement of Oxidized/Reduced Glutathione Ratio. Methods Mol. Biol. 2010, 648, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Twist, C.; Cobley, J.; Howatson, G.; Close, G. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Ochi, E.; Tsuchiya, Y. Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) in Muscle Damage and Function. Nutrients 2018, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.M. The emerging role of eicosapentaenoic acid as an important psychoactive natural product: Some answers but a lot more questions. Lipid Insights 2008, 2, 89–97. [Google Scholar] [CrossRef]
- Tipton, K.D. Nutritional Support for Exercise-Induced Injuries. Sport. Med. 2015, 45, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021, 13, 3746. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. [Google Scholar] [CrossRef]

| Study | Items | Total | % | Quality Score | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| Ávila-Gandía et al., 2020 [36] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 13 | 81.3 | VG |
| Barquilha et al., 2023 [37] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 93.8 | E |
| Brook et al., 2021 [38] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 93.8 | E |
| Heileson et al., 2023 [39] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 13 | 81.3 | VG |
| Jakeman et al., 2017 [40] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 14 | 87.5 | VG |
| Lee et al., 2022 [41] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 13 | 81.3 | VG |
| Lembke et al., 2014 [16] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 93.8 | E |
| Mullins et al., 2022 [42] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 13 | 81.3 | VG |
| Nieman et al., 2015 [43] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 13 | 81.3 | VG |
| Tomczk et al., 2024 [33] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 13 | 81.3 | VG |
| Tsuchiya et al., 2021 [34] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 13 | 81.3 | VG |
| Tsuchiya et al., 2016 [17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 15 | 93.7 | E |
| VanDusseldrorp et al., 2020 [37] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 15 | 93.7 | E |
| Study | Items | Total | % | Quality Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||
| Ávila-Gandía et al., 2020 [36] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Barquilha et al., 2023 [37] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Brook et al., 2021 [38] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Heileson et al., 2023 [39] | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | E |
| Jakeman et al., 2017 [40] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Lee et al., 2022 [41] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 | 72.7 | G |
| Lembke et al., 2014 [16] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | E |
| Mullins et al., 2022 [42] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Nieman et al., 2015 [43] | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | 72.7 | G |
| Tomczk et al., 2024 [33] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 | 72.7 | G |
| Tsuchiya et al., 2021 [34] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Tsuchiya et al., 2016 [17] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | 81.8 | E |
| VanDusseldrorp et al., 2020 [37] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| random sequence generation (selection bias) | allocation concealment (selection bias) | blinding (performance bias and detection bias) participant | blinding (performance bias and detection bias) personnel | blinding (performance bias and detection bias) outcome assessor | incomplete outcome (attrition bias) | selective reporting (reporting bias) | other bias | |
| Ávila-Gandía et al., 2020 [36] |  |  |  |  |  |  |  |  |
| Barquilha et al., 2023 [37] |  |  |  |  |  |  |  |  |
| Brook et al., 2021 [38] |  |  |  |  |  |  |  |  |
| Heileson et al., 2023 [39] |  |  |  |  |  |  |  |  |
| Jakeman et al., 2017 [40] |  |  |  |  |  |  |  |  |
| Lee et al., 2022 [41] |  |  |  |  |  |  |  |  |
| Lembke et al., 2014 [16] |  |  |  |  |  |  |  |  |
| Mullins et al., 2022 [42] |  |  |  |  |  |  |  |  |
| Nieman et al., 2015 [43] |  |  |  |  |  |  |  |  |
| Tomczk et al., 2024 [33] |  |  |  |  |  |  |  |  |
| Tsuchiya et al., 2021 [34] |  |  |  |  |  |  |  |  |
| Tsuchiya et al., 2016 [17] |  |  |  |  |  |  |  |  |
| VanDusseldrorp et al., 2020 [37] |  |  |  |  |  |  |  |  |
| Characteristics | Types | Reference |
|---|---|---|
| Level of participants | Amateur competitive | [36,42,43] |
| Amateur | [33] | |
| Recreationally | [17,33] | |
| Recreationally active | [38,39] | |
| Physically active | [16,35,37,40,41] | |
| Administration Type | Capsule | [16,17,33,34,36,38,39,40] |
| Soft gel | [35,42] | |
| Water with seed oil | [43] | |
| Unspecified | [33,38] | |
| Total dose | High * (750 mg EPA + 50 mg DHA) Low * (150 mg EPA + 100 mg DHA) | [40] |
| 2 g (1400 mg: 800 mg EPA + 600 mg DHA) 4 g (2800 mg: 1600 mg EPA + 1200 mg DHA) 6 g (4200 mg: 2400 mg EPA + 1800 mg DHA) | [35] | |
| 780 mg EPA + 606 mg DHA | [37] | |
| 1220 mg/d (975 mg DHA + 120 mg EPA) | [36] | |
| 2.1 g/d EPA + 0.78 g/d DHA | [41] | |
| 2.275 g/d EPA + 1.575 g/d DHA | [39] | |
| 2234 mg/d EPA + 930 mg/d DHA | [33] | |
| 2.4 g/d (600 mg EPA + 260 mg DHA) | [34] | |
| 2.7 g/day | [16] | |
| 3.5 g/d (1 g: 407 mg/g DHA +170 mg/g EPA) | [43] | |
| 2400 mg (1360 mg EPA + 1040 mg DHA) | [17] | |
| 3680 mg/d (1860 mg EPA +1540 mg DHA) | [38] | |
| 31 g ALA for the average | [32] | |
| Dose schedule | once a day: post-lunch morning | [34] |
| 30 min after meals with water | [17] | |
| 30 min before exercise | [43] | |
| once a day: post-exercise | [40] | |
| 3 times/days (morning, lunch, dinner) | [41] | |
| Unspecified | [16,33,35,36,37,38,39,42] | |
| Amount of supplement/d | 2/4/6 capsules | [35] |
| 3 capsules | [36,37,41] | |
| 6 capsules | [16,42] | |
| 8 capsules | [17,34] | |
| 1 g (capsule)/10 kg/BM | [40] | |
| 7 capsules | [39] | |
| 0.43 g ALA/kg BM | [43] | |
| Unspecified | [38] | |
| Duration | twice separated by two weeks | [43] |
| 1 day | [40] | |
| 30 days | [36] | |
| 4 weeks | [16] | |
| 4.5 weeks | [34] | |
| 6 weeks | [37,38] | |
| 7.5 weeks | [35] | |
| 8 week + 5 days | [17] | |
| 10 weeks | [39] | |
| 12 weeks | [33,41] | |
| 26 weeks | [42] | |
| Exercise intervention | Endurance + functional/resistance | [33,35] |
| Cycling test to exhaustion | [36] | |
| Maximum eccentric extensions of the forearm or elbow | [16,17,34] | |
| Plyometric jumps | [40] | |
| Resistance exercise training | [37,38,39,41] | |
| Running at constant speed until exhaustion | [43] | |
| Unspecified | [42] |
| First Author, Year of Publication, and Country | Study Design | Participants | Intervention | Outcomes | Results | |
|---|---|---|---|---|---|---|
| Ávila-Gandía et al. [36], 2020, Spain | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 50 ♂ Amateur cyclists competing at regional level Gn-3 n = 18 Age (mean ± SD) 35.5 ± 7.3 years Weight (mean ± SD) t 72.4 ± 4.4 kg BMI (mean ± SD) 23.83 ± 1.43 Relative VO2 max (mean ± SD) 48.5 ± 6.8 mL/min/kg CG n = 20 Age (mean ± SD) 36.0 ± 9.6 years Weight (mean ± SD) 71.1 ± 3.4 kg BMI (mean ± SD) 23.42 ± 1.31 Relative VO2 max (mean ± SD) 49.3 ± 6.1mL/min/kg Study withdrawals: 12 | Gn-3 3 soft-gels Per unit: 325 mg DHA + 40 mg EPA (Brudy plus, Brudytechnology, Barcelona, Spain) CG Sunflower oil Supplementation time: 30 days | Muscle damage Blood Lactate Physical performance Absolute VO2 HR MPO Relative VO2 RP time VO2 VT2 | Gn-3 vs. CG ↔ Blood Lactate ↓*Absolute VO2 (6’) ↓* HR ↑* MPO ↓* Relative VO2 (6’) ↑* RP ↑* Time ↔ VO2 ↑* VT2 | Gn-3 Changes from baseline ↔ Blood Lactate ↓* Absolute VO2 (6’) ↓* HR ↑* MPO ↓* Relative VO2 (6’) ↑* RP ↑* Time ↑* VO2 CG Changes from baseline ↔ Blood Lactate ↔ Absolute VO2 (6’) ↔ HR ↔ MPO ↔ Relative VO2 (6’) ↔ RP ↔ time ↔ VO2 |
| Barquilha et al. [37], 2023, Brazil | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 21 ♂ Gn-3 n = 8 CG n = 8 Physically active Age: 20–30 years Study withdrawals: Gn-3 n = 3 CG n = 2 | Gn-3 3 capsules Per unit: 260 mg EPA + 202 mg DHA 3 times daily (Capsule Naturalis Nutricao & Farma LTDA, Sao Paulo, Brazil) Supplementation time: 6 weeks | Hematology Heme Iron Iron Hormones T/C Inflammatory biomarkers CRP IL-6 Muscle damage CK LDH Oxidative stress GSH GSSG GSH/GSSG TEAC | Gn-3 vs. CG ↔ Heme Iron ↔ Iron ↓ CRP ↓ IL-6 ↓ CK ↓ LDH ↑* GSH ↓* GSSG ↑* GSH/GSSG ↔TEAC | Gn-3 Changes from baseline ↔T/C ↓* CRP ↓* IL-6 ↓ CK ↓ LDH |
| Brook et al. [38], 2021, United Kingdon | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 16 ♀ Recreationally active Gn-3 n = 8 ♀ Age (mean ± SD) 64.4 ± 0.8 years Height (mean ± SD) 162 ± 0.02 cm Weight (mean ± SD) 70.5 ± 2.5 kg BMI (mean ± SD) 26.6 ± 0.7 kg/m2 % Fat (mean ± SD) 40.8 ± 1.1% Lean Mass (mean ± SD) 39.4 ± 1.1 kg CG n = 8 ♀ Age (mean ± SD) 66.5 ± 1.4 years Height (mean ± SD) 158 ± 0.02 cm Weight (mean ± SD) 64.3 ± 1.9 kg BMI (mean ± SD) 2.8 ± 0.9 kg/m2 % Fat (mean ± SD) 39.1 ± 1.6% Lean Mass (mean ± SD) 37.1 ± 1.6 kg Study withdrawals: 0 | Gn-3 Per unit: 1860 mg EPA +1540 mg DHA (Minami Epacor) CG Cornoil Supplementation time: 6 weeks | Anthropometry BM Bone mass FFM LBM Muscle function ASR Calpain MAFbx MPS Myonuclei SC Ubiquitin VL Physical performance 1-RM MVC | Gn-3 vs. CG ↔ BM ↔ Bone mass ↔ FFM ↔ LBM ↔ ASR untrained leg (0–6 weeks) ↔ Calpain ↔ MAFbx ↔ MPS untrained leg ↔ Myonuclei type I-II fibre ↔ SC type I fibre ↔ Ubiquitin ↑ 1-RM trained leg ↔ MCV trained leg ↔ MCV untrained leg | Gn-3 Changes from baseline ↔ BM ↔ Bone mass ↔ FFM ↔ LBM ↑* ASR untrained leg (0–2 weeks) ↑ ASR untrained leg (4–6 weeks) ↔ Calpain ↔ MAFbx ↔ MPS untrained leg (0–2 weeks) ↔ MPS untrained leg (0–4 weeks) ↑* Myonuclei type I-II fibre ↔ SC type I fibre ↔ Ubiquitin ↑ 1-RM trained leg ↔ MCV trained leg ↔ MCV untrained leg CG Changes from baseline ↔ BM ↔ Bone mass ↔ FFM ↔ LBM ↑* ASR untrained leg (0–2 weeks) ↔ ASR untrained leg (4–6 weeks) ↔ Calpain ↔ MAFbx ↑* MPS untrained leg (0–2 weeks) ↔ MPS untrained leg (2–4 weeks) ↑* Myonuclei type I-II fibre ↔ SC type I fibre ↔ Ubiquitin ↑ 1-RM trained leg ↔ MCV trained leg ↔ MCV untrained leg |
| Heileson et al. [39], 2023, United States | Randomized, single-blind, placebo-controlled, parallel-group trial | n = 28 (n = 12 ♂ and n = 16 ♀) Recreationally Trained Gn-3 n = 10 n = 5 ♂ and n = 5 ♀ Age (mean ± SD) 28.0 ± 7.4 years Height (mean ± SD) 169.7 ± 9.6 cm Weight (mean ± SD) 75.1 ± 16.0 kg BMI (mean ± SD) 25.8 ± 3.5 kg/m2 % Fat (mean ± SD) 23.9 ± 6.9% CG n = 11 5 ♂ and 6 ♀ Age (mean ± SD) 30.5 ± 5.7 years Height (mean ± SD) 171.8 ± 8.9 cm Weight (mean ± SD) 79.0 ± 16.0 kg BMI (mean ± SD) 26.6 ± 4.3 kg/m2 % Fat (mean ± SD) 24.9 ± 8.0% Study withdrawals: Gn-3 n = 4 CG n = 3 | Gn-3 7 capsules 2.275 g/d EPA + 1.575 g/d DHA (Nordic Naturals, ProOmega, Watsonville, CA, USA) CG 5 capsules 4.5 g/d (NOW, Bloomingdale, IL, USA) Supplementation time: 10 weeks | Anthropometry LBM FM BF Biochemistry DBS Physical performance absolute 1RMBP absolute 1RMSQT ∆ relative 1RMBP ∆ relative 1RMSQT | Gn-3 vs. CG ↔ LBM ↔ FM ↔ BF ↑* DBS ↑* absolute1RMBP ↔ absolute 1RMSQT ↑* ∆ relative 1RMBP ↑* ∆ relative 1RMSQT | Gn-3 Changes from baseline ↑ LBM ↓ FM ↓ BF ↑* DBS ↑ absolute 1RMBP ↑ absolute 1RMSQT ↑* ∆ relative 1RMBP ↑* ∆ relative 1RMSQT CG Changes from baseline ↑ LBM ↓ FM ↔ BF ↔ DBS ↑ absolute 1RMBP ↑ absolute 1RMSQT ↑* ∆ relative 1RMBP ↑* ∆ relative 1RMSQT |
| Jakeman et al. [40], 2017, United Kingdom | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 27 ♂ Physically active > 3 h/week of vigorous athletic training + HIIT High Gn-3 n = 9 Age (mean ± SD) 25.5 ± 5.2 years Height (mean ± SD) 1.74 ± 0.06 m Weight (mean ± SD) 76.5 ± 12.6 kg Low Gn-3 n = 9 Age (mean ± SD) 25.6 ± 4.8 years Height (mean ± SD) 1.82 ± 0.09 m Weight (mean ± SD 80.2 ± 12.0 kg CG n = 9 Age (mean ± SD) 26.2 ± 4.2 years Height (mean ± SD) 1.78 ± 0.01 m Weight (mean ± SD 82.9 ± 12.1 kg Study withdrawals: 0 | Gn-3 1 g/capsule Dose: 1 g/10 kg BM High Gn-3 (EPA 750 mg + DHA 50 mg)/capsule Low Gn-3 (EPA 150 mg + DHA 100 mg)/ capsule CG Oil (flavour masker and gelatine) Supplementation time: 1 day | Inflammatory biomarkers IL-6 Muscle damage CK Perception markers VAS Physical performace CJ Knee extensor strength SJ | High Gn-3, Low Gn-3 vs. CG ↔ IL-6 ↔ CK ↔ VAS ↔ CJ ↔ Knee extensor strength ↑* SJ | High Gn-3, Low Gn-3 Changes from baseline ↔ IL-6 ↑* CK (24 h) ↑* VAS (24 h) ↓ (at 96 h) ↓ CJ (at 1 h) ↓* Knee extensor strength to 60° s−1 and 180° s−1(1 h–96 h) ↓* SJ (at 1 h) |
| Lee et al. [41], 2022, United States | Randomized, placebo-controlled trial | n = 28 (n = 10 ♂ and n = 18 ♀) Physically active RET-G n-3 n = 10 Age (mean ± SD) 67.1 ± 4.4 years Height (mean ± SD) 171.6 ± 9.3 cm Weight (mean ± SD) 70.8 ± 13.5 kg BMI (mean ± SD) 24.0 ± 3.2 kg/m2 RET n = 10 Age (mean ± SD) 66.6 ± 7.3 years Height (mean ± SD) 167.9 ± 5.7 cm Weight (mean ± SD) 66.5 ± 11.5 kg BMI (mean ± SD) 23.5 ± 3.6 kg/m2 CG n = 8 Age (mean ± SD) 66.5 ± 5.0 years Height (mean ± SD) 167.2 ± 10.24 cm Weight (mean ± SD) 68.9 ± 15.8 kg BMI (mean ± SD) 24.3 ± 3.4 kg/m2 Study withdrawals: 0 | RET- Gn-3: 3 capsules/day Per unit: 700 mg EPA + 240 mg DHA RET 3 capsules/day Safflower oil CG 3 capsules/day Safflower oil Supplementation time: 12 weeks | Inflammatory biomarkers IL-6 CRP TNF-α Metabolism TMR FAT oxidation CHO oxidation Physical Performance 1RM lat pull-dow 1RM leg-press 1RM seated row 1RM calf rise 1RM biceps curl VO2 VCO2 RER | RET-Gn-3 vs. RET vs. CG ↓* IL-6 (RET-Gn-3 vs. CG) ↓* CRP ↓* TNF-α (RET-Gn-3 vs. CG) ↔ TMR ↑* 1RM lat pull-dow (RET-Gn-3, RET) ↑* 1RM leg-press (RET-Gn-3, RET) ↑* 1RM seated row (RET-Gn-3, RET) ↑* 1RM calf rise (RET-Gn-3, RET) ↑* 1RM biceps curl (RET-Gn-3, RET) ↑* VO2 ↑* VCO2 ↓* RER | RET-Gn-3 Changes from baseline ↓* IL-6 ↓* CRP ↓ TNF-α ↑* TMR ↑* FAT oxidation ↓* CHO oxidation ↑* 1RM in lateral pull ↑* 1RM leg-press ↑* 1RM seated row ↑* 1RM calf rise ↑* 1RM biceps curl ↑* VO2 ↑* VCO2 ↓* RER RET Changes from baseline ↔ IL-6 ↔ CRP↔ TNF-α ↑* TMR↑ FAT oxidation ↓ CHO oxidation ↑* 1RM in lateral pull ↑* 1RM leg-press ↑* 1RM seated row ↑* 1RM calf rise ↑* 1RM biceps curl ↑* VO2 ↑* VCO2 ↔ RER CG Changes from baseline ↔ IL-6 ↔ CRP ↑ TNF-α ↔ TMR ↔ FAT oxidation ↔ CHO oxidation ↓* 1RM in lateral pull ↓* 1RM leg-press ↔ 1RM seated row ↓* 1RM calf rise ↓* 1RM biceps curl ↔ VO2 ↔ VCO2 ↔ RER |
| Lembke et al. [16], 2014, United States | Randomized, Single-blind, placebo-controlled, parallel-group trial | n = 69 ♂ and ♀ Physically active Gn-3 n = 42 Age (mean ± SD) 18.6 ± 1.2 years CG n = 22 Age (mean ± SD) 18.9 ± 1.1 years Study withdrawals: 5 | Gn-3 6 capsules 2.7 g/day (KD Pharma, Bexbach, Germany) CG 6 capsules High oleic sunflower oil Supplementation time: 30 days | Inflammatory biomarkers CRP Muscle damage Blood lactate CK Perception markers VAS POMS Physical Performance ROM Torque | Gn-3 vs. CG ↓* CRP ↓* Blood lactate ↔ CK (48 -96 h) ↓* VAS (at 72, at 96 h) ↑* POMS (72 h) ↔ ROM ↔ Torque | Gn-3 Changes from baseline ↓* CRP ↔ CK ↓ VAS ↓ POMS (at 48 h and 96 h) ↑ POMS (at 48 h, and at 96 h CG) ↓ ROM ↓ Torque (until 48 h) |
| Mullins et al. [42], 2022, United States | Randomized, double-blind, placebo-controlled, parallel-group trial | n =38 ♂ Competitive Gn-3 n =12 CG n = 17 Study withdrawals: Gn-3 n =7 CG n = 2 | Gn-3 Soft gel capsules Per unit: 1 g: 407 mg/g DHA+ 170 mg/g EPA Pharmavite (West Hills, California) CG Per capsule 713 mg/g oleic acid + 130 mg/g linoleic acid (safflower oil) Pharmavite (West Hills, California) Supplementation time: 26 weeks | Biochemistry Plasma AA Plasma DHA Plasma DPA Plasma EPA Inflammatory biomarkers IL-6 TNF-α Injury Neurofilament | Gn-3 vs.CG ↔ Plasma AA ↓* Plasma DHA (0–7 weeks) ↑* Plasma DHA (8–26 weeks) ↓* Plasma DPA (week 33) ↑* Plasma EPA(week 8,12,17,21) ↔ IL-6 ↔ TNF-α ↔ Neurofilament | Gn-3 Changes from baseline ↓*Plasma AA (week 8,12,17,21,26) ↑*Plasma DHA ↑*Plasma DPA (week 8) ↑*Plasma EPA (week 8,12,17,21,26) ↔IL-6 ↔TNF-α ↑Neurofilament CG Changes from baseline ↔Plasma AA ↔Plasma DHA ↔Plasma DPA ↔Plasma EPA ↔IL-6 ↔TNF-α ↑Neurofilament |
| Nieman et al. [43], 2015, United States | Randomized (1:1 allocation), placebo-controlled, crossover trial | n = 24 16 ♂ and 8 ♀ Competitive runners Age (mean ± SD) 38.0 ±1.7 year Height (mean ± SD) 1.72 ±0.02 m Weight (mean ± SD) 71.8 ± 3.0 kg % Fat (mean ± SD) 19.9 ±1.6 VO2max (mean ± SD) 47.9 ±1.6 Study withdrawals: 0 | Gn-3 0.5 L water with chia seed oil 0.43 g ALA/BM (Dole Foods California, USA), CG 0.5 L of flavored water alone Supplementation time: two occasions separated by 2 weeks | Biochemistry Plasma glucose Leukocyte Plasma ALA Hormones Cortisol Inflammatory biomarkers IL-6 IL-8 IL-10 TNF-α Muscle damage Blood lactate Perception markers RPE Physical performance HR RER VO2 | Gn-3 vs.CG ↓ Plasma glucose ↔ Leukocyte ↑* Plasma ALA ↑* Cortisol ↓ IL-6 ↓ IL-8 ↓ IL10 ↓TNF-α ↓ Blood lactate ↓ RPE ↔ HR ↔ RER ↓VO2 | Changes from baseline ↑ Plasma glucose ↑* Leukocyte ↑* Plasma ALA ↑* Cortisol ↑* IL-6 ↑* IL-8 ↑* IL10 ↑* TNF-α ↑ Blood lactate |
| Tomczyk et al. [33], 2024, Poland | Randomized, placebo-controlled, parallel-group trial | n = 40 ♂ Endurance runners Gn-3 n = 14 Age (mean ± SD) 37 ± 3 years Height (mean ± SD) 181 ± 7 cm Weight (mean ± SD) 76 ± 11 kg HRmax (mean ± SD) 190 ± 9 beats/min−1 CG n =12 Age (mean ± SD) 37 ± 4 years Height (mean ± SD) 180 ± 4 cm Weight (mean ± SD) 78 ± 8 kg HRmax (mean ± SD) 186 ± 9 beats/min−1 Study withdrawals: 14 | Gn-3 2234 mg/d EPA + 930 mg/d DHA CG 4000 mg/d MCT Supplementation time: 12 weeks | Biochemistry Red blood cell DHA Plasma DHA Red blood cell EPA Plasma EPA Plasma Trp metabolites (7) Inflammatory biomarkers IL-6 Perception markers EA HT TA | Gn-3 vs. CG IL-6 Red blood cell DHA Plasma DHA Red blood cell EPA Plasma EPA Plasma Trp metabolites ↔ EA ↔ HT ↔ TA | Gn-3 Changes from baseline ↑* Red blood cell DHA ↑* Plasma DHA ↑* Red blood cell EPA ↑* Plasma EPA ↑* Plasma Trp metabolites ↔ IL-6 ↔ EA ↔ HT ↔ TA CG Changes from baseline ↔ Red blood cell DHA ↔ Plasma DHA ↔ Red blood cell EPA ↔ Plasma EPA ↔ Plasma Trp metabolites ↔ IL-6 ↔ EA ↔ HT ↔ TA |
| Tsuchiya, et al. [34], 2021, Japan | Randomized, double-blind, placebo-controlled, parallel-group trial | n =23 ♂ Recreational Gn-3 n = 11 Age (mean ± SD) 20.2 ± 0.4 years Height (mean ± SD) 167.4 ± 5.4 cm Weight (mean ± SD) 65.0 ± 8.9 kg % Fat (mean ± SD) 17.2 ± 6.9% BMI (mean ± SD) 23.2 ± 2.9 kg/m2 CG n = 11 Age (mean ± SD) 19.8 ± 1.5 years Height (mean ± SD) 169.0 ± 7.8 cm Weight (mean ± SD) 65.4 ± 8.4 kg % Fat (mean ± SD) 15.7 ± 7.6% BMI (mean ± SD) 23.2 ± 3.3 kg/m2 Study withdrawals: 1 | Gn-3: 8 Softgel capsule of 300 mg /d Total: 2.4 g/d (600 mg EPA + 260 mg DHA) Nippon Suisan Kaisha Ltd., Tokyo, Japan CG: 8 softgel capsules of 300 mg/d corn oil Supplementation time: 4.5 weeks | Anthropometry UAC Biochemistry Blood lipids AA EPA DGLA DHA Dietary Intake Kcal CHO prot FAT Omega-3 Inflammatory biomarkers IL-6 Muscle damage CK Perception markers VAS Physical Performance Echo thickness Echo intensity MVIC ROM | Gn-3 vs. CG ↔ UAC ↑* EPA ↔ Kcal ↔ CHO ↔ prot ↔ FAT ↔ Omega-3 ↔ IL-6 ↓* CK ↔ VAS ↔ Echo thickness ↔ Echo intensity ↔ MVIC ↑* ROM (IP) | Gn-3 Changes from baseline ↑*UAC (IP) ↑* EPA (after 4 w) ↔ AA ↔ DGLA ↔ DHA ↔ Kcal ↔ CHO ↔ prot ↔ FAT ↔ Omega-3 ↔ CK ↔ IL-6 ↑* VAS (1–4 d) ↔ Echo thickness ↑ Echo intensity ↓* MVIC ROM: G n-3: ↓* IP and 1 d, after ↑ = to pre |
| Tsuchiya et al. [17], 2016, Japan | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 24 ♂ Recreational Gn-3 n = 12 Age (mean ± SD) 19.4 ± 0.7 years Height (mean ± SD) 174.4 ± 5.6 cm Weight (mean ± SD) 64.3 ± 7.7 kg % Fat (mean ± SD) 13.0 ± 3.5% CG n =12 Age (mean ± SD) 19.5 ± 0.8 years Height (mean ± SD) 174.3 ± 6.7 cm Weight (mean ± SD) 66.2 ± 8.0 kg % Fat (mean ± SD) 13.6 ± 2.8% Study withdrawals: 0 | Gn-3 8 Softgel capsule Fish oil Per unit: 300 mg EPA + 130 mg DHA (Nippon Suisan Kaisha Ltd. Tokyo) CG 8 Softgel capsule Per unit: 300 mg corn oil (Nippon Suisan Kaisha Ltd. Tokyo) Supplementation time: 8 weeks prior to exercise + 5 days after exercise | Anthropometry UAC Biochemistry AA DHA Inflammatory biomarkers IL-6 TNF-α Muscle damage CK Mb Perception markers VAS brachii VAS brachialis VAS brachioradialis Physical Performance MVC torque ROM | Gn-3 vs. CG ↔ UAC ↔ AA ↔ DHA ↓* IL-6 ↔ TNF-α ↔ CK ↔ Mb ↔ VAS brachii ↓* VAS brachialis ↔VAS brachioradialis ↑* MVC ↑* ROM | Gn-3 Changes from baseline ↔ UAC ↔ AA ↔ DHA ↔ IL-6 ↔ TNF-α ↔ CK ↔ Mb ↑ VAS brachii (at day 1–3) ↑* VAS brachialis (at day 2) ↔VAS brachioradialis ↓*MVC ↓ ROM CG Changes from baseline ↔ UAC ↔ AA ↔ DHA ↑* IL-6 (at day 3) ↔ TNF-α ↔ CK ↑* Mb ↑ VAS brachii (day 1 to day 3) ↑* VAS brachialis (day 1 to day 3) ↔ VAS brachioradialis ↓* MVC ↓* ROM (at day 3) |
| VanDusseldrorp et al. [35], 2020, United States | Randomized, double-blind, placebo-controlled, parallel-group trial | n = 32 (16 ♂ and 16 ♀) Physically active: 3 to 5 d/w, minimum of 3 h/w and a maximum of 8 h/w and no more than 2 h/w of aerobic exercise 2 g Gn-3 n = 8 (4♂ and 4♀) Age (mean ± SD) 23.5 ± 3.3 years Height (mean ± SD) 170.9 ± 6.9 cm Weight (mean ± SD) 76.1 ± 14.2 kg % Fat (mean ± SD) 20.8 ± 4.1% 4g Gn-3 n = 8 (4♂ and 4♀) Age (mean ± SD) 23.3 ± 3.0 years Height (mean ± SD) 172.9 ± 4.7 cm Weight (mean ± SD) 69.7 ± 15.9 kg % Fat (mean ± SD) 19.0 ± 6.2% 6g Gn-3 n = 8 (4♂ and 4♀) Age (mean ± SD) 23.8 ± 2.8 years Height (mean ± SD) 173.8 ± 7.6 cm Weight (mean ± SD) 72.8 ± 13.5 kg % Fat (mean ± SD) 19.4 ± 6.1% CG n = 8 (4♂ and 4♀) Age (mean ± SD) 23.0 ± 3.0 years Height (mean ± SD) 173.6 ± 6.2 cm Weight (mean ± SD) 67.9 ± 10.7 kg % Fat (mean ± SD) 20.6 ± 7.2% Study withdrawals: 0 | Gn-3 Capsule Per unit: 400 mg EPA + 300 mg DHA 2 g Gn-3 2 g/d (1400 mg: 800 mg EPA + 600 mg DHA) 4 g Gn-3 4 g/d (2800 mg: 1600 mg EPA + 1200 mg DHA) 6g Gn-3 6 g/d (4200 mg: 2400 mg EPA + 1800 mg DHA) (MusclePharm, Denver, USA) CG Safflower oil (Capsule Muscle Pharm) Supplementation time: 7.5 weeks | Muscle damage CK LDH Perception markers VAS Performance MVIC VJ height 40 yd Sprint | 2 g Gn-3, 4g Gn-, 6g Gn-3 vs. CG 24 h: ↓*6 g Gn-3 vs. 2 g Gn-3 48 h: ↓* 6 g Gn-3 vs. 4 g Gn-3 72 h: ↓* 6 g Gn-3 vs. CG LDH ↓* 6 g Gn-3 vs. CG (at to 72 h) ↓* 6 g Gn-3 vs. 2 g Gn-3 (at to 72 h) VAS 2 h: CG ↑* vs. 6 g Gn-3 24 h: ↓* 4 g Gn-3 vs. CG ↑* CG vs. 6 g Gn-3 48 h: ↑* CG vs. 6 g Gn-3 ↓* 6 g Gn-3 vs. 4 g Gn-3, 2 g Gn-3 72 h: ↑* CG vs. 4 g Gn-3 ↑* CG vs. 6 g Gn-3 ↔ MVIC ↓* VJ height CG ↔ 40 yd Sprint | Changes from baseline ↑* CK in all group ↑* LDH in all group ↓* 40 yd Sprint VAS ↓* MVIC (until 70 h) ↓ VJ height (until 48 h) ↑* in all group (24 h) ↑* CG, 2 g Gn-3, 4 g Gn-3 (48 h) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Arribalzaga, S.; Gutiérrez-Abejón, E.; Azarbayjani, M.A.; Mielgo-Ayuso, J.; Roche, E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 2044. https://doi.org/10.3390/nu16132044
Fernández-Lázaro D, Arribalzaga S, Gutiérrez-Abejón E, Azarbayjani MA, Mielgo-Ayuso J, Roche E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials. Nutrients. 2024; 16(13):2044. https://doi.org/10.3390/nu16132044
Chicago/Turabian StyleFernández-Lázaro, Diego, Soledad Arribalzaga, Eduardo Gutiérrez-Abejón, Mohammad Ali Azarbayjani, Juan Mielgo-Ayuso, and Enrique Roche. 2024. "Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials" Nutrients 16, no. 13: 2044. https://doi.org/10.3390/nu16132044
APA StyleFernández-Lázaro, D., Arribalzaga, S., Gutiérrez-Abejón, E., Azarbayjani, M. A., Mielgo-Ayuso, J., & Roche, E. (2024). Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials. Nutrients, 16(13), 2044. https://doi.org/10.3390/nu16132044










