Effect of a Combination of Lactiplantibacillus plantarum KC3 and Leonurus japonicus Extracts in Respiratory Discomfort: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
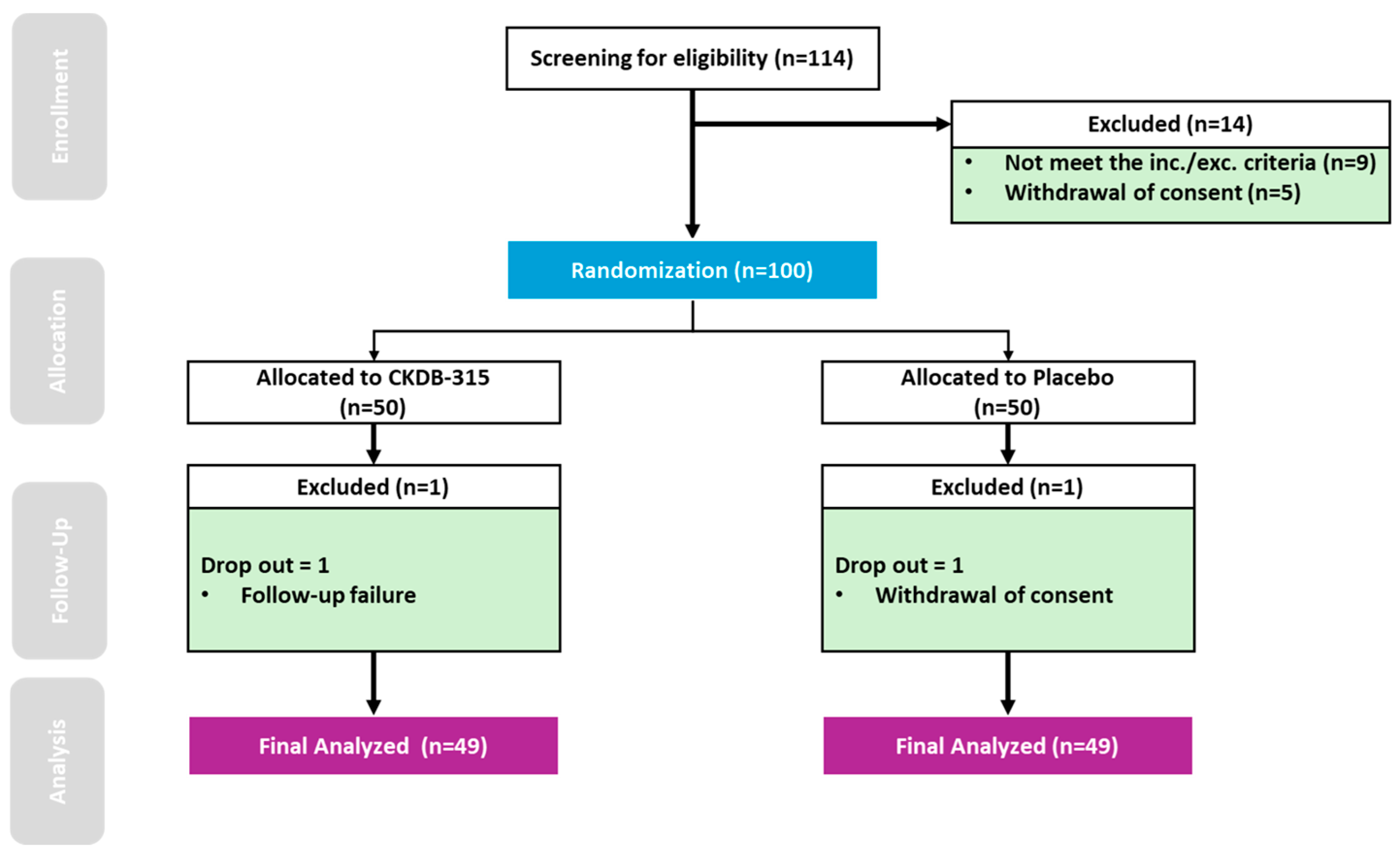
2.3. Randomization
2.4. Intervention
2.5. Efficacy Outcomes
2.6. Safety Outcomes
2.7. Exploratory Outcomes: Fecal Microbiome Analysis
2.8. Statistical Analysis
3. Results
3.1. Sub Characteristics of the Participants
3.2. Efficacy Outcomes
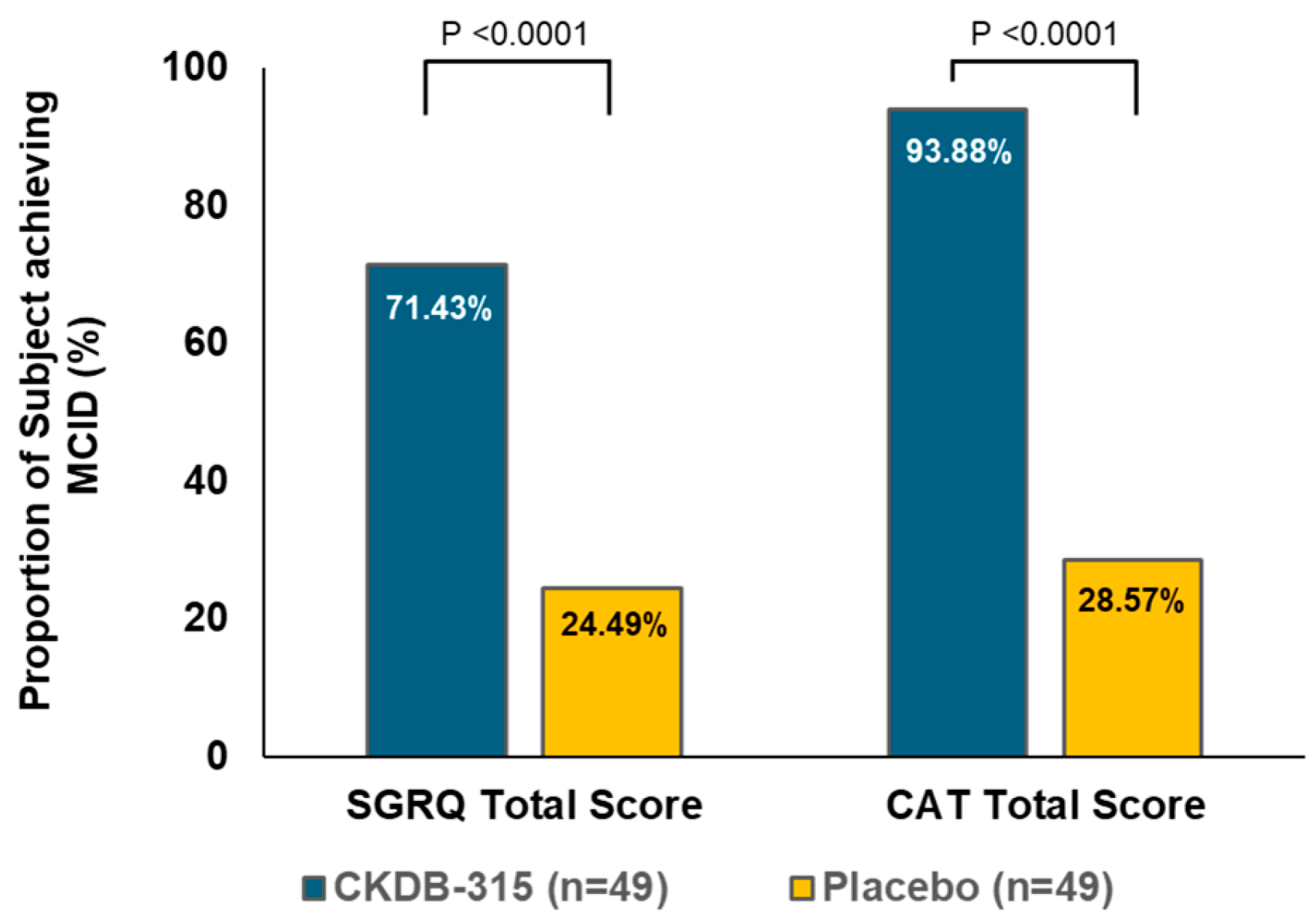
3.2.1. Changes in Health-Related Quality of Life; SGRQ and CAT
3.2.2. Changes in Dyspnea
3.2.3. Changes in Pulmonary Function Values
3.2.4. Changes in TAS and Inflammatory Cytokine Levels
3.3. Safety
3.4. Exploratory Outcomesof Microbiome Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Event |
| ALT | Alanine Transaminase |
| AST | Aspartate Transaminase |
| BMI | Body Mass Index |
| CAT | COPD Assessment Test |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRDs | Chronic Respiratory Disease |
| DVAS | Dyspnea Visual Analogue Scale |
| FAS | Full Analysis Set |
| FEV1 | Forced Expiratory Volume in 1 s |
| FVC | Forced Vital Capacity |
| GCP | Good Clinical Practice |
| HRQL | Health-Related Quality of Life |
| hs-CRP | High-Sensitivity C-Reactive Protein |
| ICS | Inhaled Corticosteroid |
| IL-6 | Interleukin-6 |
| ITT | Intention-to-Treat Principle |
| LJH | Leonurus Japonicus Houtt. |
| LS | Least Squares |
| MCID | Minimal Clinically Important Difference |
| MedDRA | Medical Dictionary for Regulatory Avtivities |
| mMRC | modified Medical Research Council Dyspnea Scale |
| PT | Preferred Term |
| SCFAs | Short-Chain Fatty Acids |
| SGRQ | St. George’s Respiratory Questionnaire |
| SOC | System Organ Class |
| SS | Safety Analysis Set |
| TAS | Total Antioxidant Status |
| TNF-α | Tumor Necrosis Factor-α |
| ULN | Upper Limit of Normal |
References
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Ahmadian Heris, J.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.A.; et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378, e069679. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Moghaddam, S.S.; Ghamari, S.H.; Rad, E.M.; Rezaei, N.; Shobeiri, P.; Aali, A.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abdelmasseh, M.; et al. Global burden of chronic respiratory diseases and risk factors, 1990–2019: An update from the Global Burden of Disease Study 2019. EClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, C.W.; Fu, Y.Y.; Li, Y.Z.; Chen, L.; Zhang, Q.W.; Chen, Y.F. Global, regional, and national burden of chronic respiratory diseases and associated risk factors, 1990–2019: Results from the Global Burden of Disease Study 2019. Front. Med. 2023, 10, 1066804. [Google Scholar] [CrossRef] [PubMed]
- Bourdrel, T.; Annesi-Maesano, I.; Alahmad, B.; Maesano, C.N.; Bind, M.A. The impact of outdoor air pollution on COVID-19: A review of evidence from in vitro, animal, and human studies. Eur. Respir. Rev. 2021, 30, 200242. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, X.; Liu, T.; Chen, D.; Jiao, K.; Wang, X.; Suo, J.; Yang, H.; Liao, J.; Ma, L. Effect of ambient fine particulates (PM2.5) on hospital admissions for respiratory and cardiovascular diseases in Wuhan, China. Respir. Res. 2021, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- Juginović, A.; Vuković, M.; Aranza, I.; Biloš, V. Health impacts of air pollution exposure from 1990 to 2019 in 43 European countries. Sci. Rep. 2021, 11, 22516. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Yasavoli-Sharahi, H.; Alsadi, N.; Ismail, N.; Matar, C. Probiotics in Treatment of Viral Respiratory Infections and Neuroinflammatory Disorders. Molecules 2020, 25, 4891. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Bibi, S.; Rahaman, M.S.; Rahman, F.; Islam, F.; Khan, M.S.; Hasan, M.M.; Parvez, A.; Hossain, M.A.; Maeesa, S.K.; et al. Natural therapeutics and nutraceuticals for lung diseases: Traditional significance, phytochemistry, and pharmacology. Biomed. Pharmacother. 2022, 150, 113041. [Google Scholar] [CrossRef] [PubMed]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Liu, A.; Ma, T.; Xu, N.; Jin, H.; Zhao, F.; Kwok, L.Y.; Zhang, H.; Zhang, S.; Sun, Z. Adjunctive Probiotics Alleviates Asthmatic Symptoms via Modulating the Gut Microbiome and Serum Metabolome. Microbiol. Spectr. 2021, 9, e0085921. [Google Scholar] [CrossRef]
- Ray, M.; Manjunath, A.; Halami, P.M. Effect of probiotics as an immune modulator for the management of COVID-19. Arch. Microbiol. 2023, 205, 182. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kim, Y.J.; Shin, H.J.; Kwon, Y.J.; Chu, J.; Lee, I.; Kim, K.H.; Kim, B.K.; Kim, S.H.; Seo, H.W.; et al. Protective Effect of Novel Lactobacillus plantarum KC3 Isolated from Fermented Kimchi on Gut and Respiratory Disorders. Microorganisms 2023, 11, 967. [Google Scholar] [CrossRef]
- Miao, L.L.; Zhou, Q.M.; Peng, C.; Liu, Z.H.; Xiong, L. Leonurus japonicus (Chinese motherwort), an excellent traditional medicine for obstetrical and gynecological diseases: A comprehensive overview. Biomed. Pharmacother. 2019, 117, 109060. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Pan, H.; Wang, X.; He, H.; Li, M. Leonurus japonicus Houtt.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 152, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Yang, W.K.; Lee, Y.C.; Kim, S.; Moon, S.O.; Kwon, Y.J.; Noh, H.J.; Kim, K.H.; Kim, B.K.; Shin, C.H.; et al. Protective effect of the mixture of Lactiplantibacillus plantarum KC3 and Leonurus japonicas Houtt extract on respiratory disorders. Ecotoxicol. Environ. Saf. 2024, 270, 115856. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Arias-Pérez, R.D.; Taborda, N.A.; Gómez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 42390–42404. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S. The Effects and Pathogenesis of PM2.5 and Its Components on Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Antkiewicz, D.S.; Shafer, M.M.; Perkins, D.A.; Sioutas, C.; Schauer, J.J. Assessing the role of chemical components in cellular responses to atmospheric particle matter (PM) through chemical fractionation of PM extracts. Anal. Bioanal. Chem. 2015, 407, 5953–5963. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated with Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Maung, T.Z.; Bishop, J.E.; Holt, E.; Turner, A.M.; Pfrang, C. Indoor Air Pollution and the Health of Vulnerable Groups: A Systematic Review Focused on Particulate Matter (PM), Volatile Organic Compounds (VOCs) and Their Effects on Children and People with Pre-Existing Lung Disease. Int. J. Environ. Res. Public Health 2022, 19, 8752. [Google Scholar] [CrossRef]
- Paulin, L.; Hansel, N. Particulate air pollution and impaired lung function. F1000Research 2016, 201. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 12, 1968257. [Google Scholar] [CrossRef]
- Loetscher, H.; Pan, Y.C.; Lahm, H.W.; Gentz, R.; Brockhaus, M.; Tabuchi, H.; Lesslauer, W. Role of TNF-α in pulmonary pathophysiology. Respir. Res. 2006, 7, 125. [Google Scholar] [CrossRef]
- Reihmane, D.; Jurka, A.; Tretjakovs, P.; Dela, F. Increase in IL-6, TNF-α, and MMP-9, but not sICAM-1, concentrations depends on exercise duration. Eur. J. Appl. Physiol. 2013, 113, 851–858. [Google Scholar] [CrossRef]
- Welte, T.; Groneberg, D.A. Asthma and COPD. Exp. Toxicol. Pathol. 2006, 57 (Suppl. S2), 35–40. [Google Scholar] [CrossRef]
- Veit, C.; Herrera, R.; Weinmayr, G.; Genuneit, J.; Windstetter, D.; Vogelberg, C.; von Mutius, E.; Nowak, D.; Radon, K.; Gerlich, J.; et al. Long-term effects of asthma medication on asthma symptoms: An application of the targeted maximum likelihood estimation. BMC Med. Res. Methodol. 2020, 20, 307. [Google Scholar] [CrossRef]
- Kahnert, K.; Jörres, R.A.; Behr, J.; Welte, T. The Diagnosis and Treatment of COPD and Its Comorbidities. Dtsch. Arztebl. Int. 2023, 120, 434–444. [Google Scholar] [CrossRef]
- Schwartz, R.H.; Neacsu, O.; Ascher, D.P.; Alpan, O. Moderate dose inhaled corticosteroid-induced symptomatic adrenal suppression: Case report and review of the literature. Clin. Pediatr. 2012, 51, 1184–1190. [Google Scholar] [CrossRef]
- Todd, G.R.; Acerini, C.L.; Ross-Russell, R.; Zahra, S.; Warner, J.T.; McCance, D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch. Dis. Child. 2002, 87, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, V. Asthma and Medicines—Long-Term Side-Effects Monitoring and Dose Titration. Indian J. Pediatr. 2018, 85, 748–756. [Google Scholar] [CrossRef]
- Festic, E.; Scanlon, P.D. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease. A double effect of inhaled corticosteroids? Am. J. Respir. Crit. Care Med. 2015, 191, 141–148. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Bourbeau, J.; Jones, P.W.; Hanania, N.A.; Mahler, D.A.; Vestbo, J.; Wachtel, A.; Martinez, F.J.; Barnhart, F.; Sanford, L.; et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: Two replicate double-blind, parallel-group, randomized controlled trials. Lancet Respir. Med. 2013, 1, 210–223. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Chen, S.; Zhang, H.; Yuan, M.; Xiao, L.; Lu, Y.; Xu, H. Trigonelline, an alkaloid from Leonurus japonicus Houtt., suppresses mast cell activation and OVA-induced allergic asthma. Front. Pharmacol. 2021, 12, 687970. [Google Scholar] [CrossRef]



| CKDB-315 (n = 49) | Placebo (n = 49) | p-Value | |
|---|---|---|---|
| Sex | 0.6018 | ||
| Male, n (%) | 8 (16.33) | 10 (20.41) | |
| Female, n (%) | 41 (83.67) | 39 (79.59) | |
| Age, yr | 50.49 ± 11.45 | 50.14 ± 8.37 | 0.8757 |
| BMI, kg/m2 | 24.37 ± 2.93 | 24.42 ± 2.42 | 0.9253 |
| History of respiratory symptoms | |||
| Past, n (%) | 0 (0.00) | 0 (0.00) | - |
| Present, n (%) | 49 (100.00) | 49 (100.00) | - |
| Cough, n (%) | 47 (95.92) | 43 (87.76) | 0.2682 |
| Duration, months | 49.37 ± 63.94 | 47.80 ± 72.77 | 0.0810 |
| Sputum, n (%) | 47 (95.92) | 49 (100.00) | 0.4948 |
| Duration, months | 57.19 ± 66.94 | 66.65 ± 101.31 | 0.2469 |
| Dyspnea, n (%) | 22 (44.90) | 21 (42.86) | 0.8387 |
| Duration, months | 45.13 ± 32.40 | 64.69 ± 111.70 | 0.8269 |
| History of smoking | 0.6171 | ||
| Non, n (%) | 46 (93.88) | 48 (97.96) | |
| Past, n (%) | 3 (6.12) | 1 (2.04) | |
| Present, n (%) | 0 (0.00) | 0 (0.00) | |
| History of drinking | 0.4527 | ||
| Non, n (%) | 40 (81.63) | 37 (75.51) | |
| Past, n (%) | 1 (2.04) | 0 (0.00) | |
| Present, n (%) | 8 (16.33) | 12 (24.49) |
| CKDB-315 (n = 49) | p-Value | Placebo (n = 48) | p-Value | |
|---|---|---|---|---|
| Acetic acid (μmol/g) | −0.54 ± 36.01 | 0.5569 | −2.28 ± 28.62 | 0.5829 |
| Butyric acid (μmol/g) | 4.79 ± 25.55 | 0.0468 | 4.80 ± 19.98 | 0.1026 |
| Propionic acid (μmol/g) | 12.13 ± 36.81 | 0.0048 | 6.59 ± 28.43 | 0.3216 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-j.; Lee, J.-H.; Kwon, S.-Y.; Kwon, Y.J.; Park, M.-S.; Kim, K.H.; Shin, C.H.; Joo, J.-C.; Park, S.-J. Effect of a Combination of Lactiplantibacillus plantarum KC3 and Leonurus japonicus Extracts in Respiratory Discomfort: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 2128. https://doi.org/10.3390/nu16132128
Kim H-j, Lee J-H, Kwon S-Y, Kwon YJ, Park M-S, Kim KH, Shin CH, Joo J-C, Park S-J. Effect of a Combination of Lactiplantibacillus plantarum KC3 and Leonurus japonicus Extracts in Respiratory Discomfort: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2024; 16(13):2128. https://doi.org/10.3390/nu16132128
Chicago/Turabian StyleKim, Hyung-jin, Jun-Ho Lee, So-Young Kwon, Yoo Jin Kwon, Mi-Sun Park, Kyung Hwan Kim, Chang Hun Shin, Jong-Cheon Joo, and Soo-Jung Park. 2024. "Effect of a Combination of Lactiplantibacillus plantarum KC3 and Leonurus japonicus Extracts in Respiratory Discomfort: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 16, no. 13: 2128. https://doi.org/10.3390/nu16132128
APA StyleKim, H.-j., Lee, J.-H., Kwon, S.-Y., Kwon, Y. J., Park, M.-S., Kim, K. H., Shin, C. H., Joo, J.-C., & Park, S.-J. (2024). Effect of a Combination of Lactiplantibacillus plantarum KC3 and Leonurus japonicus Extracts in Respiratory Discomfort: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 16(13), 2128. https://doi.org/10.3390/nu16132128





