The Impact of Dietary Carbohydrates on Inflammation-Related Cardiovascular Disease Risk: The ATTICA Study (2002–2022)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Participants
2.3. Endpoint and Follow-Up Examination
2.4. Baseline Assessment
2.4.1. Socio-Demographic Characteristics and Lifestyle
2.4.2. Lifestyle Characteristics
2.4.3. Anthropometric Measurements
2.4.4. Dietary Ascertainment
2.4.5. Biochemical Measurements and Clinical Characteristics
2.5. Follow-Up Assessment
2.6. Statistical Analysis
3. Results
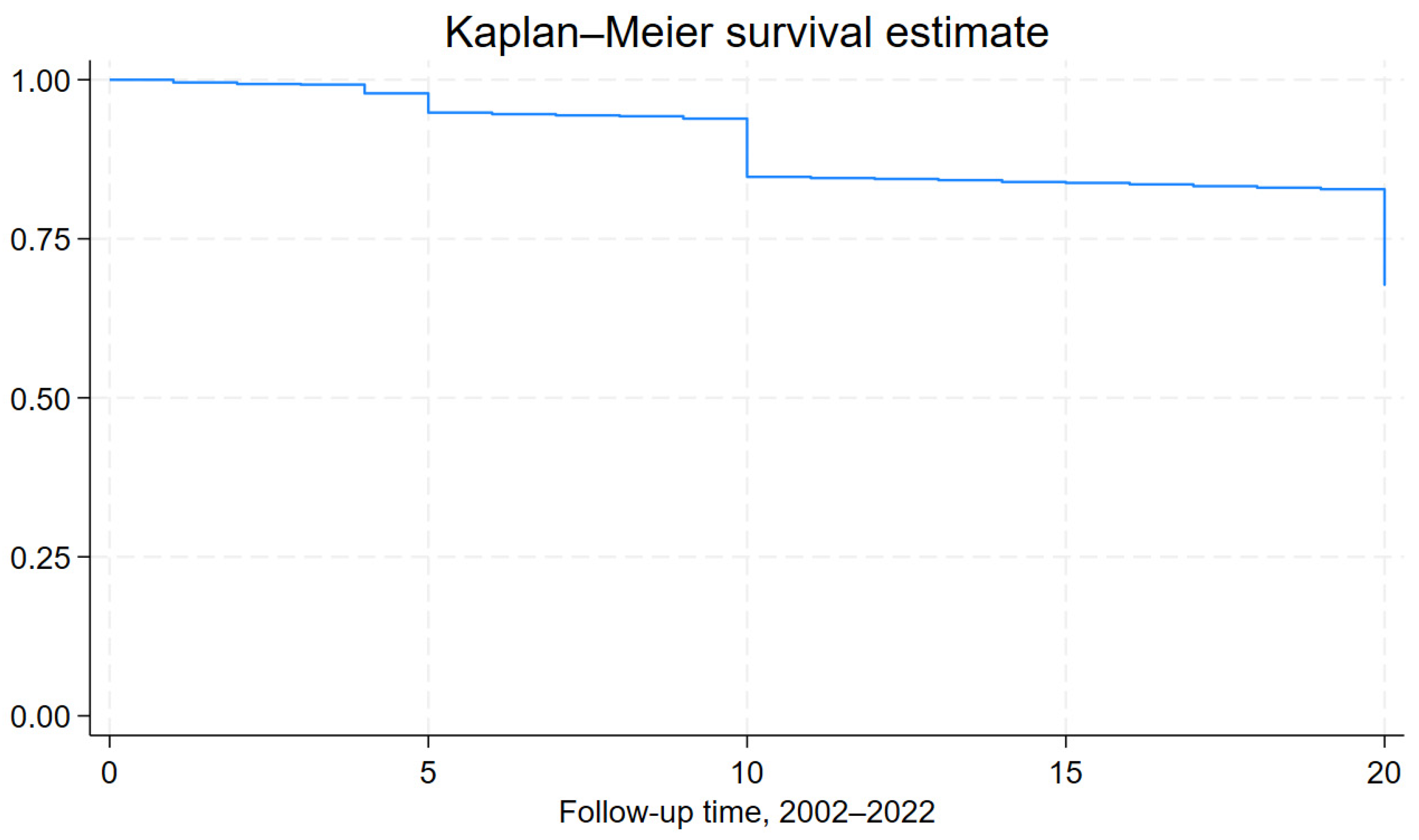
3.1. CVD Incidence and Mortality at 20-Year Follow-Up
3.2. Participants’ Characteristics by Carbohydrate Intake and Carbohydrate Quality
3.3. Inflammation Indices and 20-Year CVD Incidence
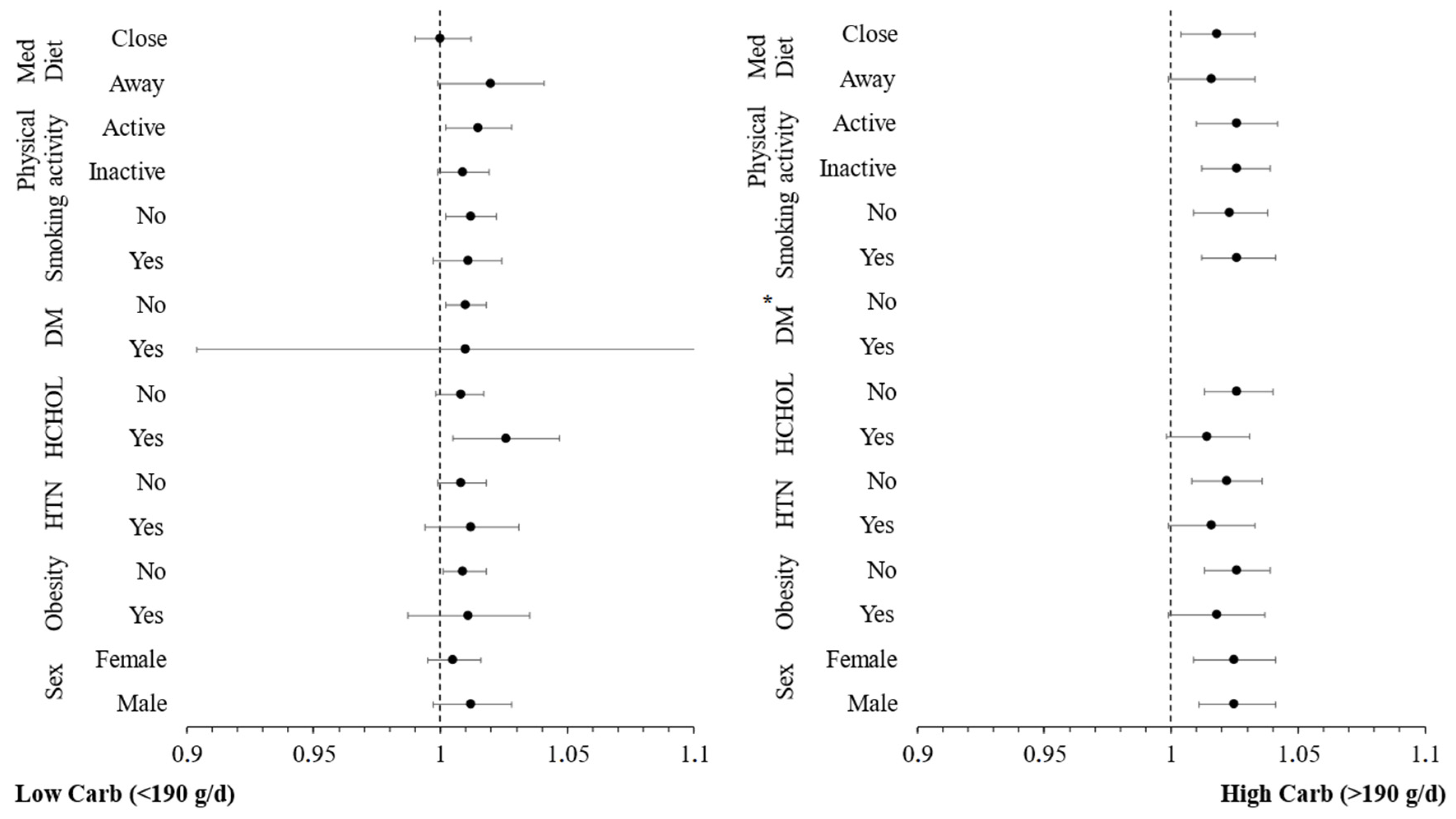
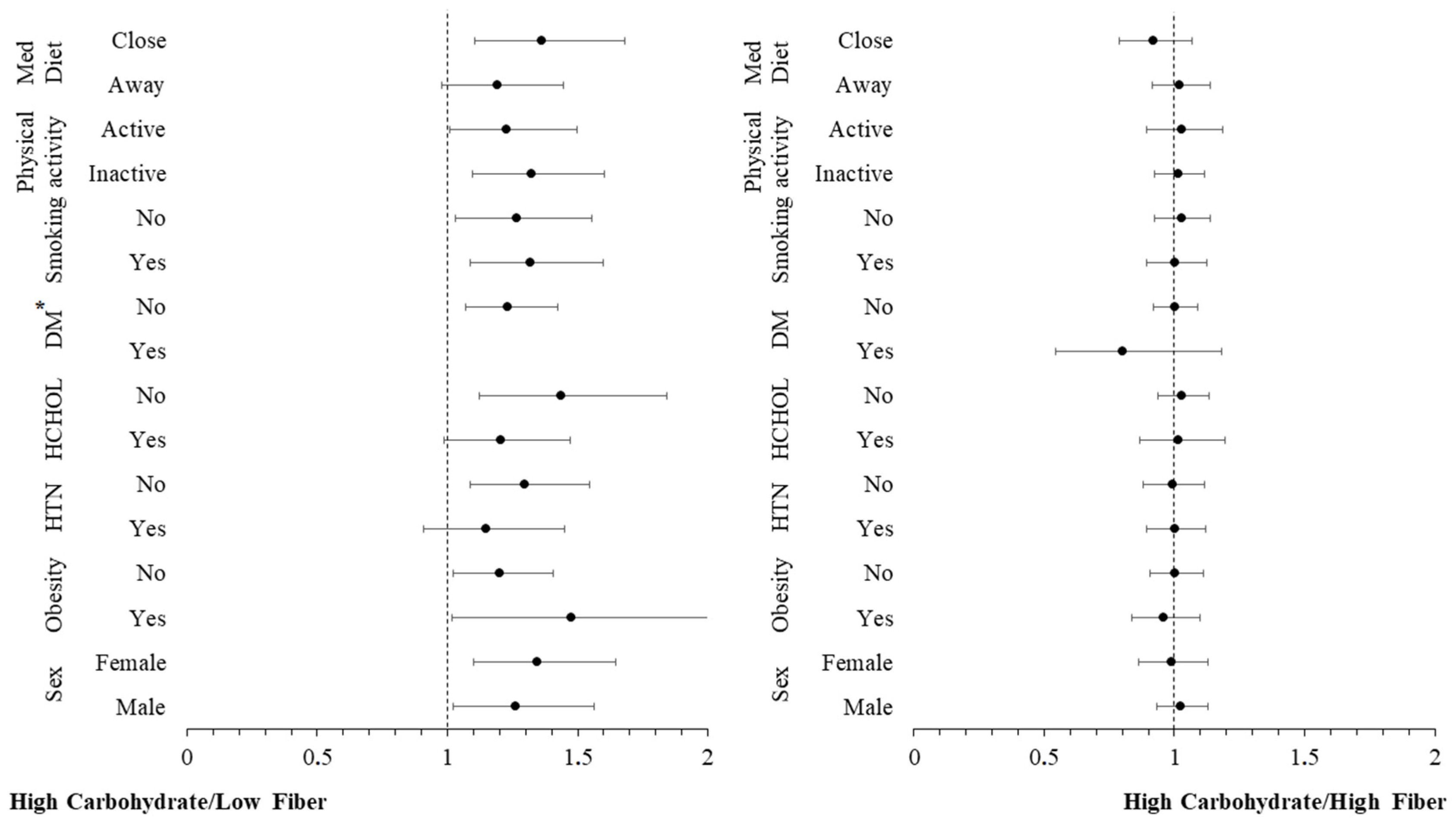
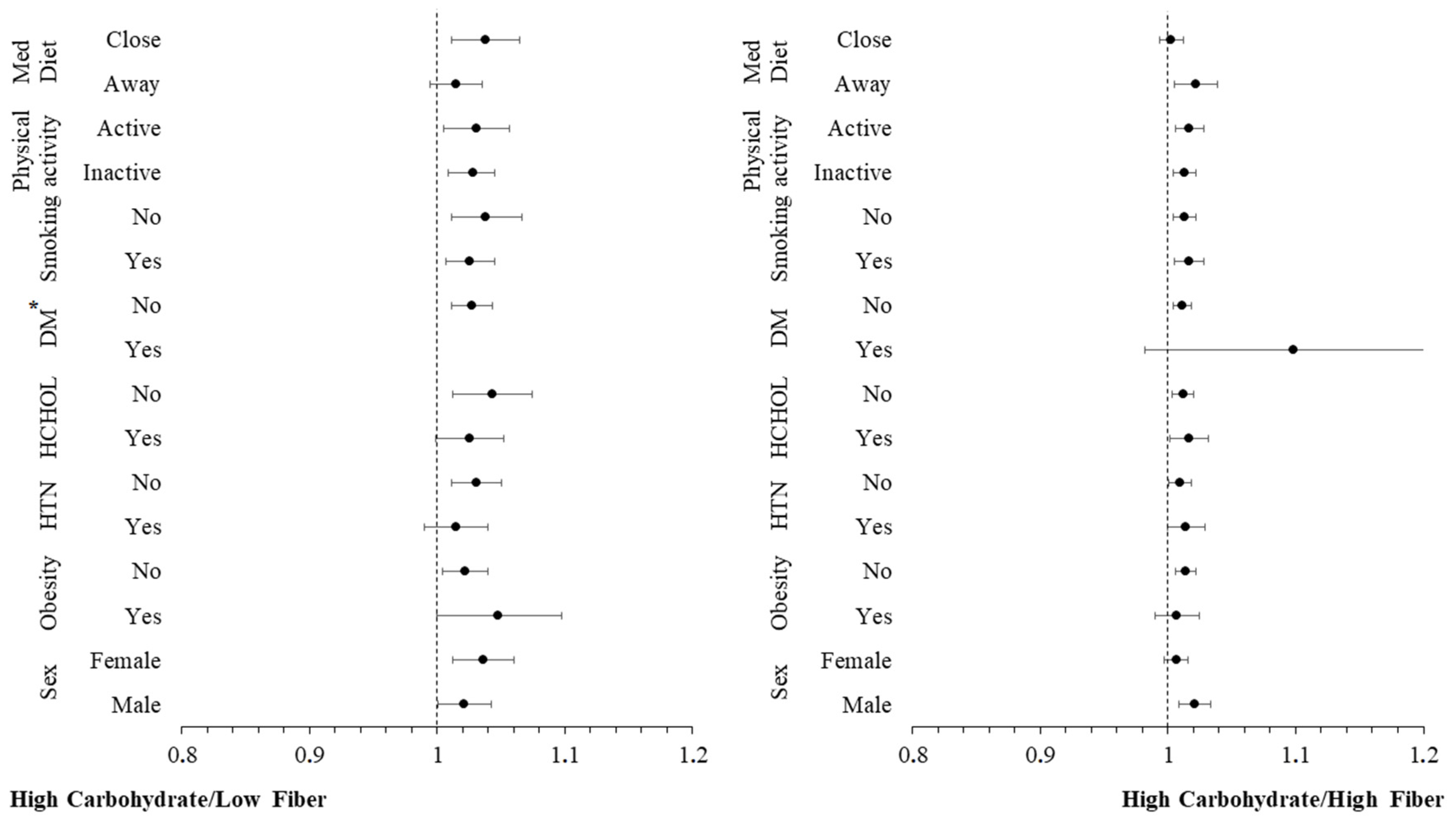
3.4. Moderation Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Huang, C.; Jiang, B.; Wang, X.; Yang, Y.; Ma, J.; Chen, S.; Hu, D.; Bo, Y. Dietary carbohydrate quantity and quality and risk of cardiovascular disease, all-cause, cardiovascular and cancer mortality: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Kasim-Karakas, S.E.; Tsodikov, A.; Singh, U.; Jialal, I. Responses of inflammatory markers to a low-fat, high-carbohydrate diet: Effects of energy intake. Am. J. Clin. Nutr. 2006, 83, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Hickling, S.; Hung, J.; Knuiman, M.; Divitini, M.; Beilby, J. Are the associations between diet and C-reactive protein independent of obesity? Prev. Med. 2008, 47, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, J.; Zhang, Z.; Zhang, H.; Wang, N.; Chen, X.; Han, X.; Lu, Q.; Chi, S. Effects of Dietary Intervention on Inflammatory Markers in Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 846591. [Google Scholar] [CrossRef] [PubMed]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, e128308. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hua, R.; Hu, K.; Wang, Z. Carbohydrates deteriorate fatty liver by activating the inflammatory response. Nutr. Res. Rev. 2022, 35, 252–267. [Google Scholar] [CrossRef]
- Tavakoli, A.; Mirzababaei, A.; Sajadi, F.; Mirzaei, K. Circulating inflammatory markers may mediate the relationship between low carbohydrate diet and circadian rhythm in overweight and obese women. BMC Women’s Health 2021, 21, 87. [Google Scholar] [CrossRef]
- Wu, S.; Jia, W.; He, H.; Yin, J.; Xu, H.; He, C.; Zhang, Q.; Peng, Y.; Cheng, R. A New Dietary Fiber Can Enhance Satiety and Reduce Postprandial Blood Glucose in Healthy Adults: A Randomized Cross-Over Trial. Nutrients 2023, 15, 4569. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.B.; Chrysohoou, C.; Stefanadis, C. Epidemiology of cardiovascular risk factors in Greece: Aims, design and baseline characteristics of the ATTICA study. BMC Public Health 2003, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Skoumas, I.; Stefanadis, C.; Study, A. Five-year incidence of cardiovascular disease and its predictors in Greece: The ATTICA study. Vasc. Med. 2008, 13, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Georgousopoulou, E.N.; Pitsavos, C.; Chrysohoou, C.; Metaxa, V.; Georgiopoulos, G.A.; Kalogeropoulou, K.; Tousoulis, D.; Stefanadis, C.; ATTICA Study Group. Ten-year (2002–2012) cardiovascular disease incidence and all-cause mortality, in urban Greek population: The ATTICA Study. Int. J. Cardiol. 2015, 180, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hell. J. Cardiol. 2009, 50, 283–294. [Google Scholar]
- Kavouras, S.A.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Anastasiou, C.A.; Lentzas, Y.; Stefanadis, C. Physical activity, obesity status, and glycemic control: The ATTICA study. Med. Sci. Sports Exerc. 2007, 39, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Katsouyanni, K.; Rimm, E.B.; Gnardellis, C.; Trichopoulos, D.; Polychronopoulos, E.; Trichopoulou, A. Reproducibility and relative validity of an extensive semi-quantitative food frequency questionnaire using dietary records and biochemical markers among Greek schoolteachers. Int. J. Epidemiol. 1997, 26 (Suppl. S1), S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Buyken, A.E.; Goletzke, J.; Joslowski, G.; Felbick, A.; Cheng, G.; Herder, C.; Brand-Miller, J.C. Association between carbohydrate quality and inflammatory markers: Systematic review of observational and interventional studies. Am. J. Clin. Nutr. 2014, 99, 813–833. [Google Scholar] [CrossRef]
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef]
- Barrea, L.; Marzullo, P.; Muscogiuri, G.; Di Somma, C.; Scacchi, M.; Orio, F.; Aimaretti, G.; Colao, A.; Savastano, S. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr. Res. Rev. 2018, 31, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Garay-Sevilla, M.E.; Rojas, A.; Portero-Otin, M.; Uribarri, J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients 2021, 13, 2802. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Sieri, S.; Agnoli, C.; Grioni, S.; Weiderpass, E.; Mattiello, A.; Sluijs, I.; Sanchez, M.J.; Jakobsen, M.U.; Sweeting, M.; van der Schouw, Y.T.; et al. Glycemic index, glycemic load, and risk of coronary heart disease: A pan-European cohort study. Am. J. Clin. Nutr. 2020, 112, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Buyken, A.E.; Flood, V.; Empson, M.; Rochtchina, E.; Barclay, A.W.; Brand-Miller, J.; Mitchell, P. Carbohydrate nutrition and inflammatory disease mortality in older adults. Am. J. Clin. Nutr. 2010, 92, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Oxidative mechanisms and atherothrombotic cardiovascular disease. Drug Discov. Today Ther. Strateg. 2008, 5, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Bermudez, E.A.; Rifai, N.; Buring, J.; Manson, J.E.; Ridker, P.M. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1668–1673. [Google Scholar] [CrossRef]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017, 173, 305–317. [Google Scholar] [CrossRef]
- Baye, E.; de Courten, M.P.; Walker, K.; Ranasinha, S.; Earnest, A.; Forbes, J.M.; de Courten, B. Effect of dietary advanced glycation end products on inflammation and cardiovascular risks in healthy overweight adults: A randomised crossover trial. Sci. Rep. 2017, 7, 4123. [Google Scholar] [CrossRef]
- Kelly, R.K.; Tong, T.Y.N.; Watling, C.Z.; Reynolds, A.; Piernas, C.; Schmidt, J.A.; Papier, K.; Carter, J.L.; Key, T.J.; Perez-Cornago, A. Associations between types and sources of dietary carbohydrates and cardiovascular disease risk: A prospective cohort study of UK Biobank participants. BMC Med. 2023, 21, 34. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Jo, U.; Park, K. Carbohydrate Intake and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2023, 15, 1740. [Google Scholar] [CrossRef]
- Pereira, M.A. Dietary carbohydrate and cardiometabolic risk: Quality over quantity. Am. J. Clin. Nutr. 2020, 111, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Slyper, A.H. The influence of carbohydrate quality on cardiovascular disease, the metabolic syndrome, type 2 diabetes, and obesity—An overview. J. Pediatr. Endocrinol. Metab. 2013, 26, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.M.; Amoutzopoulos, B.; Batterham, M.J.; Ray, S.; Beck, E.J. Whole grain intake compared with cereal fibre intake in association to CVD risk factors: A cross-sectional analysis of the National Diet and Nutrition Survey (UK). Public Health Nutr. 2020, 23, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Rangarajan, S.; Srichaikul, K.; Mohan, V.; Avezum, A.; Diaz, R.; Rosengren, A.; et al. Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N. Engl. J. Med. 2021, 384, 1312–1322. [Google Scholar] [CrossRef]
- Ma, Y.; Hébert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K.; et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Kazeminasab, F.; Miraghajani, M.; Khalafi, M.; Sakhaei, M.H.; Rosenkranz, S.K.; Santos, H.O. Effects of low-carbohydrate diets, with and without caloric restriction, on inflammatory markers in adults: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Clin. Nutr. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Forsythe, C.E.; Phinney, S.D.; Fernandez, M.L.; Quann, E.E.; Wood, R.J.; Bibus, D.M.; Kraemer, W.J.; Feinman, R.D.; Volek, J.S. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, R.; Biggs, M.L.; Djousse, L.; Durda, P.J.; Kizer, J.R.; Psaty, B.; Reiner, A.P.; Tracy, R.P.; Siscovick, D.; Mukamal, K.J. Intake and Sources of Dietary Fiber, Inflammation, and Cardiovascular Disease in Older US Adults. JAMA Netw. Open 2022, 5, e225012. [Google Scholar] [CrossRef]
- Bouzid, Y.; Alkan, Z.; Stephensen, C.; Lemay, D. Low Intake of Dietary Fiber Is Associated with Gastrointestinal Inflammation in Healthy U.S. Adults. Curr. Dev. Nutr. 2022, 6, 974. [Google Scholar] [CrossRef]
- Nash, D.; Hughes, M.G.; Butcher, L.; Aicheler, R.; Smith, P.; Cullen, T.; Webb, R. IL-6 signaling in acute exercise and chronic training: Potential consequences for health and athletic performance. Scand. J. Med. Sci. Sports 2023, 33, 4–19. [Google Scholar] [CrossRef]
- Volek, J.S.; Yancy, W.S.; Gower, B.A.; Phinney, S.D.; Slavin, J.; Koutnik, A.P.; Hurn, M.; Spinner, J.; Cucuzzella, M.; Hecht, F.M. Expert consensus on nutrition and lower-carbohydrate diets: An evidence- and equity-based approach to dietary guidance. Front. Nutr. 2024, 11, 1376098. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Aids, P.; Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; et al. Safety assessment of the substance silver nanoparticles for use in food contact materials. EFSA J. 2021, 19, e06790. [Google Scholar]
- Zello, G.A. Dietary Reference Intakes for the macronutrients and energy: Considerations for physical activity. Appl. Physiol. Nutr. Metab. 2006, 31, 74–79. [Google Scholar] [CrossRef]


| Status at 20-Year Follow-Up | ||||
|---|---|---|---|---|
| Overall (2002) | CVD Free (n = 1270) | CVD Event (n = 718) | p Value | |
| Demographic and lifestyle factors | ||||
| Age, mean ± SD | 45 ± 14 | 38 ± 9 | 58 ± 11 | <0.001 |
| Male sex | 50% | 46% | 55% | <0.001 |
| Smoking Pack years, mean ± SD | 497 ± 501 | 375 ± 340 | 703 ± 633 | <0.001 |
| Physical activity (2002–2012) | <0.001 | |||
| Remained inactive | 50% | 46% | 56% | |
| Remained active | 13% | 13% | 14% | |
| Became inactive | 28% | 27% | 29% | |
| Became active | 10% | 14% | 2% | |
| MedDietScore (0–55), mean ± SD | 26 ± 7 | 27 ± 6 | 23 ± 6 | <0.001 |
| Carbohydrate intake | ||||
| g/day | 211 ± 97 | 215 ± 97 | 201 ± 91 | 0.088 |
| % total energy intake | 36.9 ± 6.5 | 36.8 ± 6.1 | 36.8 ± 6.9 | 0.946 |
| Clinical factors | ||||
| Obesity | 18% | 14% | 27% | <0.001 |
| Diabetes at baseline | 7% | 1% | 17% | <0.001 |
| Hypertension at baseline | 30% | 20% | 51% | <0.001 |
| Hypercholesterolemia at baseline | 40% | 30% | 65% | <0.001 |
| Family history of CVD | 36% | 36% | 39% | 0.210 |
| Inflammation indices | ||||
| hs-CRP (mg/L), mean ± SD | 1.94 ± 2.42 | 1.78 ± 2.42 | 2.26 ± 2.44 | <0.001 |
| IL-6 (pg/mL), mean ± SD | 1.46 ± 0.55 | 1.36 ± 0.46 | 1.63 ± 0.62 | <0.001 |
| TNF-α (pg/mL), mean ± SD | 6.21 ± 4.90 | 5.63 ± 4.55 | 7.77 ± 5.03 | <0.001 |
| Carbohydrate Intake (g/day) | |||
|---|---|---|---|
| Low (<190 g/day) | High (>190 g/day) | p Value | |
| Demographic and lifestyle factors | |||
| Age, mean ± SD | 41 ± 11 | 39 ± 11 | 0.005 |
| Male sex | 49% | 60% | <0.001 |
| Smoking Pack years, mean ± SD | 442 ± 412 | 418 ± 420 | 0.478 |
| Physical activity (2002–2012) | 0.155 | ||
| Remained inactive | 43% | 39% | |
| Remained active | 16% | 22% | |
| Became inactive | 27% | 25% | |
| Became active | 14% | 14% | |
| MedDietScore (0–55), mean ± SD | 26 ± 6 | 29 ± 10 | <0.001 |
| Clinical factors | |||
| Obesity | 14% | 18% | 0.150 |
| Diabetes at baseline | 5% | 4% | 0.286 |
| Hypertension at baseline | 25% | 29% | 0.178 |
| Hypercholesterolemia at baseline | 33% | 29% | 0.223 |
| Family history of CVD | 36% | 35% | 0.955 |
| Inflammation indices | |||
| hs-CRP (mg/L), mean ± SD | 1.89 ± 2.39 | 2.03 ± 2.63 | 0.393 |
| IL-6 (pg/mL), mean ± SD | 1.42 ± 0.36 | 1.41 ± 0.36 | 0.778 |
| TNF-α (pg/mL), mean ± SD | 6.35 ± 3.18 | 6.31 ± 2.89 | 0.829 |
| High Carbohydrate/Low Fiber | High Carbohydrate/High Fiber | p Value | |
|---|---|---|---|
| Demographic and lifestyle factors | |||
| Age, mean ± SD | 39 ± 11 | 40 ± 11 | 0.212 |
| Male sex | 54% | 54% | 0.972 |
| Smoking Pack years, mean ± SD | 435 ± 383 | 430 ± 423 | 0.906 |
| Physical activity (2002–2012) | 0.310 | ||
| Remained inactive | 47% | 40% | |
| Remained active | 20% | 18% | |
| Became inactive | 22% | 27% | |
| Became active | 12% | 14% | |
| MedDietScore (0–55), mean ± SD | 27 ± 8 | 27 ± 8 | 0.951 |
| Clinical factors | |||
| Obesity | 16% | 16% | 0.817 |
| Diabetes at baseline | 2% | 5% | 0.093 |
| Hypertension at baseline | 20% | 29% | 0.012 |
| Hypercholesterolemia at baseline | 36% | 30% | 0.117 |
| Family history of CVD | 30% | 37% | 0.085 |
| Inflammation indices | |||
| hs-CRP (mg/L), mean ± SD | 2.17 ± 2.86 | 1.92 ± 2.43 | 0.262 |
| IL-6 (pg/mL), mean ± SD | 1.43 ± 0.39 | 1.41 ± 0.35 | 0.640 |
| TNF-α (pg/mL), mean ± SD | 6.59 ± 3.30 | 6.27 ± 2.97 | 0.216 |
| HR (95% CI) of CVD | ||||
|---|---|---|---|---|
| Overall | ||||
| Crude model | Model 1 | Model 2 | Model 3 | |
| hs-CRP (per 1 mg/L) | 1.079 (1.038–1.122) * | 1.053 (0.991–1.119) | 1.049 (0.955–1.151) | 1.051 (0.948–1.165) |
| IL-6 (per 0.01 pg/mL) | 1.012 (1.009–1.014) * | 1.000 (0.997–1.003) | 1.000 (0.996–1.004) | 1.006 (0.998–1.013) |
| TNF-α (per 0.1 pg/mL) | 1.009 (1.007–1.012) * | 1.001 (0.997–1.005) | 1.003 (0.995–1.012) | 1.001 (0.991–1.010) |
| Low Carbohydrate intake (<190 g/day) | ||||
| Crude model | Model 1 | Model 2 | Model 3 | |
| hs-CRP (per 1 mg/L) | 1.045 (0.949–1.151) | 0.996 (0.870–1.141) | 0.919 (0.782–1.079) | 0.886 (0.735–1.068) |
| IL-6 (per 0.01 pg/mL) | 1.018 (1.010–1.026) * | 1.005 (0.993–1.016) | 0.999 (0.985–1.013) | 0.998 (0.983–1.014) |
| TNF-α (per 0.1 pg/mL) | 1.012 (1.004–1.019) * | 0.996 (0.982–1.010) | 0.987 (0.968–1.006) | 0.983 (0.961–1.005) |
| High Carbohydrate intake (>190 g/day) | ||||
| Crude model | Model 1 | Model 2 | Model 3 | |
| hs-CRP (per 1 mg/L) | 1.103 (1.014–1.199) * | 1.127 (1.004–1.266) * | 1.127 (0.990–1.284) | 1.160 (1.004–1.341) * |
| IL-6 (per 0.01 pg/mL) | 1.017 (1.010–1.024) * | 1.007 (0.998–1.016) | 1.007 (0.997–1.016) | 1.010 (0.999–1.020) |
| TNF-α (per 0.1 pg/mL) | 1.024 (1.014–1.035) * | 1.012 (0.997–1.027) | 1.014 (0.998–1.031) | 1.014 (0.997–1.032) |
| HR (95% CI) of CVD | ||||
|---|---|---|---|---|
| High carbohydrate/Low fiber | ||||
| Crude model | Model 1 | Model 2 | Model 3 | |
| hs-CRP (per 1 mg/L) | 1.28 (1.11–1.47) * | 1.25 (1.04–1.505) * | 1.28 (1.03–1.60) * | 1.40 (1.04–1.88) * |
| IL-6 (per 0.01 pg/mL) | 1.03 (1.01–1.04) * | 1.02 (1.005–1.040) * | 1.03 (1.01–1.04) * | 1.03 (1.00–1.06) * |
| TNF-α (per 0.1 pg/mL) | 1.03 (1.01–1.04) * | 1.02 (0.996–1.037) | 1.02 (0.99–1.04) | 1.02 (0.98–1.04) |
| High carbohydrate/High fiber | ||||
| Crude model | Model 1 | Model 2 | Model 3 | |
| hs-CRP (per 1 mg/L) | 1.02 (0.94–1.09) | 1.01 (0.90–1.12) | 0.96 (0.85–1.09) | 0.97 (0.84–1.11) |
| IL-6 (per 0.01 pg/mL) | 1.01 (1.01–1.02) * | 1.00 (0.99–1.01) | 0.99 (0.99–1.01) | 1.001 (0.99–1.01) |
| TNF-α (per 0.1 pg/mL) | 1.01 (1.01–1.02) * | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) |
| p for interaction | ||||
| Crude model | Model 1 | Model 2 | Model 3 | |
| hs-CRP | 0.004 | 0.052 | 0.045 | 0.095 |
| IL-6 | 0.020 | 0.047 | 0.043 | 0.095 |
| TNF-α | 0.089 | 0.159 | 0.164 | 0.168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakopoulou, S.-P.; Antonopoulou, S.; Chrysohoou, C.; Barkas, F.; Tsioufis, C.; Pitsavos, C.; Liberopoulos, E.; Sfikakis, P.P.; Panagiotakos, D. The Impact of Dietary Carbohydrates on Inflammation-Related Cardiovascular Disease Risk: The ATTICA Study (2002–2022). Nutrients 2024, 16, 2051. https://doi.org/10.3390/nu16132051
Giannakopoulou S-P, Antonopoulou S, Chrysohoou C, Barkas F, Tsioufis C, Pitsavos C, Liberopoulos E, Sfikakis PP, Panagiotakos D. The Impact of Dietary Carbohydrates on Inflammation-Related Cardiovascular Disease Risk: The ATTICA Study (2002–2022). Nutrients. 2024; 16(13):2051. https://doi.org/10.3390/nu16132051
Chicago/Turabian StyleGiannakopoulou, Sofia-Panagiota, Smaragdi Antonopoulou, Christina Chrysohoou, Fotios Barkas, Costas Tsioufis, Christos Pitsavos, Evangelos Liberopoulos, Petros P. Sfikakis, and Demosthenes Panagiotakos. 2024. "The Impact of Dietary Carbohydrates on Inflammation-Related Cardiovascular Disease Risk: The ATTICA Study (2002–2022)" Nutrients 16, no. 13: 2051. https://doi.org/10.3390/nu16132051
APA StyleGiannakopoulou, S.-P., Antonopoulou, S., Chrysohoou, C., Barkas, F., Tsioufis, C., Pitsavos, C., Liberopoulos, E., Sfikakis, P. P., & Panagiotakos, D. (2024). The Impact of Dietary Carbohydrates on Inflammation-Related Cardiovascular Disease Risk: The ATTICA Study (2002–2022). Nutrients, 16(13), 2051. https://doi.org/10.3390/nu16132051







