Advances in the Anti-Atherosclerotic Mechanisms of Epigallocatechin Gallate
Abstract
:1. Introduction
2. Sources, Structure, and Physiological Functions of EGCG
2.1. Sources and Structure of EGCG
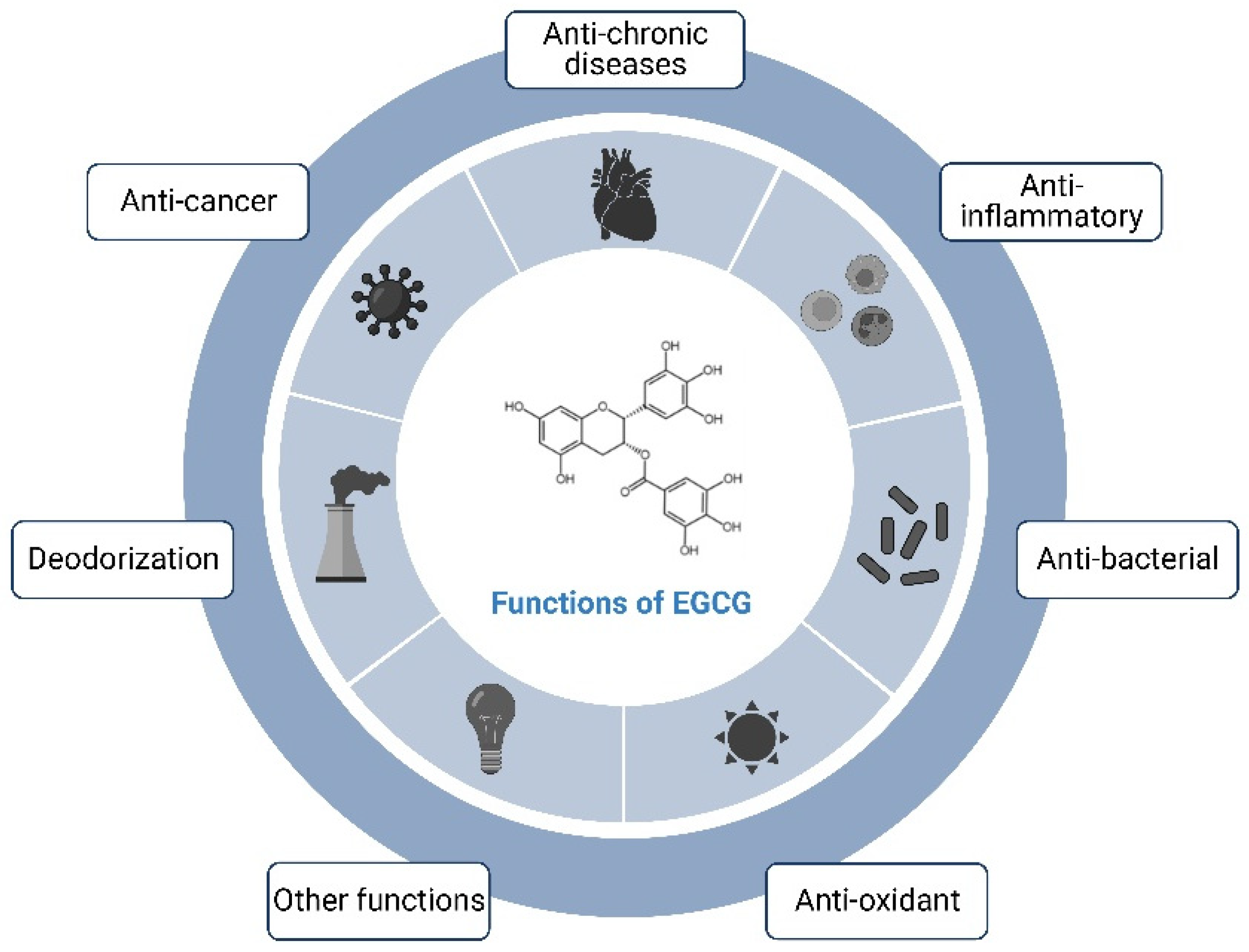
2.2. Physiological Functions of the EGCG
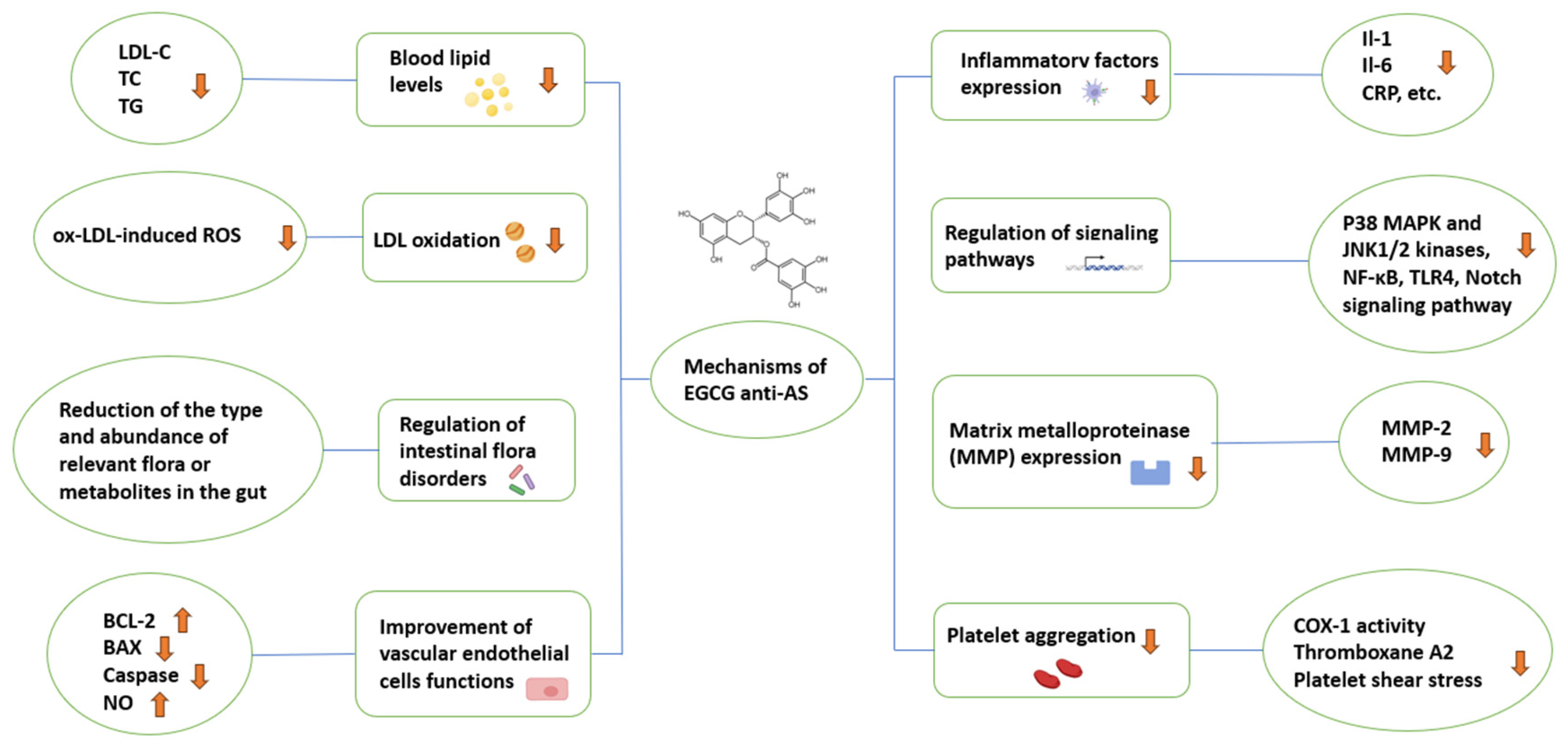
3. Studies on the Mechanisms of EGCG against AS
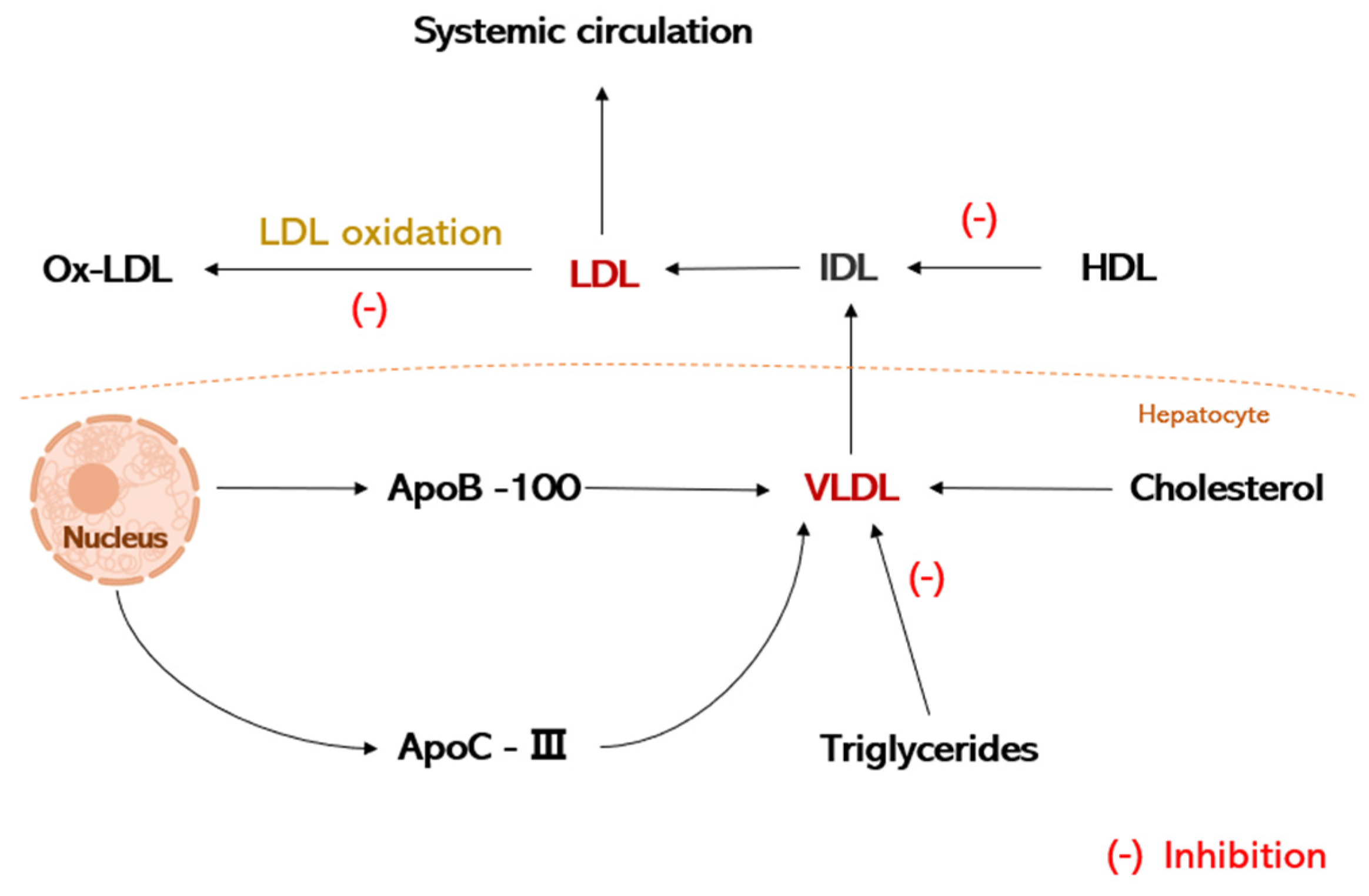
3.1. Regulation of Lipid Metabolism
3.2. Regulation of Intestinal Flora Disorders
3.3. Improvement of Vascular Endothelial Cells Functions
3.4. Inhibition of Inflammatory Factors Expression
3.5. Regulation of Inflammatory Signaling Pathways
3.6. Inhibition of Matrix Metalloproteinase (MMP) Expression
3.7. Inhibition of Platelet Aggregation
4. Summary of EGCG Anti-AS Mechanisms and Shortcomings
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tapeinos, C.; Gao, H.; Bauleth-Ramos, T.; Santos, H.A. Progress in Stimuli-Responsive Biomaterials for Treating Cardiovascular and Cerebrovascular Diseases. Small 2022, 18, e2200291. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Bi, M.; Xu, J.; Yang, H.; Zhang, Y. Noble Gases Therapy in Cardiocerebrovascular Diseases: The Novel Stars? Front. Cardiovasc. Med. 2022, 9, 802783. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Zhang, D.; Lei, X.; Ma, Y.; Cao, Y.; Chang, J. Targeting Reactive Oxygen Species in Atherosclerosis via Chinese Herbal Medicines. Oxid. Med. Cell. Longev. 2022, 2022, 1852330. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Jukema, J.W.; van der Hoorn, J.W. Amlodipine and atorvastatin in atherosclerosis: A review of the potential of combination therapy. Expert. Opin. Pharmacother. 2004, 5, 459–468. [Google Scholar] [CrossRef]
- Rognoni, A.; Cavallino, C.; Lupi, A.; Secco, G.G.; Veia, A.; Bacchini, S.; Rosso, R.; Brunelleschi, S.; Rametta, F.; Bongo, A.S. Ticagrelor: A novel drug for an old problem. Recent. Pat. Cardiovasc. Drug Discov. 2014, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mangels, D.R.; Mohler, E.R., 3rd. Catechins as Potential Mediators of Cardiovascular Health. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 757–763. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods Release 3; U.S. Department of Argiculture: Washington, DC, USA, 2011; pp. 1–156.
- Dos Santos, A.N.; de L. Nascimento, T.R.; Gondim, B.L.C.; Velo, M.; de A. Rêgo, R.I.; do C. Neto, J.R.; Machado, J.R.; da Silva, M.V.; de Araújo, H.W.C.; Fonseca, M.G.; et al. Catechins as Model Bioactive Compounds for Biomedical Applications. Curr. Pharm. Des. 2020, 26, 4032–4047. [Google Scholar] [CrossRef]
- Tian, C.; Zhen, T.; Ma, M.; Li, M.; Sun, Q. Contribution of catechin monomers in tea polyphenols to the structure and physicochemical properties of wheat gluten and its sub-fractions. J. Cereal Sci. 2021, 101, 103306. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Jing, H.; Ye, X.; Jiang, C.; Shao, J.; Ma, C.; Wang, H. Separation of epigallocatechin gallate and epicatechin gallate from tea polyphenols by macroporous resin and crystallization. Anal. Methods 2021, 13, 832–842. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Q.Y.; Tsang, D.; Huang, Y. Degradation of green tea catechins in tea drinks. J. Agric. Food Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Tian, J.; Geiss, C.; Zarse, K.; Madreiter-Sokolowski, C.T.; Ristow, M. Green tea catechins EGCG and ECG enhance the fitness and lifespan of Caenorhabditis elegans by complex I inhibition. Aging 2021, 13, 22629–22648. [Google Scholar] [CrossRef]
- Kim-Park, W.K.; Allam, E.S.; Palasuk, J.; Kowolik, M.; Park, K.K.; Windsor, L.J. Green tea catechin inhibits the activity and neutrophil release of Matrix Metalloproteinase-9. J. Tradit. Complement. Med. 2016, 6, 343–346. [Google Scholar] [CrossRef]
- Nugala, B.; Namasi, A.; Emmadi, P.; Krishna, P.M. Role of green tea as an antioxidant in periodontal disease: The Asian paradox. J. Indian Soc. Periodontol. 2012, 16, 313–316. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T. Antimicrobial Activities of Tea Polyphenol on Phytopathogens: A Review. Molecules 2019, 24, 816. [Google Scholar] [CrossRef]
- Thakur, V.S.; Gupta, K.; Gupta, S. The chemopreventive and chemotherapeutic potentials of tea polyphenols. Curr. Pharm. Biotechnol. 2012, 13, 191–199. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.T.; Gidding, S.S.; Robinson, J.G. Can atherosclerosis be cured? Curr. Opin. Lipidol. 2019, 30, 477–484. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Momose, Y.; Maeda-Yamamoto, M.; Nabetani, H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int. J. Food Sci. Nutr. 2016, 67, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Ogawa, M.; Futamatsu, H.; Kosuge, H.; Sagesaka, Y.M.; Isobe, M. Tea catechins improve left ventricular dysfunction, suppress myocardial inflammation and fibrosis, and alter cytokine expression in rat autoimmune myocarditis. Eur. J. Heart Fail. 2007, 9, 152–159. [Google Scholar] [PubMed]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Liu, J.; Zhao, W.; Wang, Y.; He, Q.; Wu, R.; Li, D.; Xu, J. Mechanistic studies for tri-targeted inhibition of enzymes involved in cholesterol biosynthesis by green tea polyphenols. Org. Biomol. Chem. 2014, 12, 4941–4951. [Google Scholar] [PubMed]
- Wang, L.; Zeng, B.; Zhang, X.; Liao, Z.; Gu, L.; Liu, Z.; Zhong, Q.; Wei, H.; Fang, X. The effect of green tea polyphenols on gut microbial diversity and fat deposition in C57BL/6J HFA mice. Food Funct. 2016, 7, 4956–4966. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Ballester, M.; Herrero-Cervera, A.; Vinué, Á.; Martínez-Hervás, S.; González-Navarro, H. Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis. Nutrients 2020, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal 2015, 23, 1144–1170. [Google Scholar]
- Steinberg, D. The LDL modification hypothesis of atherogenesis: An update. J. Lipid Res. 2009, 50, S376–S381. [Google Scholar] [CrossRef]
- Goto, T.; Saito, Y.; Morikawa, K.; Kanamaru, Y.; Nagaoka, S. Epigallocatechin gallate changes mRNA expression level of genes involved in cholesterol metabolism in hepatocytes. Br. J. Nutr. 2012, 107, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Choi, Y.J.; Shin, S.Y.; Li, J.; Kang, S.W.; Bae, J.Y.; Kim, D.S.; Ji, G.E.; Kang, J.S.; Kang, Y.H. Dietary flavonoids differentially reduce oxidized LDL-induced apoptosis in human endothelial cells: Role of MAPK- and JAK/STAT-signaling. J. Nutr. 2008, 138, 983–990. [Google Scholar]
- Li, H.L.; Huang, Y.; Zhang, C.N.; Liu, G.; Wei, Y.S.; Wang, A.B.; Liu, Y.Q.; Hui, R.T.; Wei, C.; Williams, G.M.; et al. Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic. Biol. Med. 2006, 40, 1756–1775. [Google Scholar]
- Lv, Z.; Shan, X.; Tu, Q.; Wang, J.; Chen, J.; Yang, Y. Ginkgolide B treatment regulated intestinal flora to improve high-fat diet induced atherosclerosis in ApoE−/− mice. Biomed. Pharmacother. 2021, 134, 111100. [Google Scholar]
- Anhê, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar]
- Zhu, M.Z.; Zhou, F.; Ouyang, J.; Wang, Q.Y.; Li, Y.L.; Wu, J.L.; Huang, J.A.; Liu, Z.H. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar]
- Huang, J.; Feng, S.; Liu, A.; Dai, Z.; Wang, H.; Reuhl, K.; Lu, W.; Yang, C.S. Green Tea Polyphenol EGCG Alleviates Metabolic Abnormality and Fatty Liver by Decreasing Bile Acid and Lipid Absorption in Mice. Mol. Nutr. Food Res. 2018, 62, 1700696. [Google Scholar]
- Sheng, L.; Jena, P.K.; Liu, H.X.; Hu, Y.; Nagar, N.; Bronner, D.N.; Settles, M.L.; Bäumler, A.J.; Wan, Y.Y. Obesity treatment by epigallocatechin-3-gallate-regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. 2018, 32, fj201800370R. [Google Scholar]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; et al. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar]
- Perrotta, I. The microscopic anatomy of endothelial cells in human atherosclerosis: Focus on ER and mitochondria. J. Anat. 2020, 237, 1015–1025. [Google Scholar]
- Duan, H.; Zhang, Q.; Liu, J.; Li, R.; Wang, D.; Peng, W.; Wu, C. Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis. Pharmacol. Res. 2021, 168, 105599. [Google Scholar]
- Dlugosz, P.J.; Billen, L.P.; Annis, M.G.; Zhu, W.; Zhang, Z.; Lin, J.; Leber, B.; Andrews, D.W. Bcl-2 changes conformation to inhibit Bax oligomerization. Embo J. 2006, 25, 2287–2296. [Google Scholar] [CrossRef]
- Salvayre, R.; Auge, N.; Benoist, H.; Negre-Salvayre, A. Oxidized low-density lipoprotein-induced apoptosis. Biochim. Biophys. Acta 2002, 1585, 213–221. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.; Yu, H.; Wang, W.; Wu, C.; Yang, Y.; Hu, Y.; Shi, X.; Li, J. Epigallocatechin-3-gallate inhibits H2O2-induced apoptosis in Mouse Vascular Smooth Muscle Cells via 67kD Laminin Receptor. Sci. Rep. 2017, 7, 7774. [Google Scholar] [CrossRef]
- Meng, J.; Chen, Y.; Wang, J.; Qiu, J.; Chang, C.; Bi, F.; Wu, X.; Liu, W. EGCG protects vascular endothelial cells from oxidative stress-induced damage by targeting the autophagy-dependent PI3K-AKT-mTOR pathway. Ann. Transl. Med. 2020, 8, 200. [Google Scholar]
- Hong, F.F.; Liang, X.Y.; Liu, W.; Lv, S.; He, S.J.; Kuang, H.B.; Yang, S.L. Roles of eNOS in atherosclerosis treatment. Inflamm. Res. 2019, 68, 429–441. [Google Scholar] [CrossRef]
- Comuzzo, P.; Toniolo, R.; Battistutta, F.; Lizee, M.; Svigelj, R.; Zironi, R. Oxidative behavior of (+)-catechin in the presence of inactive dry yeasts: A comparison with sulfur dioxide, ascorbic acid and glutathione. J. Sci. Food Agric. 2017, 97, 5158–5167. [Google Scholar]
- Yamagata, K. Protective Effect of Epigallocatechin Gallate on Endothelial Disorders in Atherosclerosis. J. Cardiovasc. Pharmacol. 2020, 75, 292–298. [Google Scholar]
- Xuan, F.; Jian, J. Epigallocatechin gallate exerts protective effects against myocardial ischemia/reperfusion injury through the PI3K/Akt pathway-mediated inhibition of apoptosis and the restoration of the autophagic flux. Int. J. Mol. Med. 2016, 38, 328–336. [Google Scholar]
- Liu, S.; Sun, Z.; Chu, P.; Li, H.; Ahsan, A.; Zhou, Z.; Zhang, Z.; Sun, B.; Wu, J.; Xi, Y.; et al. EGCG protects against homocysteine-induced human umbilical vein endothelial cells apoptosis by modulating mitochondrial-dependent apoptotic signaling and PI3K/Akt/eNOS signaling pathways. Apoptosis 2017, 22, 672–680. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, L.L.; Maimaitirexiati, X.M.; Zhang, Y.; Wu, L. Irbesartan attenuates TNF-α-induced ICAM-1, VCAM-1, and E-selectin expression through suppression of NF-κB pathway in HUVECs. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3295–3302. [Google Scholar] [PubMed]
- Suzuki, J.; Isobe, M.; Morishita, R.; Nagai, R. Tea polyphenols regulate key mediators on inflammatory cardiovascular diseases. Mediat. Inflamm. 2009, 2009, 494928. [Google Scholar] [CrossRef]
- Wang, Z.M.; Gao, W.; Wang, H.; Zhao, D.; Nie, Z.L.; Shi, J.Q.; Zhao, S.; Lu, X.; Wang, L.S.; Yang, Z.J. Green tea polyphenol epigallocatechin-3-gallate inhibits TNF-α-induced production of monocyte chemoattractant protein-1 in human umbilical vein endothelial cells. Cell Physiol. Biochem. 2014, 33, 1349–1358. [Google Scholar] [CrossRef]
- Ramesh, E.; Geraldine, P.; Thomas, P.A. Regulatory effect of epigallocatechin gallate on the expression of C-reactive protein and other inflammatory markers in an experimental model of atherosclerosis. Chem. Biol. Interact. 2010, 183, 125–132. [Google Scholar] [CrossRef]
- Chae, Y.J.; Kim, C.H.; Ha, T.S.; Hescheler, J.; Ahn, H.Y.; Sachinidis, A. Epigallocatechin-3-O-gallate inhibits the angiotensin II-induced adhesion molecule expression in human umbilical vein endothelial cell via inhibition of MAPK pathways. Cell Physiol. Biochem. 2007, 20, 859–866. [Google Scholar] [CrossRef]
- Ludwig, A.; Lorenz, M.; Grimbo, N.; Steinle, F.; Meiners, S.; Bartsch, C.; Stangl, K.; Baumann, G.; Stangl, V. The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 2004, 316, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar]
- Yang, D.; Liu, J.; Tian, C.; Zeng, Y.; Zheng, Y.H.; Fang, Q.; Li, H.H. Epigallocatechin gallate inhibits angiotensin II-induced endothelial barrier dysfunction via inhibition of the p38 MAPK/HSP27 pathway. Acta Pharmacol. Sin. 2010, 31, 1401–1406. [Google Scholar] [CrossRef]
- Yang, L.; Gao, C. MiR-590 Inhibits Endothelial Cell Apoptosis by Inactivating the TLR4/NF-κB Pathway in Atherosclerosis. Yonsei Med. J. 2019, 60, 298–307. [Google Scholar] [CrossRef]
- Hong Byun, E.; Fujimura, Y.; Yamada, K.; Tachibana, H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J. Immunol. 2010, 185, 33–45. [Google Scholar] [CrossRef]
- Vieceli Dalla Sega, F.; Fortini, F.; Aquila, G.; Campo, G.; Vaccarezza, M.; Rizzo, P. Notch Signaling Regulates Immune Responses in Atherosclerosis. Front. Immunol. 2019, 10, 1130. [Google Scholar] [CrossRef]
- Wang, T.; Xiang, Z.; Wang, Y.; Li, X.; Fang, C.; Song, S.; Li, C.; Yu, H.; Wang, H.; Yan, L.; et al. (-)-Epigallocatechin Gallate Targets Notch to Attenuate the Inflammatory Response in the Immediate Early Stage in Human Macrophages. Front. Immunol. 2017, 8, 433. [Google Scholar] [CrossRef]
- Yin, J.; Huang, F.; Yi, Y.; Yin, L.; Peng, D. EGCG attenuates atherosclerosis through the Jagged-1/Notch pathway. Int. J. Mol. Med. 2016, 37, 398–406. [Google Scholar] [CrossRef]
- Xie, H.; Sun, J.; Chen, Y.; Zong, M.; Li, S.; Wang, Y. EGCG Attenuates Uric Acid-Induced Inflammatory and Oxidative Stress Responses by Medicating the NOTCH Pathway. Oxid. Med. Cell. Longev. 2015, 2015, 214836. [Google Scholar] [CrossRef]
- Huang, Y.W.; Zhu, Q.Q.; Yang, X.Y.; Xu, H.H.; Sun, B.; Wang, X.J.; Sheng, J. Wound healing can be improved by (-)-epigallocatechin gallate through targeting Notch in streptozotocin-induced diabetic mice. FASEB J. 2019, 33, 953–964. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar]
- Johnson, J.L. Metalloproteinases in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 93–106. [Google Scholar] [CrossRef]
- Zhou, M.; Qin, S.; Chu, Y.; Wang, F.; Chen, L.; Lu, Y. Immunolocalization of MMP-2 and MMP-9 in human rheumatoid synovium. Int. J. Clin. Exp. Pathol. 2014, 7, 3048–3056. [Google Scholar]
- Krychtiuk, K.A.; Kastl, S.P.; Speidl, W.S.; Wojta, J. Inflammation and coagulation in atherosclerosis. Hamostaseologie 2013, 33, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, A.; Hulsmans, M.; Holvoet, P.; Monaco, C. Biomechanical factors and macrophages in plaque stability. Cardiovasc. Res. 2013, 99, 284–293. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Li, Y.; Shi, H.; Wang, H.; Chen, B.; Wang, F.; Wang, Z.; Yang, Z.; Wang, L. Green tea polyphenol epigallocatechin-3-gallate increases atherosclerotic plaque stability in apolipoprotein E-deficient mice fed a high-fat diet. Kardiol. Pol. 2018, 76, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Wang, H.; Fan, Y.; Shi, H.J.; Wang, Q.M.; Chen, B.R.; Khurwolah, M.R.; Long, Q.Q.; Wang, S.B.; Wang, Z.M.; et al. Epigallocatechin-3-Gallate Inhibits Matrix Metalloproteinase-9 and Monocyte Chemotactic Protein-1 Expression Through the 67-κDa Laminin Receptor and the TLR4/MAPK/NF-κB Signalling Pathway in Lipopolysaccharide-Induced Macrophages. Cell Physiol. Biochem. 2017, 43, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Moon, S.K. Epigallocatechin-3-gallate causes the p21/WAF1-mediated G(1)-phase arrest of cell cycle and inhibits matrix metalloproteinase-9 expression in TNF-alpha-induced vascular smooth muscle cells. Arch. Biochem. Biophys. 2005, 435, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, V.; Baraghis, E.; Duquette, N.; Thorin-Trescases, N.; Lambert, J.; Lesage, F.; Thorin, E. Catechin prevents severe dyslipidemia-associated changes in wall biomechanics of cerebral arteries in LDLr−/−:hApoB+/+ mice and improves cerebral blood flow. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1330–H1339. [Google Scholar] [CrossRef]
- Cheng, X.W.; Kuzuya, M.; Nakamura, K.; Liu, Z.; Di, Q.; Hasegawa, J.; Iwata, M.; Murohara, T.; Yokota, M.; Iguchi, A. Mechanisms of the inhibitory effect of epigallocatechin-3-gallate on cultured human vascular smooth muscle cell invasion. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1864–1870. [Google Scholar] [CrossRef]
- Filkova, A.A.; Martyanov, A.A.; Garzon Dasgupta, A.K.; Panteleev, M.A.; Sveshnikova, A.N. Quantitative dynamics of reversible platelet aggregation: Mathematical modelling and experiments. Sci. Rep. 2019, 9, 6217. [Google Scholar] [CrossRef]
- Custodio-Chablé, S.J.; Lezama, R.A.; Reyes-Maldonado, E. Platelet activation as a trigger factor for inflammation and atherosclerosis. Cir. Cir. 2020, 88, 233–243. [Google Scholar] [CrossRef]
- Xie, Z.; Tian, Y.; Ma, L.; Lv, X.; Cheng, K.; Li, S.; Huang, X.; Kong, H.; Huang, L.; Wu, B.; et al. Developments in inhibiting platelet aggregation based on different design strategies. Future Med. Chem. 2019, 11, 1757–1775. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Ebrahimi, F.; Farzaei, M.H.; Baratpourmoghaddam, A.; Ahmadi, P.; Rostamiasrabadi, P.; Rasouli Amirabadi, A.H.; Rahimi, R. Dietary polyphenols for atherosclerosis: A comprehensive review and future perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Lill, G.; Voit, S.; Schrör, K.; Weber, A.A. Complex effects of different green tea catechins on human platelets. FEBS Lett. 2003, 546, 265–270. [Google Scholar] [CrossRef]
- Ok, W.J.; Cho, H.J.; Kim, H.H.; Lee, D.H.; Kang, H.Y.; Kwon, H.W.; Rhee, M.H.; Kim, M.; Park, H.J. Epigallocatechin-3-gallate has an anti-platelet effect in a cyclic AMP-dependent manner. J. Atheroscler. Thromb. 2012, 19, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, Y.J.; Kim, H.H.; Cho, H.J.; Ryu, J.H.; Rhee, M.H.; Park, H.J. Inhibitory effects of epigallocatechin-3-gallate on microsomal cyclooxygenase-1 activity in platelets. Biomol. Ther. 2013, 21, 54–59. [Google Scholar] [CrossRef]
- Guo, J.; Li, K.; Lin, Y.; Liu, Y. Protective effects and molecular mechanisms of tea polyphenols on cardiovascular diseases. Front. Nutr. 2023, 10, 1202378. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.J.; Park, J.Y.; Hong, S.J.; Kim, K.A.; Lee, S.H.; Cho, J.Y.; Park, J.H.; Yu, C.W.; Lim, D.S. Anti-platelet effects of epigallocatechin-3-gallate in addition to the concomitant aspirin, clopidogrel or ticagrelor treatment. Korean J. Intern. Med. 2018, 33, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wu, X.; He, W. (-)-Epigallocatechin-3-gallate plays an antagonistic role in the antitumor effect of bortezomib in myeloma cells via activating Wnt/β-catenin signaling pathway. Adv. Clin. Exp. Med. 2022, 31, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Frasheri, L.; Schielein, M.C.; Tizek, L.; Mikschl, P.; Biedermann, T.; Zink, A. Great green tea ingredient? A narrative literature review on epigallocatechin gallate and its biophysical properties for topical use in dermatology. Phytother. Res. 2020, 34, 2170–2179. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, e14189. [Google Scholar] [CrossRef]
- Gawande, S.; Kale, A.; Kotwal, S. Effect of nutrient mixture and black grapes on the pharmacokinetics of orally administered (-)epigallocatechin-3-gallate from green tea extract: A human study. Phytother. Res. 2008, 22, 802–808. [Google Scholar] [CrossRef]
- Liao, Z.H.; Zhu, H.Q.; Chen, Y.Y.; Chen, R.L.; Fu, L.X.; Li, L.; Zhou, H.; Zhou, J.L.; Liang, G. The epigallocatechin gallate derivative Y6 inhibits human hepatocellular carcinoma by inhibiting angiogenesis in MAPK/ERK1/2 and PI3K/AKT/HIF-1α/VEGF dependent pathways. J. Ethnopharmacol. 2020, 259, 112852. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Faridi Esfanjani, A.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef]


| Food Sources | Types | EGCG Content (mg/100 g) |
|---|---|---|
| Tea | Green | 7380 (dry leaves) |
| 64.15 (brew) | ||
| White | 4245 (dry leaves) | |
| 46.00 (brew) | ||
| Oolong | 34.48 (brew) | |
| Black | 9.36 (brew) | |
| Nuts | Hazelnuts | 1.06 |
| Pistachio nuts | 0.40 | |
| Pecans | 2.30 | |
| Carob flour | 109.46 | |
| Fruit | Apples | 1.93 |
| Blackberries | 0.68 | |
| Cranberries | 0.97 | |
| Kiwifruit | 0.09 | |
| Peaches | 0.30 | |
| Pears | 0.17 | |
| Plums | 0.40 | |
| Strawberries | 0.11 | |
| Vegetables | Onions | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Long, Y.; Fang, J.; Liu, G. Advances in the Anti-Atherosclerotic Mechanisms of Epigallocatechin Gallate. Nutrients 2024, 16, 2074. https://doi.org/10.3390/nu16132074
Liu Y, Long Y, Fang J, Liu G. Advances in the Anti-Atherosclerotic Mechanisms of Epigallocatechin Gallate. Nutrients. 2024; 16(13):2074. https://doi.org/10.3390/nu16132074
Chicago/Turabian StyleLiu, Yihui, Yiling Long, Jun Fang, and Gang Liu. 2024. "Advances in the Anti-Atherosclerotic Mechanisms of Epigallocatechin Gallate" Nutrients 16, no. 13: 2074. https://doi.org/10.3390/nu16132074





