A Plea for Monitoring Serum Selenium Levels in Breast Cancer Patients: Selenium Deficiency Is Rare during the First Year of Therapy, and Selenium Supplementation Is Associated with Elevated Risk of Overdosing
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistics
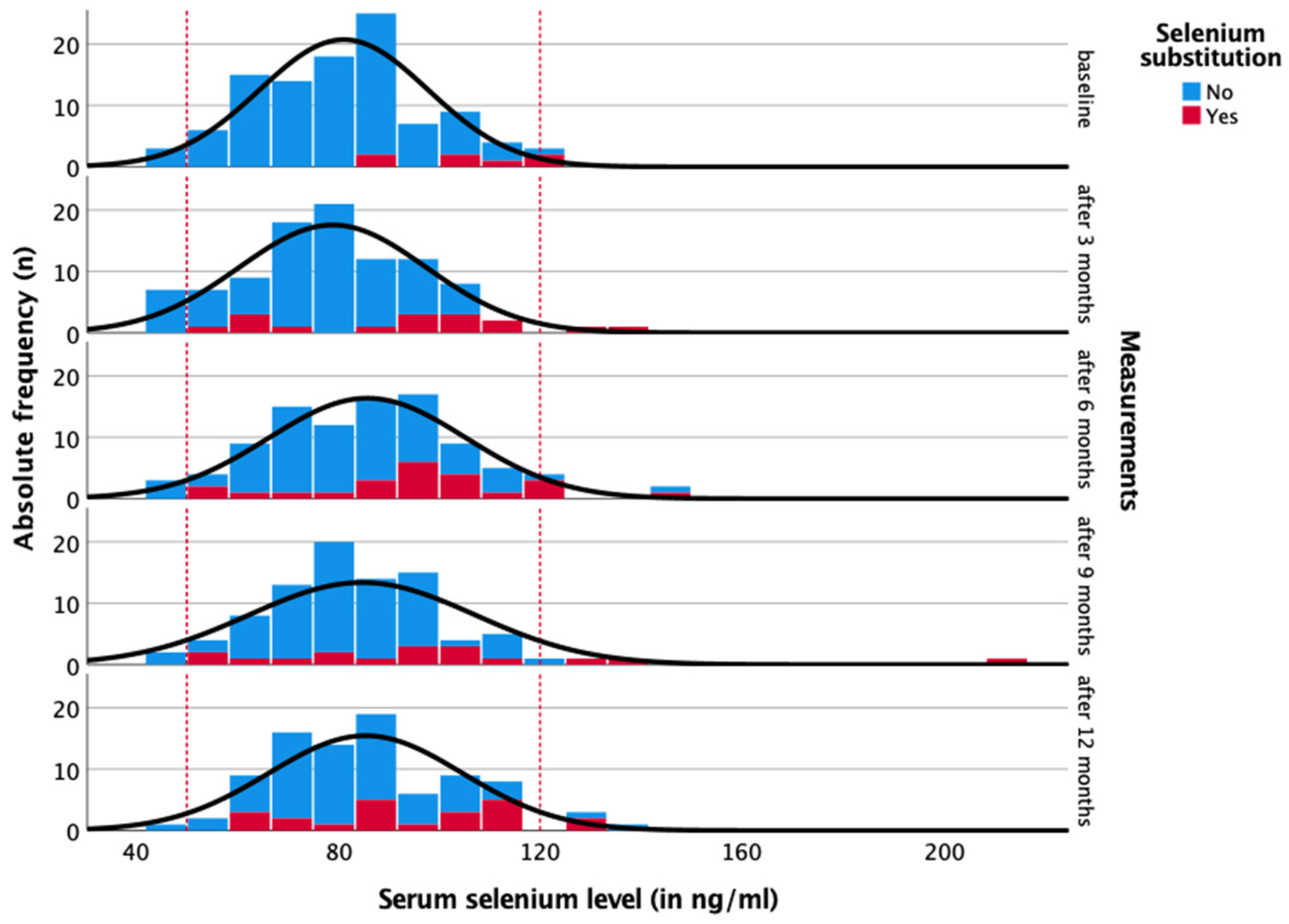
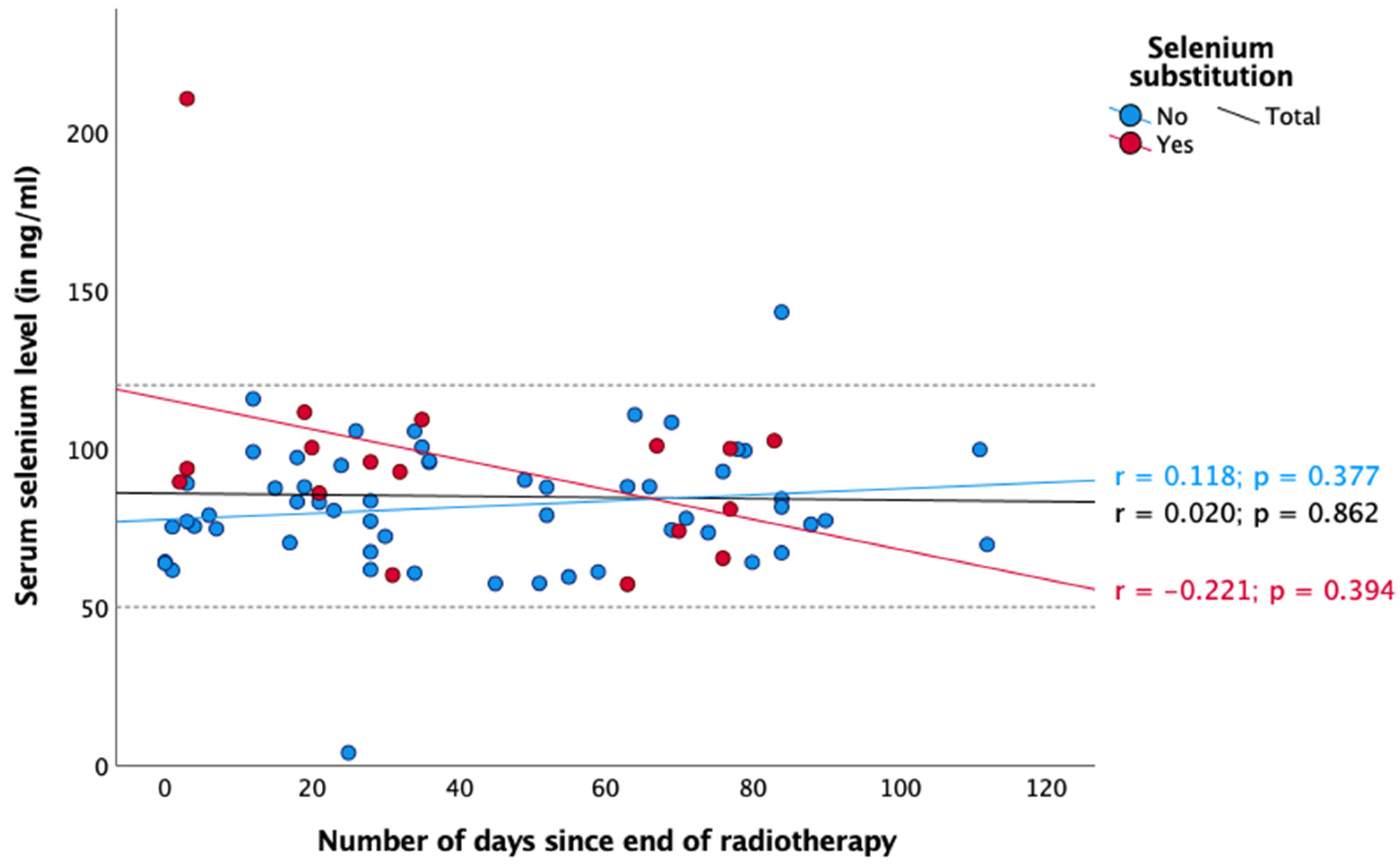
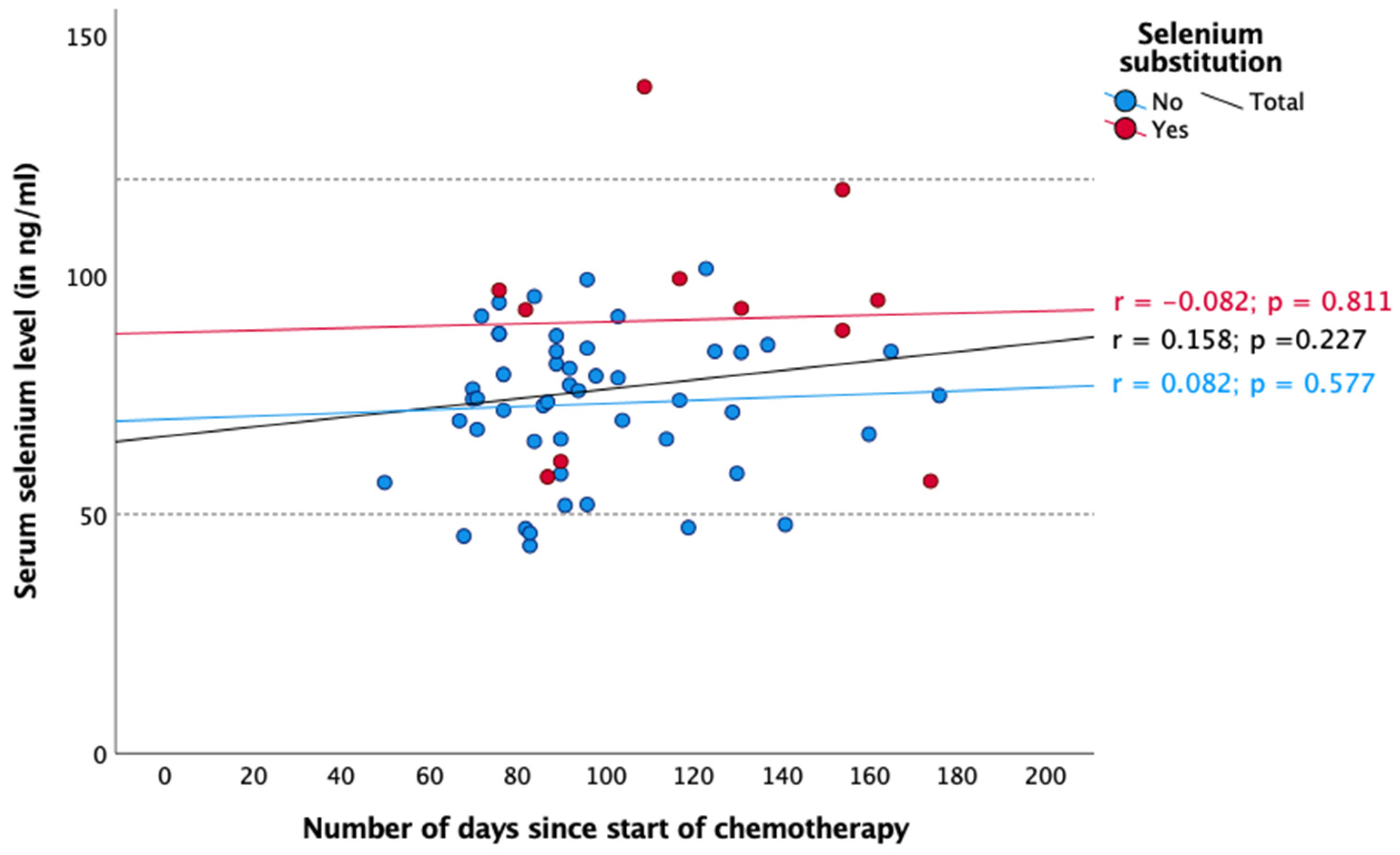
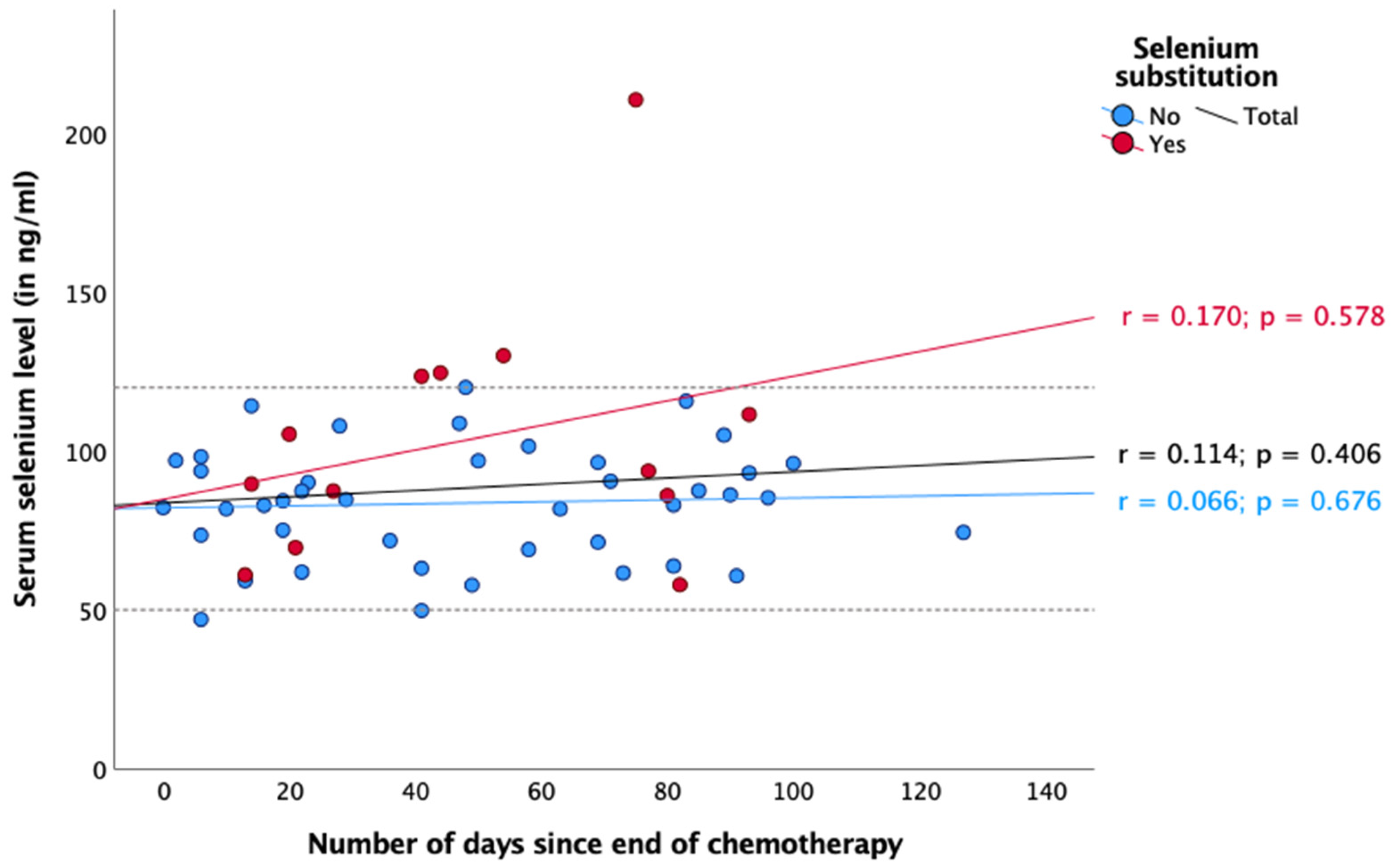
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| ER | estrogen receptor |
| PR | progesterone receptor |
| HER2 | human epidermal growth factor 2 |
| GEE | generalized estimating equations analysis |
| NST | no special type |
| UICC/AJCC | Union for International Cancer Control/American Joint Committee on Cancer |
References
- World Health Organization. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 21 February 2024).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Holzhauer, P.; Kisters, K.; Holick, M.F.; Adamietz, I.A. Micronutrients in Oncological Intervention. Nutrients 2016, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Hübner, J.; Holzhauer, P.; Kleeberg, U.R. Antioxidants and Other Micronutrients in Complementary Oncology. Der Onkol. 2010, 16, 73–79. [Google Scholar] [CrossRef]
- Zirpoli, G.R.; Brennan, P.M.; Hong, C.C.; McCann, S.E.; Ciupak, G.; Davis, W.; Unger, J.M.; Budd, G.T.; Hershman, D.L.; Moore, H.C.; et al. Supplement Use during an Intergroup Clinical Trial for Breast Cancer (S0221). Breast Cancer Res. Treat. 2013, 137, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Oncology Guideline Programme (German Cancer Society; German Health Aid; AWMF). S3 Guideline Early Detection, Diagnosis, Therapy and Aftercare of Breast Cancer, Version 4.4. Available online: http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ (accessed on 20 February 2024).
- Oncology Guideline Programme (German Cancer Society; German Health Aid; AWMF). S3 Guideline on Complementary Medicine in the Treatment of Oncological Patients. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/komplementaermedizin (accessed on 21 February 2024).
- Robert Koch Institute. Recommendation of the Robert Koch Institute. Fed. Health Bull. Health Res. Health Prot. 2005, 49, 88–101. [Google Scholar] [CrossRef]
- Federal Environment Agency. Statement on Selenium and Human Biomonitoring. Fed. Health Bull. Health Res. Health Prot. 2002, 45, 190–195. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Müller, M.; Graeve, L. Löffler/Petrides Biochemie Und Pathobiochemie; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Burk, R.F.; Hill, K.E.; Motley, A.K. Selenoprotein Metabolism and Function: Evidence for More than One Function for Selenoprotein P. J. Nutr. 2003, 133, 1517S–1520S. [Google Scholar] [CrossRef] [PubMed]
- Domke, A.; Großklaus, R.; Niemann, B.; Przyrembel, H.; Richter, K.; Schmidt, E.; Weißenborn, A.; Wörner, B.; Ziegenhagen, R. Verwendung von Mineralstoffen in Lebensmitteln; Bundesinstitut für Risikobewertung: Berlin, Germany, 2004; ISBN 3931675882. [Google Scholar]
- Köhrle, J. Selenium and the Thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Mattmiller, S.A.; Carlson, B.A.; Sordillo, L.M. Regulation of Inflammation by Selenium and Selenoproteins: Impact on Eicosanoid Biosynthesis. J. Nutr. Sci. 2013, 2, e28. [Google Scholar] [CrossRef] [PubMed]
- German Society for Nutrition. Selected Questions and Answers about Selenium. Available online: https://www.dge.de/gesunde-ernaehrung/faq/selen/ (accessed on 20 February 2024).
- German Health Care. Nutrition and Vital Substances. In Nutritional Value Table: Selenium in Food; German Health Care: Frankfurt, Germany, 2011. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for Selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- German Society for Nutrition. Selen. Available online: https://www.dge.de/wissenschaft/referenzwerte/selen/ (accessed on 20 February 2024).
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised Reference Values for Selenium Intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Tolerable Upper Intake Levels Scientific Committee on Food Scientific Panel on Dietetic Products, Nutrition and Allergies; EFSA: Parma, Italy, 2006; ISBN 9291990140. [Google Scholar]
- Yang, G.; Zhou, R.; Yin, S.; Gu, L.; Yan, B.; Liu, Y.; Liu, Y.; Li, X. Studies of Safe Maximal Daily Dietary Selenium Intake in a Seleniferous Area in China. I. Selenium Intake and Tissue Selenium Levels of the Inhabitants. J. Trace Elem. Electrolytes Health Dis. 1989, 3, 77–87. [Google Scholar]
- Raines, A.M.; Sunde, R.A. Selenium Toxicity but Not Deficient or Super-Nutritional Selenium Status Vastly Alters the Transcriptome in Rodents. BMC Genom. 2011, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Trippe, R.C.; Pilon-Smits, E.A.H. Selenium Transport and Metabolism in Plants: Phytoremediation and Biofortification Implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.; Crane, S.B. Selenium Toxicity from a Misformulated Dietary Supplement, Adverse Health Effects, and the Temporal Response in the Nail Biologic Monitor. Nutrients 2008, 5, 1024–1057. [Google Scholar] [CrossRef] [PubMed]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute Selenium Toxicity Associated with a Dietary Supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef]
- Merian, E.; Clarkson, T.W. Metals and Their Compounds in the Environment: Occurrence, Analysis, and Biological Relevance; VCH: Weinheim, Germany, 1991; ISBN 9780895735621. [Google Scholar]
- Shamberger, R.J.; Frost, D.V. Possible Protective Effect of Selenium against Human Cancer. Can. Med. Assoc. J. 1969, 100, 682. [Google Scholar] [PubMed]
- Rayman, M.P. Selenium in Cancer Prevention: A Review of the Evidence and Mechanism of Action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F. Chemopreventive Mechanisms of Selenium. Med. Klin. 1999, 94 (Suppl. S3), 18–24. [Google Scholar] [CrossRef] [PubMed]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A Double-Edged Sword for Defense and Offence in Cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef]
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.; Falco, M.; et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients 2021, 13, 953. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.; Horneber, M.; D’Amico, R.; Crespi, C. Selenium for Preventing Cancer (Cochrane Review). Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef] [PubMed]
- Kipp, A.P. Selenium in Colorectal and Differentiated Thyroid Cancer. Hormones 2020, 19, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Rodemann, H.P.; Hehr, T.; Bamberg, M. Relevance of the Radioprotective Effect of Natrium Selenite. Preclinical Results. Med. Klin. 1999, 94, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Franca, C.A.S.; Nogueira, C.R.; Ramalho, A.; Carvalho, A.C.P.; Vieira, S.L.; Penna, A.B.R.C. Serum Levels of Selenium in Patients with Breast Cancer before and after Treatment of External Beam Radiotherapy. Ann. Oncol. 2010, 22, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Narod, S.A.; Huzarski, T.; Zajaczek, S.; Huzarska, J.; Gorski, B.; Lubinski, J. Increased Rates of Chromosome Breakage in BRCA1 Carriers Are Normalized by Oral Selenium Supplementation. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.; Pedram, P.; Shahidi, M.; Du, J.; Yi, Y.; Gulliver, W.; Zhang, H.; Sun, G. Significant Beneficial Association of High Dietary Selenium Intake with Reduced Body Fat in the CODING Study. Nutrients 2016, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, L.C.; Cardoso de Araújo, D.S.; da Cunha Soares, T.; Clímaco Cruz, K.J.; Henriques, G.S.; Marreiro, D. do N. Nutritional Status of Selenium in Overweight and Obesity: A Systematic Review and Meta-Analysis. Clin. Nutr. 2022, 41, 862–884. [Google Scholar] [CrossRef] [PubMed]
- Zemlin, C.; Schleicher, J.T.; Altmayer, L.; Stuhlert, C.; Wörmann, C.; Lang, M.; Scherer, L.S.; Thul, I.C.; Spenner, L.S.; Simon, J.A.; et al. Improved Awareness of Physical Activities Is Associated with a Gain of Fitness and a Stable Body Weight in Breast Cancer Patients during the First Year of Antineoplastic Therapy: The BEGYN-1 Study. Front. Oncol. 2023, 13, 1198157. [Google Scholar] [CrossRef] [PubMed]
- Zemlin, C.; Stuhlert, C.; Schleicher, J.T.; Wörmann, C.; Altmayer, L.; Lang, M.; Scherer, L.S.; Thul, I.C.; Müller, C.; Kaiser, E.; et al. Longitudinal Assessment of Physical Activity, Fitness, Body Composition, Immunological Biomarkers, and Psychological Parameters during the First Year After Diagnosis in Women With Non-Metastatic Breast Cancer: The BEGYN Study Protocol. Front. Oncol. 2021, 11, 762709. [Google Scholar] [CrossRef] [PubMed]
- Zemlin, C.; Altmayer, L.; Stuhlert, C.; Schleicher, J.T.; Wörmann, C.; Lang, M.; Scherer, L.S.; Thul, I.C.; Spenner, L.S.; Simon, J.A.; et al. Prevalence and Relevance of Vitamin D Deficiency in Newly Diagnosed Breast Cancer Patients: A Pilot Study. Nutrients 2023, 15, 1450. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Saez, J.B.; Senra-Varela, A.; Pousa-Estevez, L. Selenium in Breast Cancer. Oncology 2003, 64, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Marian, M.J. Dietary Supplements Commonly Used by Cancer Survivors: Are There Any Benefits? Nutr. Clin. Pract. 2017, 32, 607–627. [Google Scholar] [CrossRef]
- Huguenin, G.V.B.; Oliveira, G.M.M.; Moreira, A.S.B.; Saint’Pierre, T.D.; Gonçalves, R.A.; Pinheiro-Mulder, A.R.; Teodoro, A.J.; Luiz, R.R.; Rosa, G. Improvement of Antioxidant Status after Brazil Nut Intake in Hypertensive and Dyslipidemic Subjects. Nutr. J. 2015, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.; Da Silva, A.; Kelly, B.; Silveira, S.; Vieira, B.; De Freitas, M.; Hermana, H.; Hermsdorff, M.; Bressan, J. Effects of Regular Brazil Nut (Bertholletia Excelsa H.B.K.) Consumption on Health: A Systematic Review of Clinical Trials. Foods 2022, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Dayan, D.; Leinert, E.; Singer, S.; Janni, W.; Kühn, T.; Flock, F.; Felberbaum, R.; Herbert, S.L.; Wöckel, A.; Schwentner, L. Association of Social Service Counseling in Breast Cancer Patients with Financial Problems, Role Functioning and Employment-Results from the Prospective Multicenter BRENDA II Study. Arch. Gynecol. Obstet. 2023, 307, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Aviki, E.M.; Manning-Geist, B.L.; Sokolowski, S.S.; Newman, T.; Blinder, V.S.; Chino, F.; Doyle, S.M.; Liebhaber, A.; Gordhandas, S.B.; Brown, C.L.; et al. Risk Factors for Financial Toxicity in Patients with Gynecologic Cancer. Am. J. Obstet. Gynecol. 2022, 226, 817.e1–817.e9. [Google Scholar] [CrossRef] [PubMed]
- Seifart, U. Socioeconomic Risks for People with Cancer-Possible Consequences and Assistance. Fed. Health Bull. Health Res. Health Prot. 2022, 65, 439–445. [Google Scholar] [CrossRef]
- Gagnon, M.A.; Lexchin, J. The Cost of Pushing Pills: A New Estimate of Pharmaceutical Promotion Expenditures in the United States. PLoS Med. 2008, 5, e1. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium Contaminated Waters: An Overview of Analytical Methods, Treatment Options and Recent Advances in Sorption Methods. Sci. Total Environ. 2015, 521–522, 246–260. [Google Scholar] [CrossRef] [PubMed]



| Clinical Characteristics | n | % | Serum Selenium Level (in ng/mL) | p-Value | |
|---|---|---|---|---|---|
| Tumor entity | NST | 91 | 82.7% | 84.0 (44.4–123.2) | 0.858 |
| Invasive lobular | 12 | 10.9% | 81.5 (52.0–108.7) | ||
| Others 1) | 7 | 6.4% | 83.9 (62.2–110.3) | ||
| UICC-/AJCC stage | I | 46 | 41.8% | 84.0 (44.4–123.2) | 0.629 |
| II | 56 | 50.9% | 78.3 (52.0–120.8) | ||
| III | 8 | 7.3% | 82.4 (46.1–93.3) | ||
| Grading | G1 | 10 | 9.2% | 83.8 (62.2–110.3) | 0.506 |
| G2 | 56 | 51.4% | 80.6 (44.4–116.9) | ||
| G3 | 43 | 39.4% | 84.2 (52.0–123.2) | ||
| Tumor biology | Luminal A | 48 | 43.6% | 81.2 (44.4–110.3) | 0.497 |
| Luminal B | 24 | 21.8% | 79.6 (57.3–123.2) | ||
| HER2/neu-positive | 27 | 24.5% | 78.3 (46.1–116.9) | ||
| Triple-negative | 11 | 10.0% | 85.9 (54.9–102.2) | ||
| ER status | Positive | 89 | 80.9% | 81.5 (44.4–123.2) | 0.811 |
| Negative | 21 | 19.1% | 81.1 (54.9–102.2) | ||
| PR status | Positive | 72 | 65.5% | 80.4 (44.4–123.2) | 0.325 |
| Negative | 38 | 34.5% | 84.2 (54.9.1–116.9) | ||
| Mutations | No | 30 | 71.4% | 79.6 (44.4–114.6) | 0.070 2) |
| BRCA1/2 | 8 | 19.1% | 89.0 (66.2–123.2) | ||
| CHECK/ATM | 4 | 9.5% | 69.6 (64.1–74.6) | ||
| Menopause | No | 33 | 30.0% | 81.5 (52.0–120.8) | 0.464 |
| Yes | 77 | 70.0% | 81.2 (44.4–123.2) |
| Measurements | Serum Selenium Level in ng/ml | Frequency of Substitution | Overdose with Substitution |
|---|---|---|---|
| Baseline | 81.5 (44.4–123.2) | 7 of 110 (6.4%) | 2 of 7 (28.6%) |
| After 3 months | 78.7 (41.9–139.3) | 17 of 101 (16.8%) | 2 of 17 (11.8%) |
| After 6 months | 84.5 (47.2–143.1) | 23 of 96 (24.0%) | 3 of 23 (13.0%) |
| After 9 months | 82.4 (40.7–210.5) | 17 of 90 (18.9%) | 3 of 17 (17.6%) |
| After 12 months | 84.3 (38.4–138.7) | 23 of 91 (25.3%) | 2 of 23 (8.7%) |
| Dosage | 0 Months n = 7/110 (6.4%) | 3 Months n = 17/101 (16.8%) | 6 Months n = 23/96 (24.0%) | 9 Months n = 17/90 (18.9%) | 12 Months n = 23/91 (25.3%) |
|---|---|---|---|---|---|
| 100 µg/day | 0 (0.0%) | 3 (17.6%) | 3 (13.0%) | 2 (11.8%) | 3 (13.0%) |
| 200 µg/day | 1 (14.3%) | 2 (11.8%) | 4 (17.4%) | 3 (17.6%) | 5 (21.7%) |
| 300 µg/day | 1 (14.3%) | 1 (5.9%) | 6 (26.1%) | 4 (23.5%) | 7 (30.4%) |
| 50 µg/day | 0 (0.0%) | 2 (11.8%) | 2 (8.7%) | 0 (0.0%) | 1 (4.3%) |
| 30 µg/day | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (4.3%) |
| Variable, 100–300 µg/day | 0 (0.0%) | 1 (5.9%) | 1 (4.3%) | 1 (5.9%) | 1 (4.3%) |
| 35 µg/day LaVita juice (1 tbsp) | 0 (0.0%) | 2 (11.8%) | 2 (8.7%) | 3 (17.6%) | 4 (17.4%) |
| 113 µg Sinekrin (2 tablets) | 1 (14.3%) | 1 (5.9%) | 1 (4.3%) | 1 (5.9%) | 2 (8.7%) |
| No information on exact dosage | 4 (57.1%) | 5 (29.4%) | 6 (26.1%) | 3 (17.6%) | 1 (4.3%) |
| Various Food Products | Dietary Intake (in Days per Month) | Selenium Content (in μg/kg) | Spearman Correlation |
|---|---|---|---|
| Herring/trout/salmon | 3.0 (0–12) | 250–430 | r = 0.044 p = 0.694 |
| Mackerel/tuna | 1.0 (0–8) | 390–820 | r = 0.132 p = 0.239 |
| Eggs/margarine | 12.0 (0–28) | Eggs: 100–200 Margarine: no data available | r = 0.104 p = 0.355 |
| Cream/gouda/butter | 20.0 (0–28) | No data available | r = 0.003 p = 0.978 |
| Whole milk/quark/yogurt | 20.0 (0–28) | 5–20 | r = 0.156 p = 0.166 |
| Chanterelles/champignons/ porcini mushrooms | 3.0 (0–28) | 10–1800 | r = 0.324 p = 0.003 |
| Beef/veal liver | 0 (0–5) | 20–200 | r = 0.198 p = 0.077 |
| Body Composition | Selenium Level at Baseline | Selenium Level after 3 Months | Selenium Level after 6 Months | Selenium Level after 9 months | Selenium Level after 12 Months |
|---|---|---|---|---|---|
| Weight | r = −0.06 p = 0.52 | r = 0.05 p = 0.63 | r = 0.07 p = 0.49 | r = 0.10 p = 0.35 | r = 0.06 p = 0.61 |
| Body Mass Index | r = −0.12 p = 0.24 | r = −0.01 p = 0.89 | r = 0.11 p = 0.30 | r = 0.12 p = 0.26 | r = 0.07 p = 0.50 |
| Muscle mass | r = 0.04 p = 0.677 | r = 0.04 p = 0.69 | r = −0.01 p = 0.94 | r = −0.08 p = 0.44 | r = 0.06 p = 0.60 |
| Body fat percentage | r = −0.14 p = 0.16 | r = 0.07 p = 0.52 | r = 0.15 p = 0.16 | r = 0.20 p = 0.06 | r = 0.07 p = 0.54 |
| Visceral fat | r = −0.17 p = 0.08 | r = −0.02 p = 0.84 | r = 0.14 p = 0.18 | r = 0.09 p = 0.40 | r = 0.09 p = 0.41 |
| Bone mass | r = 0.05 p = 0.63 | r = 0.05 p = 0.61 | r = −0.02 p = 0.85 | r = −0.09 p = 0.40 | r = 0.02 p = 0.84 |
| Thyroid Hormones | Selenium Level at Baseline | Selenium Level after 3 Months | Selenium Level after 6 Months | Selenium Level after 9 Months | Selenium Level after 12 Months |
|---|---|---|---|---|---|
| TSH | r = −0.07 p = 0.51 | r = −0.13 p = 0.26 | r = −0.09 p = 0.43 | r = 0.02 p = 0.88 | r = 0.00 p = 0.98 |
| T3 | r = 0.11 p = 0.30 | r = −0.14 p = 0.20 | r = 0.05 p = 0.65 | r = 0.11 p = 0.37 | r = 0.01 p = 0.95 |
| T4 | r = 0.05 p = 0.67 | r = 0.15 p = 0.17 | r = 0.15 p = 0.20 | r = −0.03 p = 0.82 | r = −0.04 p = 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altmayer, L.A.; Lang, M.; Schleicher, J.T.; Stuhlert, C.; Wörmann, C.; Scherer, L.-S.; Thul, I.C.; Spenner, L.S.; Simon, J.A.; Wind, A.; et al. A Plea for Monitoring Serum Selenium Levels in Breast Cancer Patients: Selenium Deficiency Is Rare during the First Year of Therapy, and Selenium Supplementation Is Associated with Elevated Risk of Overdosing. Nutrients 2024, 16, 2134. https://doi.org/10.3390/nu16132134
Altmayer LA, Lang M, Schleicher JT, Stuhlert C, Wörmann C, Scherer L-S, Thul IC, Spenner LS, Simon JA, Wind A, et al. A Plea for Monitoring Serum Selenium Levels in Breast Cancer Patients: Selenium Deficiency Is Rare during the First Year of Therapy, and Selenium Supplementation Is Associated with Elevated Risk of Overdosing. Nutrients. 2024; 16(13):2134. https://doi.org/10.3390/nu16132134
Chicago/Turabian StyleAltmayer, Laura Alicia, Marina Lang, Julia Theresa Schleicher, Caroline Stuhlert, Carolin Wörmann, Laura-Sophie Scherer, Ida Clara Thul, Lisanne Sophie Spenner, Jana Alisa Simon, Alina Wind, and et al. 2024. "A Plea for Monitoring Serum Selenium Levels in Breast Cancer Patients: Selenium Deficiency Is Rare during the First Year of Therapy, and Selenium Supplementation Is Associated with Elevated Risk of Overdosing" Nutrients 16, no. 13: 2134. https://doi.org/10.3390/nu16132134
APA StyleAltmayer, L. A., Lang, M., Schleicher, J. T., Stuhlert, C., Wörmann, C., Scherer, L.-S., Thul, I. C., Spenner, L. S., Simon, J. A., Wind, A., Tokcan, M., Kaiser, E., Weber, R., Goedicke-Fritz, S., Wagenpfeil, G., Zemlin, M., Solomayer, E.-F., Reichrath, J., Müller, C., & Zemlin, C. (2024). A Plea for Monitoring Serum Selenium Levels in Breast Cancer Patients: Selenium Deficiency Is Rare during the First Year of Therapy, and Selenium Supplementation Is Associated with Elevated Risk of Overdosing. Nutrients, 16(13), 2134. https://doi.org/10.3390/nu16132134









