Effects of Short-Term Nighttime Carbohydrate Restriction Method on Exercise Performance and Fat Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants
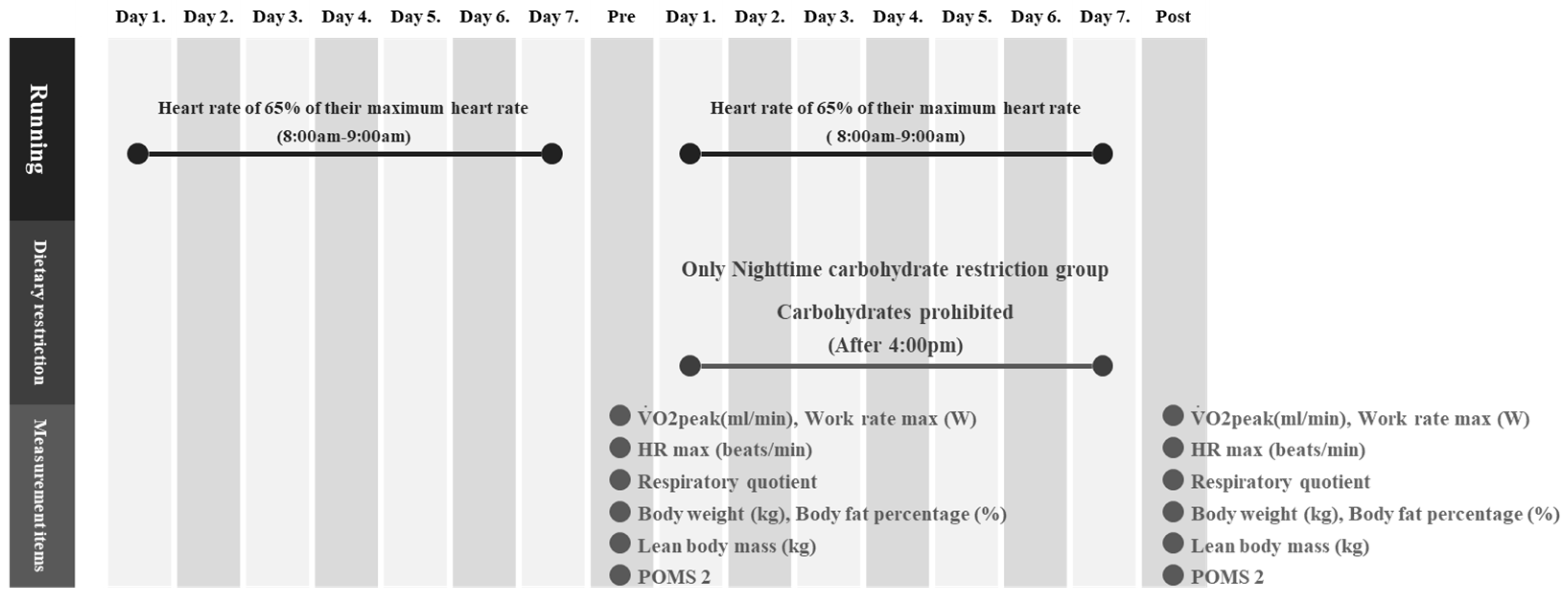
2.3. Experimental Protocol
2.4. Measurement Items and Methods
2.5. Statistical Analysis
3. Results
3.1. Calorie Intake
3.2. Carbohydrate Intake
3.3. POMS 2 Score
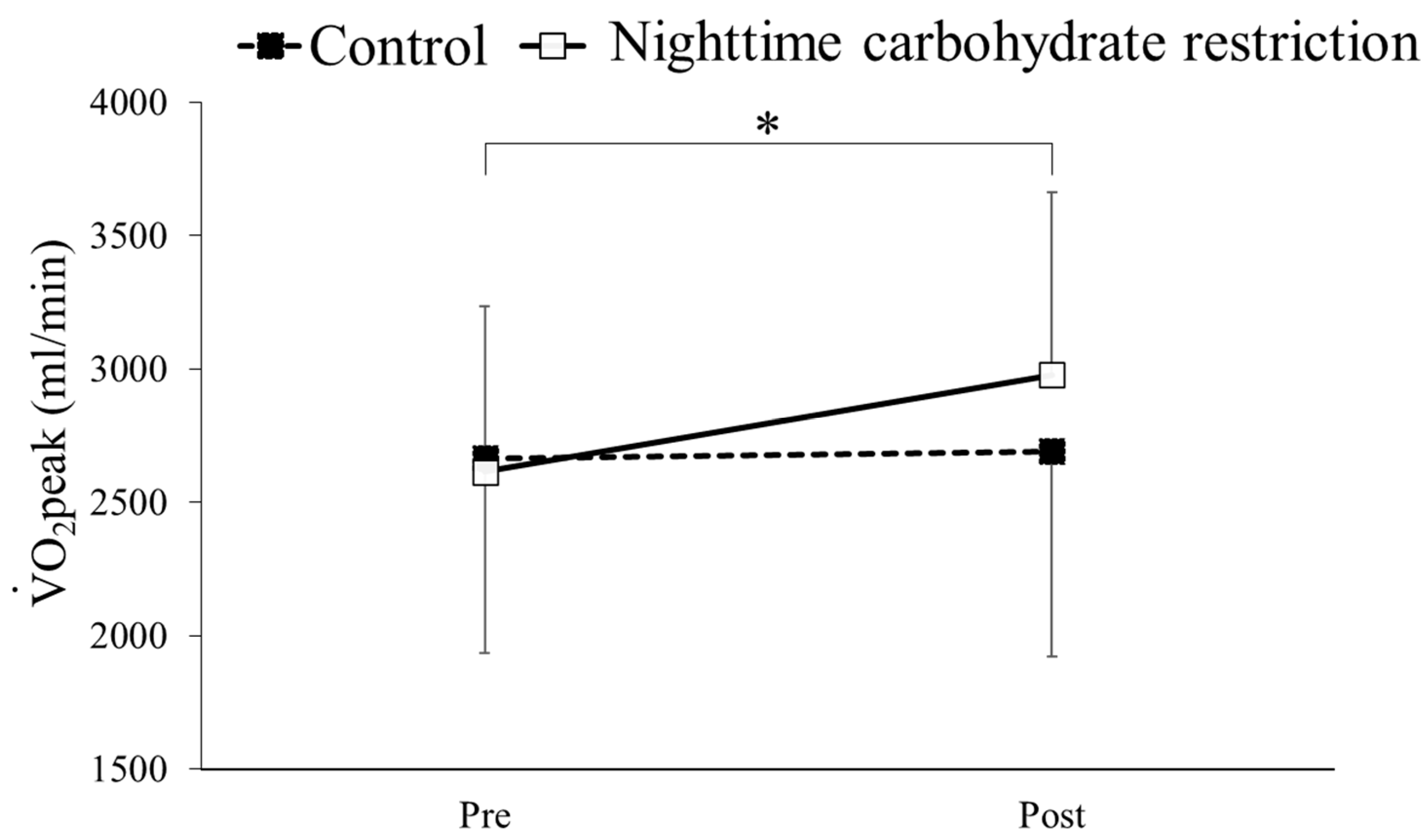
3.4. O2peak
3.5. HRmax
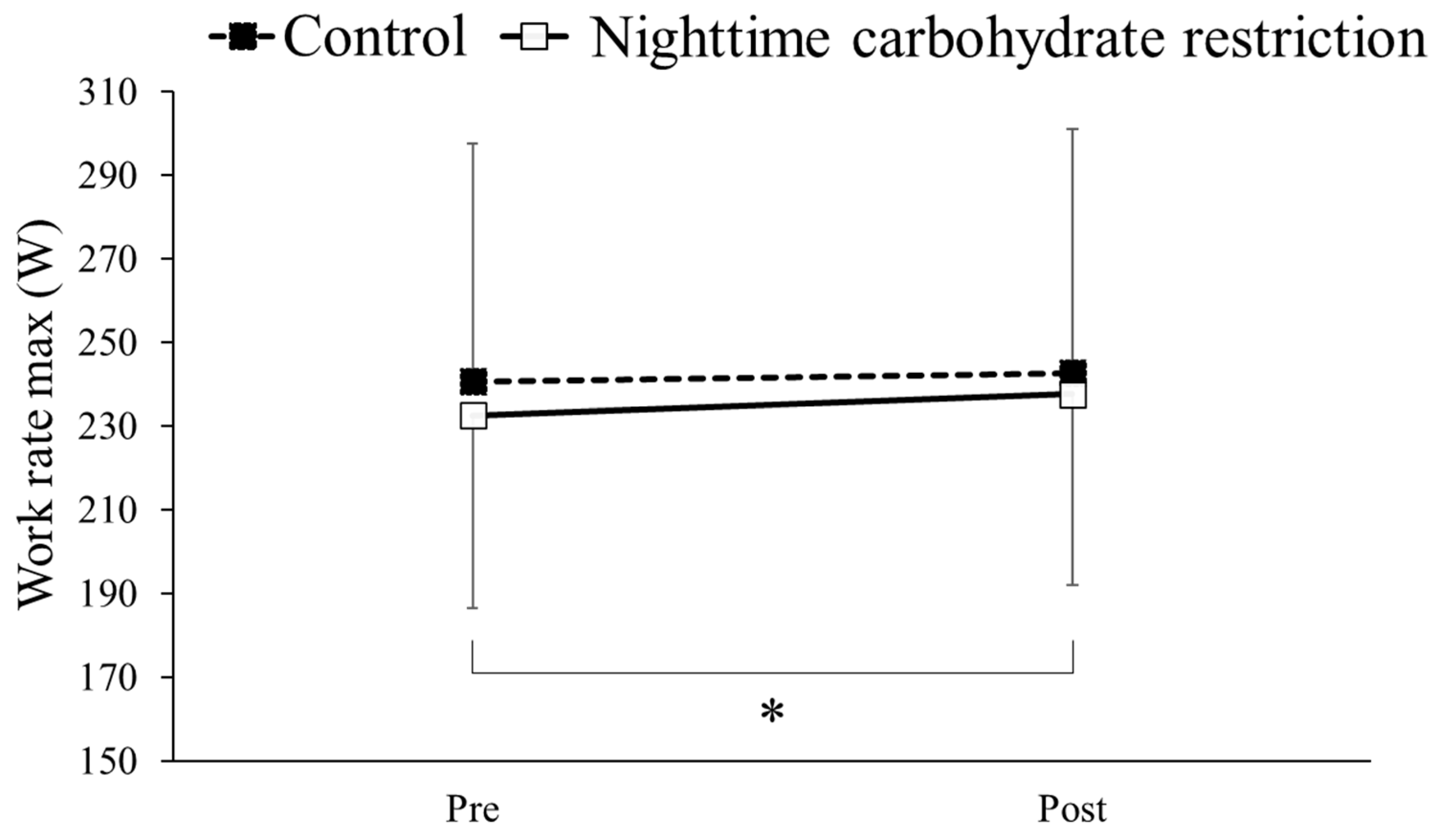
3.6. Work Rate Max
3.7. RQ
3.8. Body Weight
3.9. Body Fat Percentage
3.10. Lean Body Mass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDowall, J.A. Supplement use by young athletes. J. Sports Sci. Med. 2007, 6, 337–342. [Google Scholar] [PubMed]
- Molinero, O.; Márquez, S. Use of nutritional supplements in sports: Risks, knowledge, and behavioural-related factors. Nutr. Hosp. 2009, 24, 128–134. [Google Scholar]
- Stellingwerff, T. Contemporary nutrition approaches to optimize elite marathon performance. Int. J. Sports Physiol. Perform. 2013, 8, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Louhelainen, J.; Iqbal, Z.; Cochran, A.J.; Gibala, M.J.; Gregson, W.; Close, G.L.; Drust, B.; Morton, J.P. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: Implications for mitochondrial biogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, 450–458. [Google Scholar] [CrossRef]
- Hulston, C.J.; Venables, M.C.; Mann, C.H.; Martin, C.; Philp, A.; Baar, K.; Jeukendrup, A.E. Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Med. Sci. Sports Exerc. 2010, 42, 2046–2055. [Google Scholar] [CrossRef]
- Morton, J.P.; Croft, L.; Bartlett, J.D.; Maclaren, D.P.; Reilly, T.; Evans, L.; McArdle, A.; Drust, B. Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. J. Appl. Physiol. 2009, 106, 1513–1521. [Google Scholar] [CrossRef]
- Pilegaard, H.; Keller, C.; Steensberg, A.; Helge, J.W.; Pedersen, B.K.; Saltin, B.; Neufer, P.D. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J. Physiol. 2002, 541, 261–271. [Google Scholar] [CrossRef]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Ramaekers, M.; Hespel, P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J. Appl. Physiol. 2011, 110, 236–245. [Google Scholar] [CrossRef]
- Yeo, W.K.; Paton, C.D.; Garnham, A.P.; Burke, L.M.; Carey, A.L.; Hawley, J.A. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J. Appl. Physiol. 2008, 105, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M. Metabolic responses to carbohydrate ingestion: Effect on exercise performance. In Perspectives in Exercise Science and Sports Medicine Vol 12. The Metabolic Basis of Performance in Sport and Exercise; Lamb, D.R., Murray, R., Eds.; Cooper Publishing Company: Traverse City, MI, USA, 1999; pp. 93–124. [Google Scholar]
- Holloszy, J.O.; Kohrt, W.M.; Hansen, P.A. The regulation of carbohydrate and fat metabolism during and after exercise. Front. Biosci. 1998, 15, 1011–1027. [Google Scholar] [CrossRef]
- Rapoport, B.I. Metabolic factors limiting performance in marathon runners. PLoS Comput. Biol. 2010, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- American Dietetic Association; Dietitians of Canada; American College of Sports Medicine; Rodriguez, N.R.; Di Marco, N.M.; Langley, S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar] [CrossRef]
- Chryssanthopoulos, C.; Williams, C. Pre-exercise carbohydrate meal and endurance running capacity when carbohydrates are ingested during exercise. Int. J. Sports Med. 1997, 18, 543–548. [Google Scholar] [CrossRef]
- Fallowfield, J.L.; Williams, C. Carbohydrate intake and recovery from prolonged exercise. Int. J. Sport Nutr. 1993, 3, 150–164. [Google Scholar] [CrossRef]
- Wright, D.A.; Sherman, W.M.; Dernbach, A.R. Carbohydrate feedings before, during, or in combination improve cycling endurance performance. J. Appl. Physiol. 1991, 71, 1082–1088. [Google Scholar] [CrossRef]
- Akermark, C.; Jacobs, I.; Rasmussen, M.; Karlsson, J. Diet and muscle glycogen concentration in relation to physical performance in Swedish elite ice hockey players. Int. J. Sport Nutr. 1996, 6, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Balsom, P.D.; Wood, K.; Olsson, P.; Ekblom, B. Carbohydrate intake and multiple sprint sports: With special reference to football (soccer). Int. J. Sports Med. 1999, 20, 48–52. [Google Scholar] [CrossRef]
- Karlsson, J.; Saltin, B. Diet, muscle glycogen, and endurance performance. J. Appl. Physiol. 1971, 31, 203–206. [Google Scholar] [CrossRef]
- Burke, L.M. Fueling strategies to optimize performance: Training high or training low? Scand. J. Med. Sci. Sports 2010, 20, 248–258. [Google Scholar] [CrossRef]
- Louis, J.; Marquet, L.A.; Tiollier, E.; Bermon, S.; Hausswirth, C.; Brisswalter, J. The impact of sleeping with reduced glycogen stores on immunity and sleep in triathletes. Eur. J. Appl. Physiol. 2016, 116, 1941–1954. [Google Scholar] [CrossRef]
- Marquet, L.A.; Brisswalter, J.; Louis, J.; Tiollier, E.; Burke, L.M.; Hawley, J.A.; Hausswirth, C. Enhanced Endurance Performance by Periodization of Carbohydrate Intake: “Sleep Low” Strategy. Med. Sci. Sports Exerc. 2016, 48, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Marquet, L.A.; Hausswirth, C.; Molle, O.; Hawley, J.A.; Burke, L.M.; Tiollier, E.; Brisswalter, J. Periodization of Carbohydrate Intake: Short-Term Effect on Performance. Nutrients 2016, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.; Ghilagaber, S.; Nikolaev, A.; Hardie, D.G. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009, 9, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Wojtaszewski, J.F.; MacDonald, C.; Nielsen, J.N.; Hellsten, Y.; Hardie, D.G.; Kemp, B.E.; Kiens, B.; Richter, E.A. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2003, 284, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Ruuska, P.S.; Hautala, A.J.; Kiviniemi, A.M.; Mäkikallio, T.H.; Tulppo, M.P. Self-rated mental stress and exercise training response in healthy subjects. Front. Physiol. 2012, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Stults-Kolehmainen, M.A.; Sinha, R. The effects of stress on physical activity and exercise. Sports Med. 2014, 44, 81–121. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, 17–27. [Google Scholar] [CrossRef]
- Lane, S.C.; Camera, D.M.; Lassiter, D.G.; Areta, J.L.; Bird, S.R.; Yeo, W.K.; Jeacocke, N.A.; Krook, A.; Zierath, J.R.; Burke, L.M.; et al. Effects of sleeping with reduced carbohydrate availability on acute training responses. J. Appl. Physiol. 2015, 119, 643–655. [Google Scholar] [CrossRef]
- Stellingwerf, T. Case study: Nutrition and training periodization inthree elite marathon runners. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 392–400. [Google Scholar] [CrossRef]
- Pilegaard, H.; Ordway, G.A.; Saltin, B.; Neufer, P.D. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am. J. Physiol. Endocrinol. Metab. 2000, 279, 806–814. [Google Scholar] [CrossRef]
- Pilegaard, H.; Osada, T.; Andersen, L.T.; Helge, J.W.; Saltin, B.; Neufer, P.D. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 2005, 54, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1α: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Jeukendrup, A.E.; Wagenmakers, A.; Saris, W.H. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am. J. Physiol. 1997, 273 Pt 1, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Morton, J.P. Ramping up the signal: Promoting endurance training adaptation in skeletal muscle by nutritional manipulation. Clin. Exp. Pharmacol. Physiol. 2014, 41, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Fischer, C.P.; Plomgaard, P.; Andersen, J.L.; Saltin, B.; Pedersen, B.K. Skeletal muscle adaptation: Training twice every second day vs. training once daily. J. Appl. Physiol. 2005, 98, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Riis, S.; Møller, A.B.; Dollerup, O.; Høffner, L.; Jessen, N.; Madsen, K. Acute and sustained effects of a periodized carbohydrate intake using the sleep-low model in endurance-trained males. Scand. J. Med. Sci. Sports 2019, 29, 1866–1880. [Google Scholar] [CrossRef]
- Gejl, K.D.; Thams, L.B.; Hansen, M.; Rokkedal-Lausch, T.; Plomgaard, P.; Nybo, L.; Larsen, F.J.; Cardinale, D.A.; Jensen, K.; Holmberg, H.C.; et al. No superior adaptations to carbohydrate periodization in elite endurance athletes. Med. Sci. Sports Exerc. 2017, 49, 2486–2497. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Hawley, J.A.; Morton, J.P. Carbohydrate availability and exercise training adaptation: Too much of a good thing? Eur. J. Sport Sci. 2015, 15, 3–12. [Google Scholar] [CrossRef]
- Impey, S.G.; Hammond, K.M.; Shepherd, S.O.; Sharples, A.P.; Stewart, C.; Limb, M.; Smith, K.; Philp, A.; Jeromson, S.; Hamilton, D.L.; et al. Fuel for the work required: A practical approach to amalgamating train-low paradigms for endurance athletes. Physiol. Rep. 2016, 4, 12803. [Google Scholar] [CrossRef]
- Yeo, W.K.; Lessard, S.J.; Chen, Z.P.; Garnham, A.P.; Burke, L.M.; Rivas, D.A.; Kemp, B.E.; Hawley, J.A. Fat adaptation followed by carbohydrate restoration increases AMPK activity in skeletal muscle from trained humans. J. Appl. Physiol. 2008, 105, 1519–1526. [Google Scholar] [CrossRef]
- Yeo, W.K.; McGee, S.L.; Carey, A.L.; Paton, C.D.; Garnham, A.P.; Hargreaves, M.; Hawley, J.A. Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen. Exp. Physiol. 2010, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Areta, J.L.; Iraki, J.; Owens, D.J.; Joanisse, S.; Philp, A.; Morton, J.P.; Hallén, J. Achieving energy balance with a high-fat meal does not enhance skeletal muscle adaptation and impairs glycaemic response in a sleep-low training model. Exp. Physiol. 2020, 105, 1778–1791. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Cheuvront, S.N.; Carter, R. 3rd. Human water needs. Nutr. Rev. 2005, 63 Pt 2, 30–39. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate: Glycolytic End Product and Oxidative Substrate During Sustained Exercise in Mammals—The “Lactate Shuttle”. In Circulation, Respiration and Metabolism; Springer: Berlin/Heidelberg, Germany, 1985; pp. 208–218. [Google Scholar]
- Brooks, G.A. The lactate shuttle during exercise and recovery. Med. Sci. Sports Exerc. 1986, 18, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, N.; van Loon, L.J.; Baar, K. The role of amino acids in skeletal muscle adaptation to exercise. Nestle. Nutr. Inst. Workshop. Ser. 2013, 76, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Camera, D.M.; Areta, J.L.; Hawley, J.A. Beyond muscle hypertrophy: Why dietary protein is important for endurance athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Kido, T.; Yoshizawa, F.; Ito, Y.; Nishizawa, N. Rapid suppression of protein degradation in skeletal muscle after oral feeding of leucine in rats. J. Nutr. Biochem. 2002, 13, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Ito, Y.; Nishizawa, N.; Nagasawa, T. Supplementation with dietary leucine to a protein deficient diet suppresses myofibrillar protein degradation in rats. J. Nutr. Sci. Vitaminol. 2007, 53, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Ito, Y.; Nishizawa, N.; Nagasawa, T. Regulation of muscle protein degradation, not synthesis, by dietary leucine in rats fed a protein-deficient diet. Amino Acids. 2009, 37, 609–616. [Google Scholar] [CrossRef]
- Higashida, K.; Terada, S.; Li, X.; Inoue, S.; Iida, N.; Kitai, S.; Nakai, N. Low-carbohydrate high-protein diet diminishes the insulin response to glucose load via suppression of SGLT-1 in mice. Biosci. Biotechnol. Biochem. 2019, 83, 365–371. [Google Scholar] [CrossRef]


| Control Group | Nighttime Carbohydrate Restriction Group | |||
|---|---|---|---|---|
| (n = 11) | (n = 11) | |||
| Pre | Post | Pre | Post | |
| Calorie intake (kcal) | 2349.5 ± 549.7 | 2437.8 ± 669.8 | 2238.5 ± 418.6 | 2234.5 ± 431.0 |
| Carbohydrate intake (g) | 290.2 ± 64.2 | 287.3 ± 59.6 | 270.4 ± 69.9 | 267.3 ± 68.3 |
| Control Group | Nighttime Carbohydrate Restriction Group | |||
|---|---|---|---|---|
| (n = 11) | (n = 11) | |||
| Pre | Post | Pre | Post | |
| Anger—Hostility | 39.9 ± 2.6 | 38.3 ± 0.5 | 40.3 ± 2.6 | 42.7 ± 5.4 |
| Confusion—Bewilderment | 42.9 ± 6.2 | 43.1 ± 6.9 | 47.7 ± 9.4 | 49.4 ± 10.2 |
| Depression—Dejection | 42.4 ± 4.3 | 42.4 ± 2.4 | 45.9 ± 4.8 | 45.4 ± 6.4 |
| Fatigue—Inertia | 49.6 ± 8.1 | 47.8 ± 7.5 | 53.9 ± 9.5 | 51.1 ± 9.3 |
| Tension—Anxiety | 45.0 ± 8.4 | 41.0 ± 5.6 | 46.1 ± 8.9 | 46.1 ± 7.9 |
| Vigor—Activity | 55.3 ± 11.2 | 53.6 ± 8.7 | 57.7 ± 11.3 | 55.1 ± 12.9 |
| Friendliness | 56.5 ± 11.4 | 55.5 ± 9.0 | 56.4 ± 10.5 | 54 ± 11.7 |
| Total Mood Disturbance Score | 42.3 ± 6.3 | 41.1 ± 4.5 | 44.6 ± 7.7 | 45.3 ± 8.2 |
| Control Group | Nighttime Carbohydrate Restriction Group | |||
|---|---|---|---|---|
| (n = 11) | (n = 11) | |||
| Pre | Post | Pre | Post | |
| Body weight (kg) | 61.7 ± 11.0 | 61.5 ± 10.9 | 62.9 ± 7.7 | 61.5 ± 7.4 * |
| Body fat percentage (%) | 19.3 ± 4.8 | 19.0 ± 4.5 | 19.0 ± 5.2 | 18.7 ± 6.2 |
| Lean body mass (kg) | 50.0 ± 10.3 | 50.0 ± 10.2 | 50.9 ± 6.9 | 50.0 ± 6.6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, T.; Ueda, S.-y.; Nakahara, H. Effects of Short-Term Nighttime Carbohydrate Restriction Method on Exercise Performance and Fat Metabolism. Nutrients 2024, 16, 2138. https://doi.org/10.3390/nu16132138
Sakamoto T, Ueda S-y, Nakahara H. Effects of Short-Term Nighttime Carbohydrate Restriction Method on Exercise Performance and Fat Metabolism. Nutrients. 2024; 16(13):2138. https://doi.org/10.3390/nu16132138
Chicago/Turabian StyleSakamoto, Takumi, Shin-ya Ueda, and Hidehiro Nakahara. 2024. "Effects of Short-Term Nighttime Carbohydrate Restriction Method on Exercise Performance and Fat Metabolism" Nutrients 16, no. 13: 2138. https://doi.org/10.3390/nu16132138






