Importance of Gut Microbiota in Patients with Inflammatory Bowel Disease
Abstract
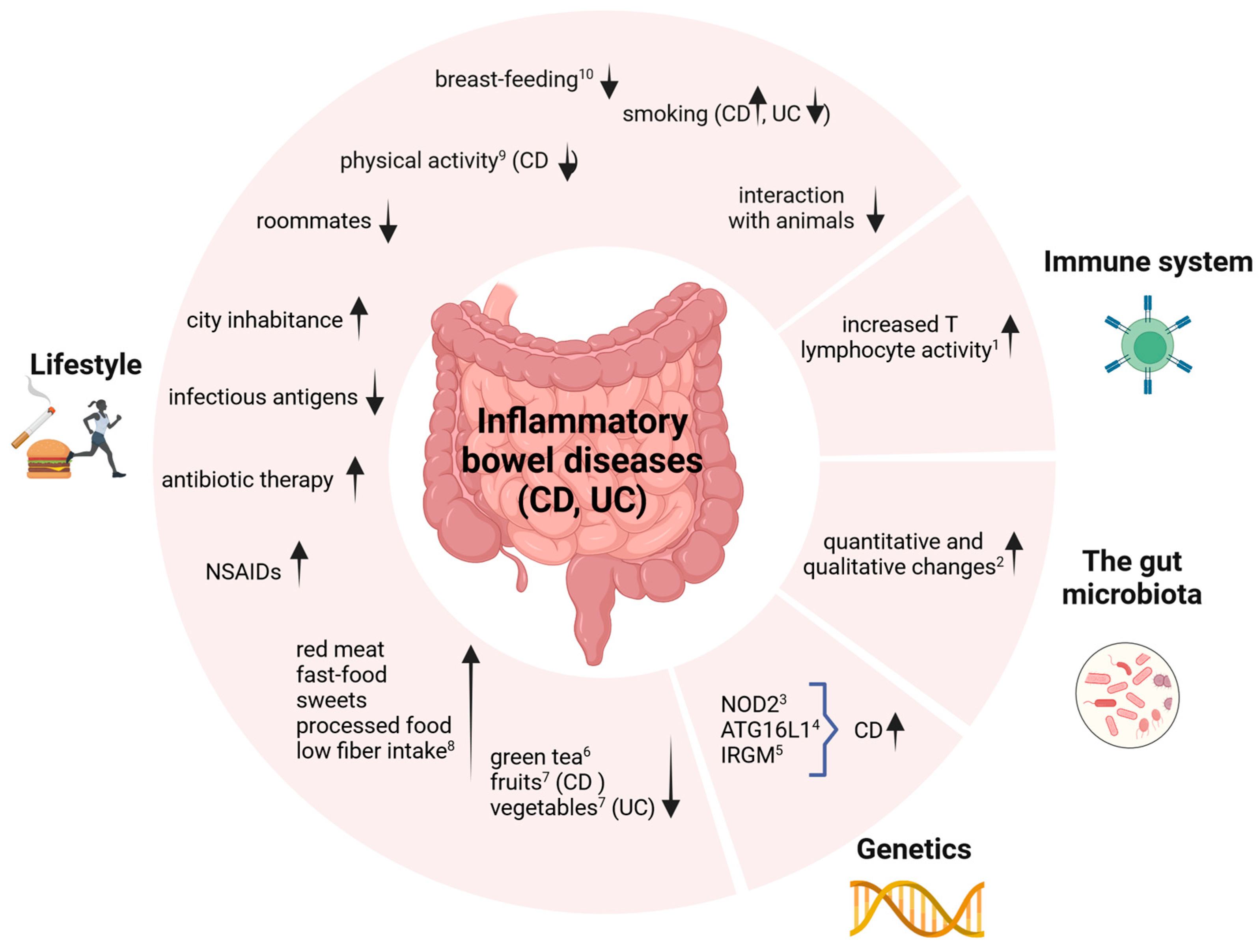
:1. Introduction
2. Etiological Factors
3. Relationship of IBD with Gut Microflora and Immune System
4. Gut Microbiota in Patients with Inflammatory Bowel Disease
5. Importance of Particular Strains of Probiotic Microorganisms in the Treatment of Inflammatory Bowel Diseases
5.1. Saccharomyces Boulardii
5.2. Lactic Acid Bacteria
5.3. Escherichia coli Strain Nissle 1917
5.4. A Mixture of Bacterial Strains from the Genus Bifidobacterium, Lactobacillus, and Streptococcus
6. Use of Prebiotics in IBD
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, V.; Abbas, A.K.; Aster, J.C.; Robbins, S.L.; Perkins, J.A. Robbins Basic Pathology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2018; ISBN 978-0-323-35317-5. [Google Scholar]
- Yu, Y.R.; Rodriguez, J.R. Clinical Presentation of Crohn’s, Ulcerative Colitis, and Indeterminate Colitis: Symptoms, Extraintestinal Manifestations, and Disease Phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef]
- M’Koma, A.E. Inflammatory Bowel Disease: Clinical Diagnosis and Surgical Treatment-Overview. Medicina 2022, 58, 567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106, Erratum in Gut 2021, 70, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-Analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef]
- Lundin, A.; Bok, C.M.; Aronsson, L.; Björkholm, B.; Gustafsson, J.-A.; Pott, S.; Arulampalam, V.; Hibberd, M.; Rafter, J.; Pettersson, S. Gut Flora, Toll-like Receptors and Nuclear Receptors: A Tripartite Communication That Tunes Innate Immunity in Large Intestine. Cell. Microbiol. 2008, 10, 1093–1103. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The Role of Probiotic Lactic Acid Bacteria and Bifidobacteria in the Prevention and Treatment of Inflammatory Bowel Disease and Other Related Diseases: A Systematic Review of Randomized Human Clinical Trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Takeda, K. Regulation of Intestinal Homeostasis by Innate and Adaptive Immunity. Int. Immunol. 2012, 24, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Lucas López, R.; Grande Burgos, M.J.; Gálvez, A.; Pérez Pulido, R. The Human Gastrointestinal Tract and Oral Microbiota in Inflammatory Bowel Disease: A State of the Science Review. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2017, 125, 3–10. [Google Scholar] [CrossRef]
- Niezgódka-Klósak, A.; Eder, P. Therapy in Inflammatory Bowel Disease-Use of Special-Purpose Foods. In Nutrition in Intestinal Microbiota Disorders; Stachowska, E., Ed.; PZWL Wydawnictwo Lekarskie: Warsaw, Poland, 2021; pp. 83–100. [Google Scholar]
- Greenblum, S.; Turnbaugh, P.J.; Borenstein, E. Metagenomic Systems Biology of the Human Gut Microbiome Reveals Topological Shifts Associated with Obesity and Inflammatory Bowel Disease. Proc. Natl. Acad. Sci. USA 2012, 109, 594–599. [Google Scholar] [CrossRef]
- Prosberg, M.; Bendtsen, F.; Vind, I.; Petersen, A.M.; Gluud, L.L. The Association between the Gut Microbiota and the Inflammatory Bowel Disease Activity: A Systematic Review and Meta-Analysis. Scand. J. Gastroenterol. 2016, 51, 1407–1415. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhi, F. Lower Level of Bacteroides in the Gut Microbiota Is Associated with Inflammatory Bowel Disease: A Meta-Analysis. BioMed Res. Int. 2016, 2016, e5828959. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-Like Receptor 2 Pathway Establishes Colonization by a Commensal of the Human Microbiota. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics Are a Good Choice in Remission of Inflammatory Bowel Diseases: A Meta Analysis and Systematic Review. J. Cell. Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zuo, Z.-X.; Mao, A.-P. Effect of Probiotics on Inducing Remission and Maintaining Therapy in Ulcerative Colitis, Crohn’s Disease, and Pouchitis: Meta-Analysis of Randomized Controlled Trials. Inflamm. Bowel Dis. 2014, 20, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.M.K.; Nair, L.; Alegre, M.-L. The Interplay between the Intestinal Microbiota and the Immune System. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 9–19. [Google Scholar] [CrossRef]
- Akutko, K.; Stawarski, A. Probiotics, Prebiotics and Synbiotics in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 2466. [Google Scholar] [CrossRef]
- Biagioli, M.; Carino, A.; Di Giorgio, C.; Marchianò, S.; Bordoni, M.; Roselli, R.; Distrutti, E.; Fiorucci, S. Discovery of a Novel Multi-Strains Probiotic Formulation with Improved Efficacy toward Intestinal Inflammation. Nutrients 2020, 12, 1945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Guan, X.-X.; Tang, Y.-J.; Sun, J.-F.; Wang, X.-K.; Wang, W.-D.; Fan, J.-M. Clinical Effects and Gut Microbiota Changes of Using Probiotics, Prebiotics or Synbiotics in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef]
- Szajewska, H.; Kołodziej, M. Systematic Review with Meta-Analysis: Saccharomyces Boulardii in the Prevention of Antibiotic-Associated Diarrhoea. Aliment. Pharmacol. Ther. 2015, 42, 793–801. [Google Scholar] [CrossRef]
- Plein, K.; Hotz, J. Therapeutic Effects of Saccharomyces Boulardii on Mild Residual Symptoms in a Stable Phase of Crohn’s Disease with Special Respect to Chronic Diarrhea—A Pilot Study. Z. Gastroenterol. 1993, 31, 129–134. [Google Scholar]
- McFarland, L.V. Systematic Review and Meta-Analysis of Saccharomyces Boulardii in Adult Patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Darewicz, M.; Iwaniak, A.; Borawska, J.; Bucholska, J.; Hrynkiewicz, M. Biologically active peptides from food proteins: In silico, in vitro and in vivo studies, application aspects, and safety evaluation. Zywnosc. Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2015, 5, 5–22. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the Dairy Industry-Advances and Opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Gerardi, V.; Mangiola, F.; Lopetuso, L.R.; Pizzoferrato, M.; Petito, V.; Papa, A.; Stojanovic, J.; Poscia, A.; Cammarota, G.; et al. Role and Mechanisms of Action of Escherichia coli Nissle 1917 in the Maintenance of Remission in Ulcerative Colitis Patients: An Update. World J. Gastroenterol. 2016, 22, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Strauch, U.G.; Linde, H.-J.; Watzl, S.; Obermeier, F.; Göttl, C.; Dunger, N.; Grunwald, N.; Schölmerich, J.; Rath, H.C. Preventive Effects of Escherichia Coli Strain Nissle 1917 on Acute and Chronic Intestinal Inflammation in Two Different Murine Models of Colitis. Clin. Diagn. Lab. Immunol. 2004, 11, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukás, M.; Fixa, B.; Kascák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining Remission of Ulcerative Colitis with the Probiotic Escherichia Coli Nissle 1917 Is as Effective as with Standard Mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Schütz, E.; Fric, P.; Fixa, B.; Judmaier, G.; Stolte, M. Double-Blind Comparison of an Oral Escherichia Coli Preparation and Mesalazine in Maintaining Remission of Ulcerative Colitis. Aliment. Pharmacol. Ther. 1997, 11, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Rembacken, B.J.; Snelling, A.M.; Hawkey, P.M.; Chalmers, D.M.; Axon, A.T. Non-Pathogenic Escherichia coli versus Mesalazine for the Treatment of Ulcerative Colitis: A Randomised Trial. Lancet Lond. Engl. 1999, 354, 635–639. [Google Scholar] [CrossRef]
- Losurdo, G.; Iannone, A.; Contaldo, A.; Ierardi, E.; Di Leo, A.; Principi, M. Escherichia Coli Nissle 1917 in Ulcerative Colitis Treatment: Systematic Review and Meta-Analysis. J. Gastrointest. Liver Dis. JGLD 2015, 24, 499–505. [Google Scholar] [CrossRef]
- Fan, H.; Du, J.; Liu, X.; Zheng, W.-W.; Zhuang, Z.-H.; Wang, C.-D.; Gao, R. Effects of Pentasa-Combined Probiotics on the Microflora Structure and Prognosis of Patients with Inflammatory Bowel Disease. Turk. J. Gastroenterol. 2019, 30, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.-E.; Li, W.-B.; Wang, H.-Y.; Ma, Y.-M.; Zhao, X.-H.; Yang, H.; Qian, J.-M.; Li, J.-N. VSL#3 Can Prevent Ulcerative Colitis-Associated Carcinogenesis in Mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef] [PubMed]
- Parang, B.; Barrett, C.W.; Williams, C.S. AOM/DSS Model of Colitis-Associated Cancer. Methods Mol. Biol. Clifton NJ 2016, 1422, 297–307. [Google Scholar] [CrossRef]
- Mardini, H.E.; Grigorian, A.Y. Probiotic Mix VSL#3 Is Effective Adjunctive Therapy for Mild to Moderately Active Ulcerative Colitis: A Meta-Analysis. Inflamm. Bowel Dis. 2014, 20, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Xu, M.; Liu, D.; Zhou, D.; Yang, W. Assessing the Efficacy and Safety of Fecal Microbiota Transplantation and Probiotic VSL#3 for Active Ulcerative Colitis: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0228846. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.L.; Hedin, C.R.H.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, Double-Blind, Placebo-Controlled Trial of Fructo-Oligosaccharides in Active Crohn’s Disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Hafer, A.; Krämer, S.; Duncker, S.; Krüger, M.; Manns, M.P.; Bischoff, S.C. Effect of Oral Lactulose on Clinical and Immunohistochemical Parameters in Patients with Inflammatory Bowel Disease: A Pilot Study. BMC Gastroenterol. 2007, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Godoy-Brewer, G.; Shah, N.D.; Maas, L.; White, J.; Parian, A.M.; Mullin, G.E. Prebiotics for Induction and Maintenance of Remission in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2024, izae115. [Google Scholar] [CrossRef]
- Gao, X.; Xu, F.; Li, T.; Huang, P.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Zhai, Q. CAZymes-Associated Method to Explore Glycans That Mitigate DSS-Induced Colitis via Targeting Bacteroides Cellulosilyticus. Int. J. Biol. Macromol. 2024, 258, 128694. [Google Scholar] [CrossRef]
- Zheng, J.; Gong, S.; Han, J. Arabinogalactan Alleviates Lipopolysaccharide-Induced Intestinal Epithelial Barrier Damage through Adenosine Monophosphate-Activated Protein Kinase/Silent Information Regulator 1/Nuclear Factor Kappa-B Signaling Pathways in Caco-2 Cells. Int. J. Mol. Sci. 2023, 24, 15337. [Google Scholar] [CrossRef] [PubMed]
- Łodyga, M.; Eder, P.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the management of patients with Crohn’s disease. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Gastroenterol. Rev. 2021, 16, 257–296. [Google Scholar] [CrossRef] [PubMed]
- Eder, P.; Łodyga, M.; Gawron-Kiszka, M.; Dobrowolska, A.; Gonciarz, M.; Hartleb, M.; Kłopocka, M.; Małecka-Wojciesko, E.; Radwan, P.; Reguła, J.; et al. Guidelines for the management of ulcerative colitis. Recommendations of the Polish Society of Gastroenterology and the Polish National Consultant in Gastroenterology. Gastroenterol. Rev. 2023, 18, 1–42. [Google Scholar] [CrossRef] [PubMed]
| Ulcerative Colitis (UC) | Crohn’s Disease (CD) | |
|---|---|---|
| Gastrointestinal symptoms | Chronic diarrhea with admixture of fresh blood in almost 90% of patients; severe bleeding from the lower part of the gastrointestinal tract; sudden and intense pushing on the stool; expulsion of mucopurulent contents; severe abdominal pain of a spasmodic nature, located mostly in the left side of the iliac fossa, usually subsiding after defecation; in patients with the proctitis form, symptoms are usually limited to violent pushing on the stool with the presence of fresh blood; constipation instead of diarrhea may also occur. | Chronic, watery diarrhea, often with admixture of mucus and blood; often with occult blood from the bleeding small intestine; bowel movements also at night; with the constrictive form, the symptom is constipation with symptoms of incomplete obstruction; crampy abdominal pain located on the right side of the iliac fossa and near the umbilicus; flatulence; pain in the lower abdomen often occurs with a feeling of pushing on the stool; perianal lesions (including anal fissures and fistulas) that are asymptomatic or with burning pain at the anus aggravated during bowel movements and sitting; active fistulas discharge purulent contents; symptoms in the upper gastrointestinal region usually occur along with other symptoms (rarely the only complaints the patient has); lesions of the stomach and duodenum, nausea, vomiting, and pain in the epigastrium; pathologically involved esophagus—dysphagia and odynophagia (swallowing disorders, pain when swallowing); in the oral cavity, ulcers, as well as aphthous ulcers. |
| Other symptoms | With more severe episodes: fever, tachycardia, weight loss, nausea, and vomiting; osteoarticular system: arthritis, osteopenia, and osteoporosis; skin lesions (erythema nodosum, gangrenous dermatitis); pathological changes in the liver and biliary tract, such as hepatic steatosis and primary sclerosing cholangitis; venous thromboembolism. | Usually lasting chronically (more than 6 weeks): weakness, weight loss, lack of appetite, subfebrile states and fever, night sweats; extraintestinal symptoms: cholelithiasis—in up to 30% of patients with ileus, urolithiasis, clubbed fingers—occurs in 40–60% of patients with severe flares, pain in axial and peripheral joints and peripheral joints, erythema nodosum, pyoderma gangrenosum; in 3–5%, visual problems such as conjunctivitis, or iritis. |
| Long-term effects of the disease | Chronic inflammation of the colon strongly associated with an increased risk of colorectal cancer in the future, especially if the period of active stage of the disease is more than 10 years, which promotes dysplasia within the intestinal epithelium; the risk of malignancy increases, with concomitant primary sclerosing cholangitis [6]; pancolectomy is necessary in about 20% of patients (10 to 15% develop intestinal reservoir inflammation (pouchitis)). | Increased risk of future colorectal cancer in some cases; inflammatory subtype may develop into a fistulizing or narrowing form; most patients are forced to undergo surgery, which can lead to short bowel syndrome (nutritional deficiencies due to a smaller surface area for nutrient absorption); comorbidities and extraintestinal symptoms due to exacerbation or side effects of medications. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekstedt, N.; Jamioł-Milc, D.; Pieczyńska, J. Importance of Gut Microbiota in Patients with Inflammatory Bowel Disease. Nutrients 2024, 16, 2092. https://doi.org/10.3390/nu16132092
Ekstedt N, Jamioł-Milc D, Pieczyńska J. Importance of Gut Microbiota in Patients with Inflammatory Bowel Disease. Nutrients. 2024; 16(13):2092. https://doi.org/10.3390/nu16132092
Chicago/Turabian StyleEkstedt, Natalia, Dominika Jamioł-Milc, and Joanna Pieczyńska. 2024. "Importance of Gut Microbiota in Patients with Inflammatory Bowel Disease" Nutrients 16, no. 13: 2092. https://doi.org/10.3390/nu16132092
APA StyleEkstedt, N., Jamioł-Milc, D., & Pieczyńska, J. (2024). Importance of Gut Microbiota in Patients with Inflammatory Bowel Disease. Nutrients, 16(13), 2092. https://doi.org/10.3390/nu16132092






