Silkworm (Bombyx mori L.) Has Beneficial Effects on Menopausal Symptoms by Enhancing Estrogen Receptor Signaling in Ovariectomized Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Silkworm Powder
2.2. Evaluation of ABTS Radical Scavenging Activity
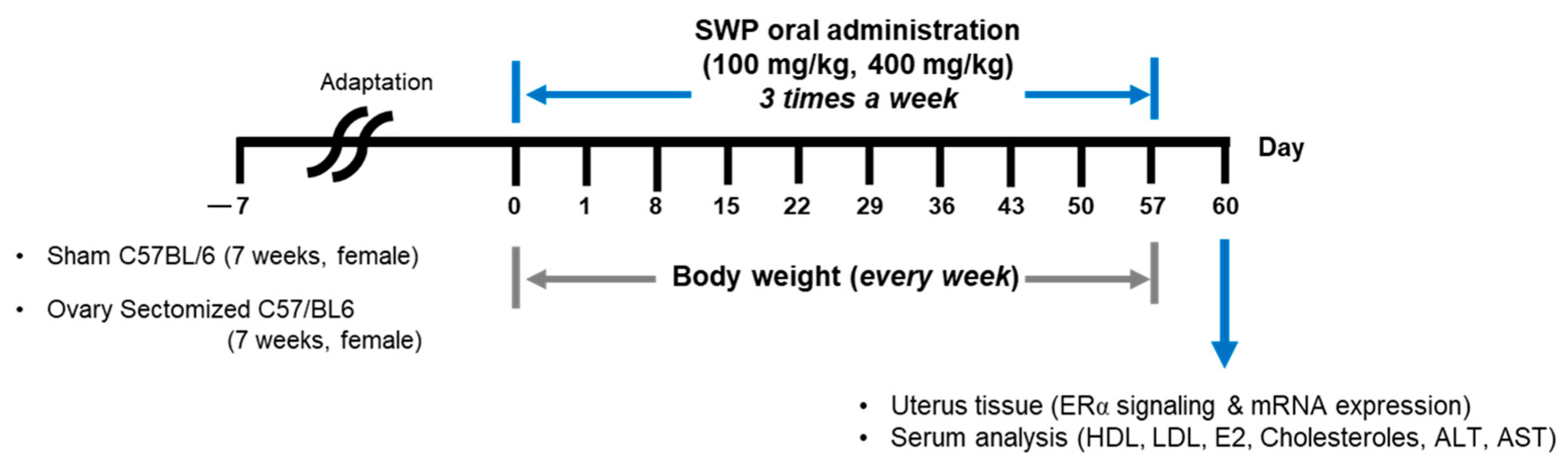
2.3. Experimental Design
2.4. Uterine Tissue and Blood Collection
2.5. Assay of Serum 17β-Estradiol
2.6. Preparation of Tissue Lysate for Immunoblotting
2.7. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.8. Blood Biochemical Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Freeze-Dried SWP
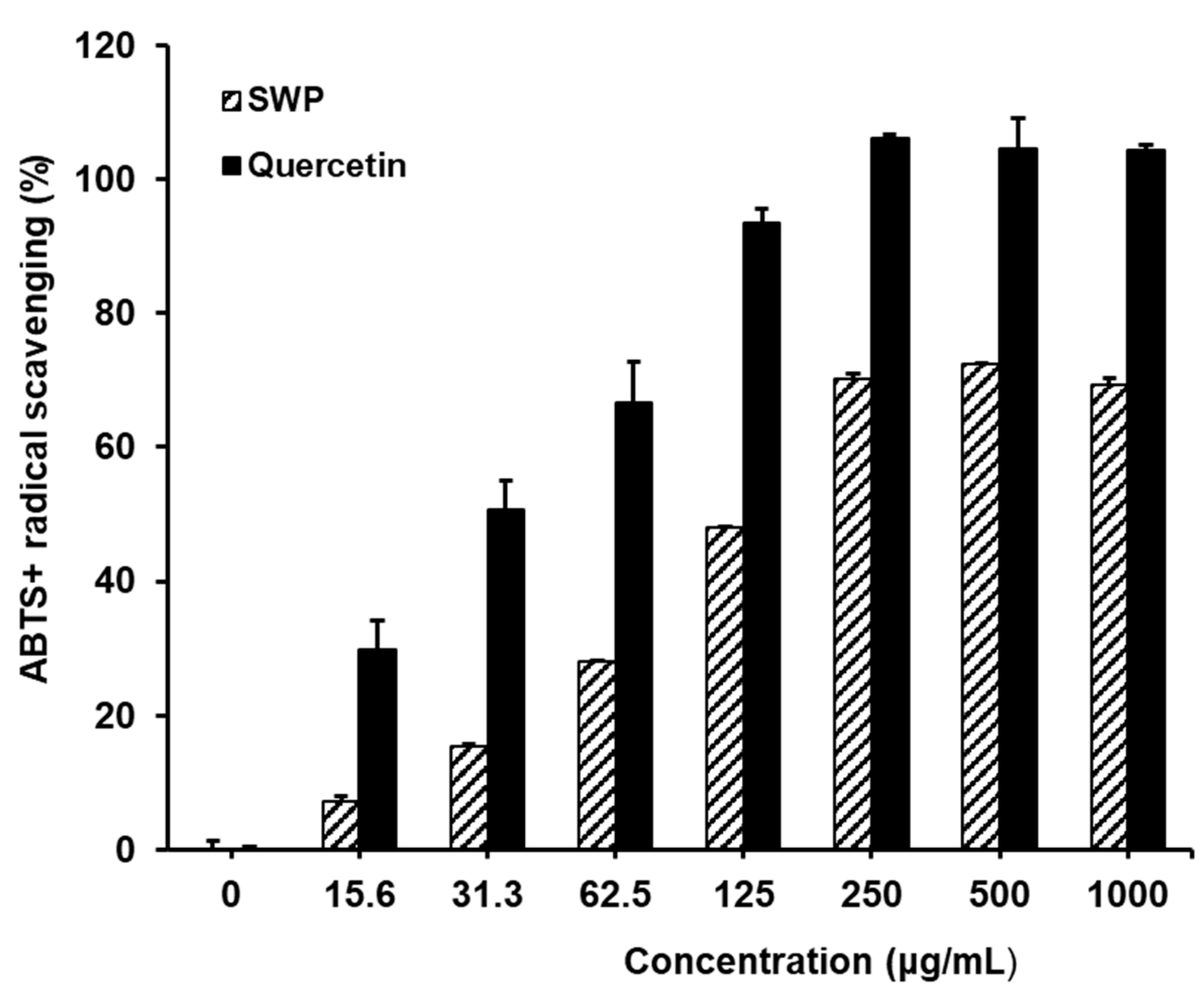
3.2. The Effect of SWP on ABTS+ Free Radical Scavenging Activity
3.3. Change in the Body Weight of OVX Mice Following the Administration of Freeze-Dried Silkworm Powder
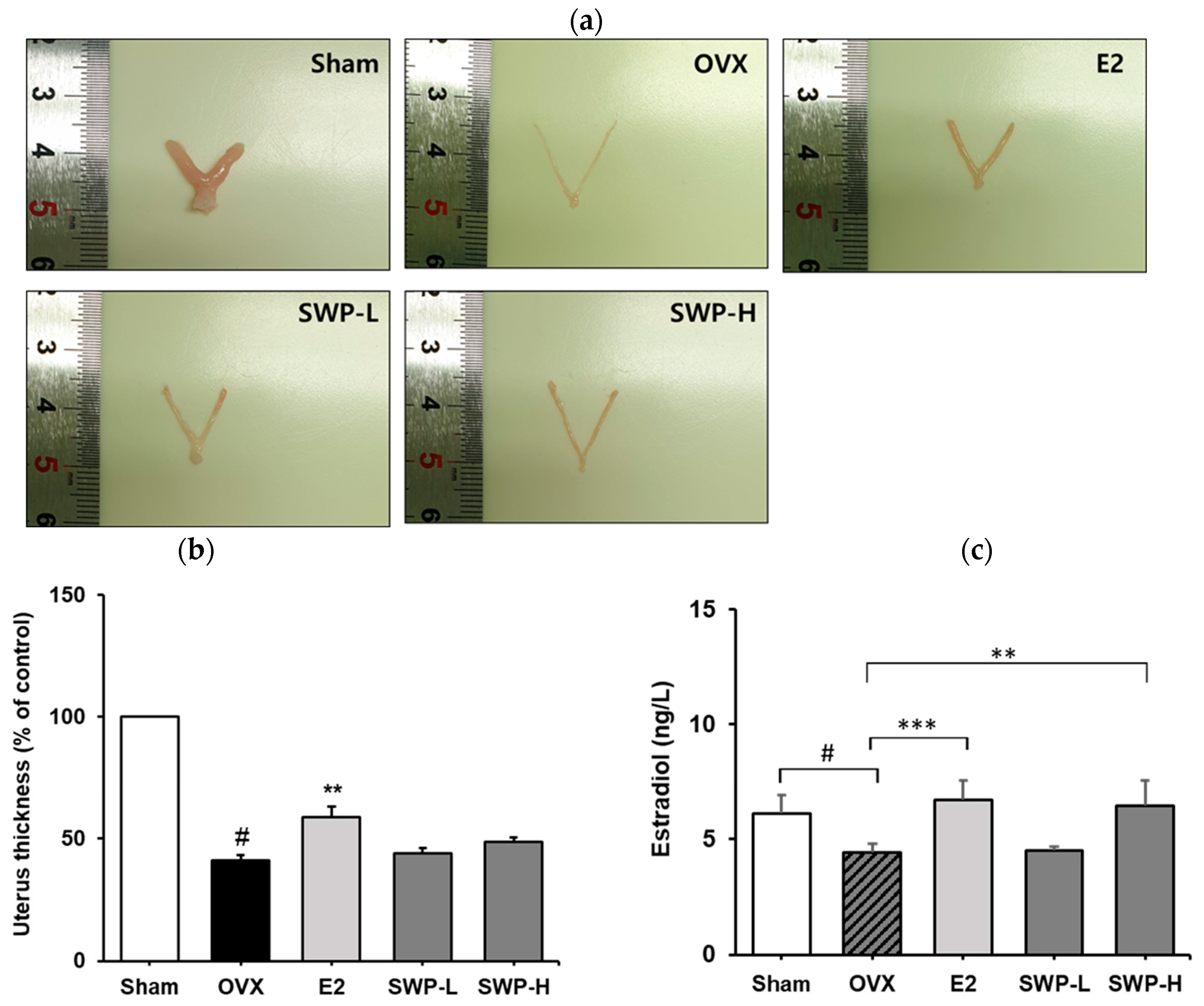
3.4. Effect of Freeze-Dried Silkworm Powder on Uterus Weight in the OVX Mouse Model
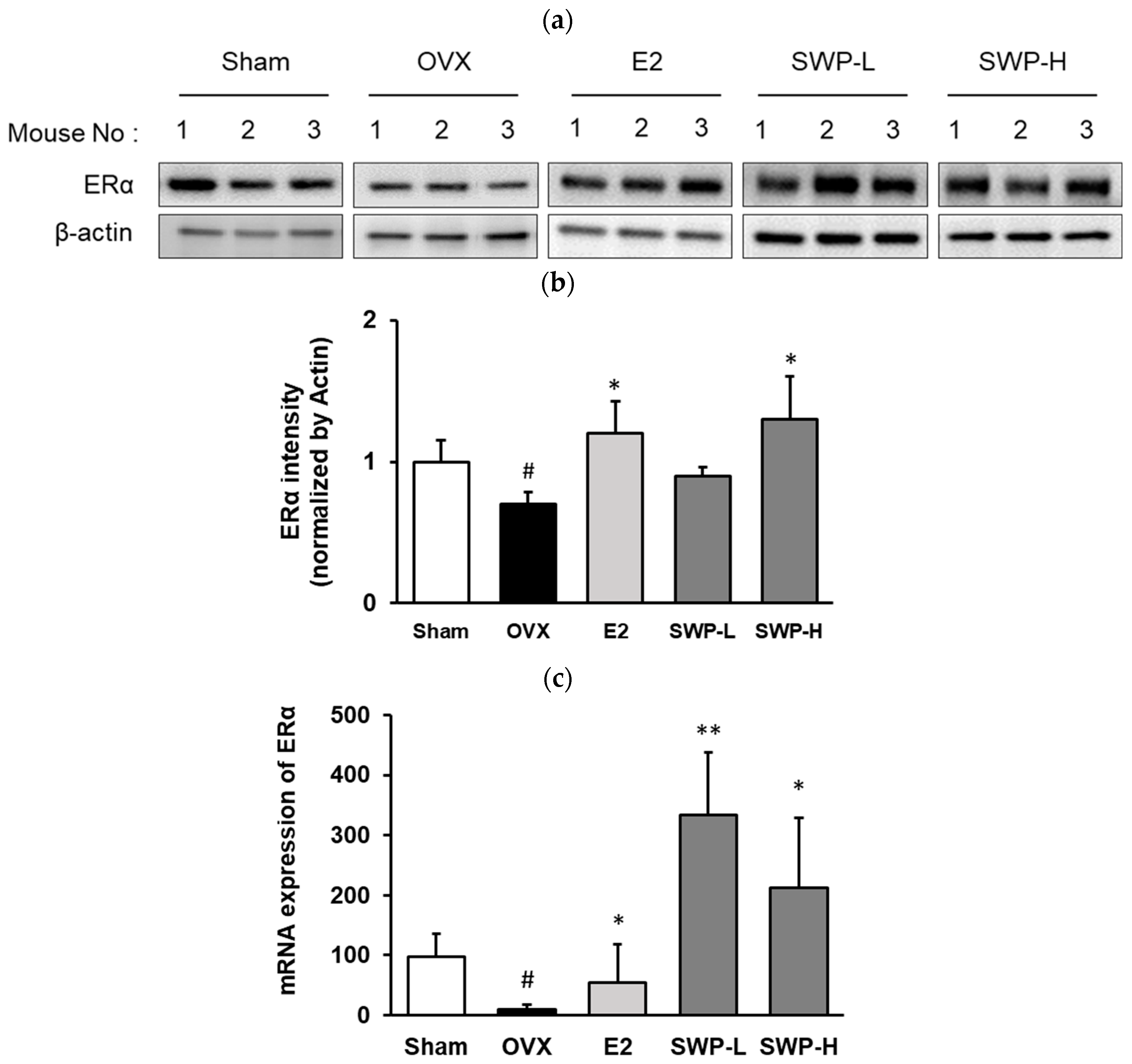
3.5. Effect of Freeze-Dried SWP on the Expression of Estrogen Receptor Alpha (ERα) and mRNA in Uterine Tissues of OVX Mice
3.6. Effect of Freeze-Dried SWP on the Phosphorylation of AKT and ERK in the Uterine Tissues of OVX Mice
3.7. Effect of Freeze-Dried SWP on Serum Levels in OVX Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, T.A.; Johnson, K.M. Menopause. Med. Clin. N. Am. 2015, 99, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Delamater, L.; Santoro, N. Management of the perimenopause. Clin. Obstet. Gynecol. 2018, 61, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Rymer, J.; Morris, E.P. Extracts from “clinical evidence”: Menopausal symptoms. BMJ 2000, 321, 1516–1519. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.J.; Tchernof, A.; Sites, C.K.; Poehlman, E.T. Menopause-related changes in body fat distribution. Ann. N. Y. Acad. Sci. 2000, 904, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Armellini, F.; Milani, M.P.; De Marchi, M.; Todesco, T.; Robbi, R. Body fat distribution in pre- and post-menopausal women: Metabolic and anthropometric variables and their inter-relationships. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 495–504. [Google Scholar] [PubMed]
- Ryu, K.S.; Lee, H.S.; Kim, S.Y. Pharmacodynamic study of silkworm powder in mice administered to maltose, sucrose and lactose. Korean J. Seric. Sci. 1999, 41, 9–13. [Google Scholar]
- Cha, J.Y.; Kim, Y.S.; Kang, P.D.; Ahn, H.Y.; Eom, K.E.; Cho, Y.S. Biological activity and chemical characteristics of fermented silkworm powder by mold. J. Life Sci. 2010, 20, 237–244. [Google Scholar] [CrossRef]
- Chon, J.W.; Kweon, H.Y.; Jo, Y.Y.; Park, M.K.; Son, Y.H.; Lee, H.S. A study on the development of functional cosmetics using silk-gland powder of silkworm. J. Soc. Cosmet. Sci. Korea 2012, 38, 163–169. [Google Scholar] [CrossRef][Green Version]
- Hyun, J.W.; Lee, K.G.; Yeo, J.H.; Choe, T.B. Hair care effects of hair cosmetics including low molecular weight silk peptide component and micro structure analysis. Korean J. Biotechnol. Bioeng. 2008, 23, 439–444. [Google Scholar]
- Kim, S.H.; Je, Y.H.; Yun, E.Y.; Kang, S.W.; Kim, K.Y.; Kang, S.K. Isolation and characterization of inducible genes from Bombyx mori injected with E. coli by differential screening. Korean J. Seric. Sci. 1996, 38, 19–24. [Google Scholar]
- Kwon, H.J.; Lee, K.H.; Kim, J.H.; Chun, S.S.; Cho, Y.J.; Cha, W.S. Effect of protease on the extraction and properties of the protein from silkworm pupa. J. Korean Soc. Appl. Biol. Chem. 2006, 49, 304–308. [Google Scholar]
- Lee, A.Y.; Lee, J.; Kim, H.S.; Choi, G. Inorganic components of insect origin medicines. Korean Herb. Med. Inform. 2019, 7, 189–194. [Google Scholar]
- Choi, H.S.; Kim, S.A.; Shin, H.J. Present and perspective on insect biotechnology. Korean Soc. Biotechnol. Bioeng. J. 2015, 30, 257–267. [Google Scholar]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Li, W.; Mu, L.; Zou, Y.; Wang, W.; Zhao, H.; Wu, X.; Liao, S. Effect of silkworm pupa protein hydrolysates on proliferation of gastric cancer cells in vitro. Foods 2022, 11, 2367. [Google Scholar] [CrossRef]
- Wang, W.; Shen, S.; Chen, Q.; Tanga, B.; He, G.; Ruan, H.; Das, U.N. Hydrolyzates of silkworm pupae (Bombyx mori) protein is a new source of angiotensin I-converting enzyme inhibitory peptides (ACEIP). Curr. Pharm. Biotechnol. 2008, 9, 307–314. [Google Scholar] [CrossRef]
- Yu, J.S.; Woo, K.S.; Hwang, I.G.; Lee, Y.R.; Kang, T.S.; Jeong, H.S. ACE inhibitory and antioxidative activities of silkworm larvae (Bombyx mori) hydrolysate. J. Korean Soc. Food Sci. Nutr. 2008, 37, 136–140. [Google Scholar] [CrossRef]
- Joo-Wha, Y. Effects of silkworm extract powder on plasma lipids and glucose in rats. Korean J. Food Nutr. 2005, 18, 140–145. [Google Scholar]
- Biganeh, H.; Kabiri, M.; Zeynalpourfattahi, Y.; Brancalhão RM, C.; Karimi, M.; Ardekani MR, S.; Rahimi, R. Bombyx mori cocoon as a promising pharmacological agent: A review of ethnopharmacology, chemistry, and biological activities. Heliyon 2022, 8, e10496. [Google Scholar] [CrossRef]
- Vegunta, S.; Kling, J.M.; Kapoor, E. Androgen therapy in women. J. Women Health 2020, 29, 57–64. [Google Scholar] [CrossRef]
- Cho, S.H.; Kang, S.E.; Cho, J.Y.; Kim, A.R.; Park, S.M.; Hong, Y.K.; Ahn, D.H. The antioxidant properties of brown seaweed (Sargassum siliquastrum) extracts. J. Med. Food 2007, 10, 479–485. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Biondi, D.M.; Amico, V. Antioxidant activity of extracts of the marine algae genus Cystoseira in a micellar model system. J. Appl. Phycol. 2001, 13, 403–407. [Google Scholar] [CrossRef]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef]
- Park, M.R.; Kim, M.H. Effects of Punica granatum L. extracts on serum lipids in ovariectomized rats. J. Life Sci. 2007, 18, 46–51. [Google Scholar] [CrossRef]
- Lee, S.; Nguyen, Q.N.; Kim, S.J.; Lee, J.; Shin, M.S. Estrogenic activity of freeze-dried silkworm extracts through the activation of estrogen receptors in MCF-7 cells. Appl. Biol. Chem. 2022, 65, 43. [Google Scholar] [CrossRef]
- Yoo, J.M.; Hwang, J.S.; Goo, T.W.; Yun, E.Y. Comparative analysis of nutritional and harmful components in Korean and Chinese mealworms (Tenebrio molitor). J. Korean Soc. Food Sci. Nutr. 2013, 42, 249–254. [Google Scholar] [CrossRef]
- Eom, K.E. Improvement Effect of Fermented Silkworm (Bombyx mori L.) Powder on the Alcoholic Hepatotoxicity. Master’s Thesis, Dong-A. University of Medical Bioscience, Busan, Republic of Korea, 2011; pp. 2–3. [Google Scholar]
- Castelli, W.P.; Abbott, R.D.; McNamara, P.M. Summary estimates of cholesterol used to predict coronary heart disease. Circulation 1983, 67, 730–734. [Google Scholar] [CrossRef]
- Noh, Y.H.; Lee, J.W.; Park, J.A.; Lee, S.H.; Lee, J.Y.; Kim, S.S.; Jeong, Y.H. Natural substance MS-10 improves women’s health via regulation of estrogen receptor. J. Korean Soc. Food Sci. Nutr. 2016, 45, 903–910. [Google Scholar] [CrossRef]
- Meli, R.; Pacilio, M.; Raso, G.M.; Esposito, E.; Coppola, A.; Nasti, A.; Di Carlo, R. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology 2004, 145, 3115–3121. [Google Scholar] [CrossRef]
- Kim, C.S.; Ha, H.K.; Lee, J.H.; Kim, J.S.; Song, K.Y.; Park, S.W. Herbal extract prevents bone loss in ovariectomized rats. Arch. Pharm. Res. 2003, 26, 917–924. [Google Scholar] [CrossRef]
- Maeng, Y.S.; Choi, M.S.; Ahn, I.S.; Kim, D.I. Effects of Sagunjatang-Gami on uterine and ovarian function in the ovariectomized rat postmenopause model. J. Orient. Obstet. Gynecol. 2012, 25, 12–26. [Google Scholar]
- Dizavandi, F.; Ghazanfarpour, M.; Roozbeh, N.; Kargarfard, L.; Khadivzadeh, T.; Dashti, S. An overview of the phytoestrogen effect on vaginal health and dyspareunia in peri-and post-menopausal women. Post Reprod. Health 2019, 25, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]

| Antibodies | Source | Catalog Number | Dilution |
|---|---|---|---|
| Estrogen Receptor (ERα) | Cell Signaling Technology | #8644 | 1/3000 |
| ERK | Cell Signaling Technology | #9102 | 1/5000 |
| phospho-ERK | Cell Signaling Technology | #9101 | 1/3000 |
| AKT | Cell Signaling Technology | #9272 | 1/5000 |
| Phospho-AKT | Cell Signaling Technology | #4058 | 1/3000 |
| β-Actin | Cell Signaling Technology | #4967 | 1/5000 |
| Initial Weight (g) | Final Weight (g) | Body Weight Gain (g) | |
|---|---|---|---|
| Sham | 17.3 ± 0.56 | 23.1 ± 1.46 | 5.86 |
| OVX | 20.8 ± 0.46 | 28.4 ± 1.14 | 7.66 # |
| Estradiol | 20.6 ± 0.5 | 26.9 ± 2.3 | 6.3 |
| SWP-Low | 21.4 ± 0.45 | 28.9 ± 1.84 | 7.52 |
| SWP-High | 22.4 ± 0.61 | 29.86 ± 1.04 | 7.48 |
| Sham | OVX | E2 | SWP-L | SWP-H | |
|---|---|---|---|---|---|
| TG (mg/dL) | 20.0 ± 2.6 | 34.3 ± 0.6 # | 15.3 ± 0.6 *** | 32.0 ± 1.0 | 29.0 ± 3.0 |
| TC (mg/dL) | 65.7 ± 0.6 | 94.7 ± 10.0 # | 71.0 ± 1.7 ** | 88.7 ± 2.3 | 85.7 ± 1.2 * |
| HDL (mg/dL) | 38.5 ± 0.5 | 45.0 ± 2.9 # | 43.4 ± 2.6 | 50.6 ± 1.5 * | 44.4 ± 0.6 |
| LDL (mg/dL) | 5.8 ± 0.7 | 7.5 ± 0.8 # | 4.4 ± 0.4 ** | 6.5 ± 0.5 | 6.6 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Lee, M.-G.; Lee, J.; Shin, M.-S. Silkworm (Bombyx mori L.) Has Beneficial Effects on Menopausal Symptoms by Enhancing Estrogen Receptor Signaling in Ovariectomized Mice. Nutrients 2024, 16, 2164. https://doi.org/10.3390/nu16132164
Kim SJ, Lee M-G, Lee J, Shin M-S. Silkworm (Bombyx mori L.) Has Beneficial Effects on Menopausal Symptoms by Enhancing Estrogen Receptor Signaling in Ovariectomized Mice. Nutrients. 2024; 16(13):2164. https://doi.org/10.3390/nu16132164
Chicago/Turabian StyleKim, Sung Jin, Mi-Gi Lee, Joohwan Lee, and Myoung-Sook Shin. 2024. "Silkworm (Bombyx mori L.) Has Beneficial Effects on Menopausal Symptoms by Enhancing Estrogen Receptor Signaling in Ovariectomized Mice" Nutrients 16, no. 13: 2164. https://doi.org/10.3390/nu16132164
APA StyleKim, S. J., Lee, M.-G., Lee, J., & Shin, M.-S. (2024). Silkworm (Bombyx mori L.) Has Beneficial Effects on Menopausal Symptoms by Enhancing Estrogen Receptor Signaling in Ovariectomized Mice. Nutrients, 16(13), 2164. https://doi.org/10.3390/nu16132164








