Citrullus mucosospermus Extract Reduces Weight Gain in Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Extraction of Citrullus mucosospermus
2.3. HPLC Analysis
2.4. Adipocyte Culture and Differentiation
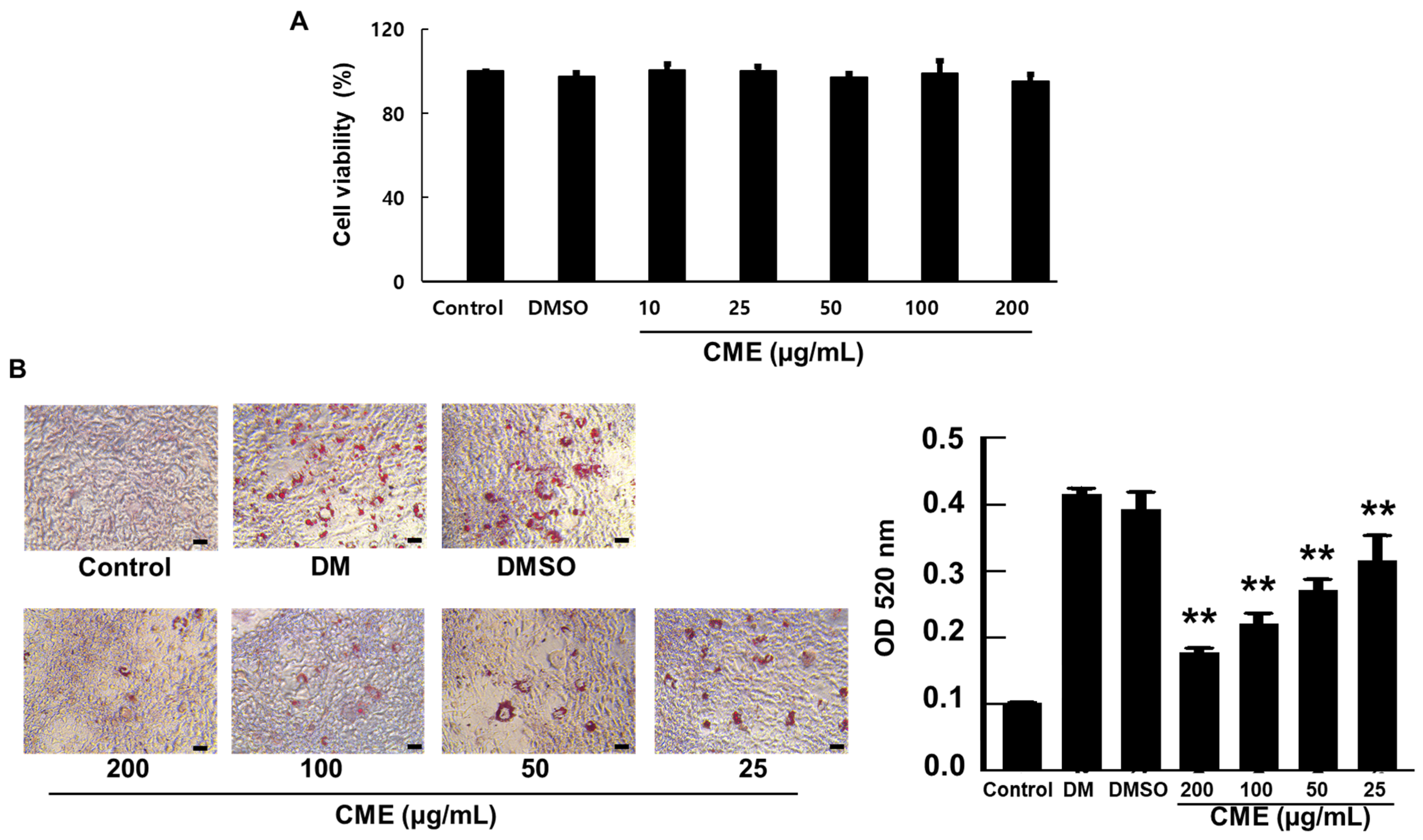
2.5. Cell Viability
2.6. Oil Red O Staining
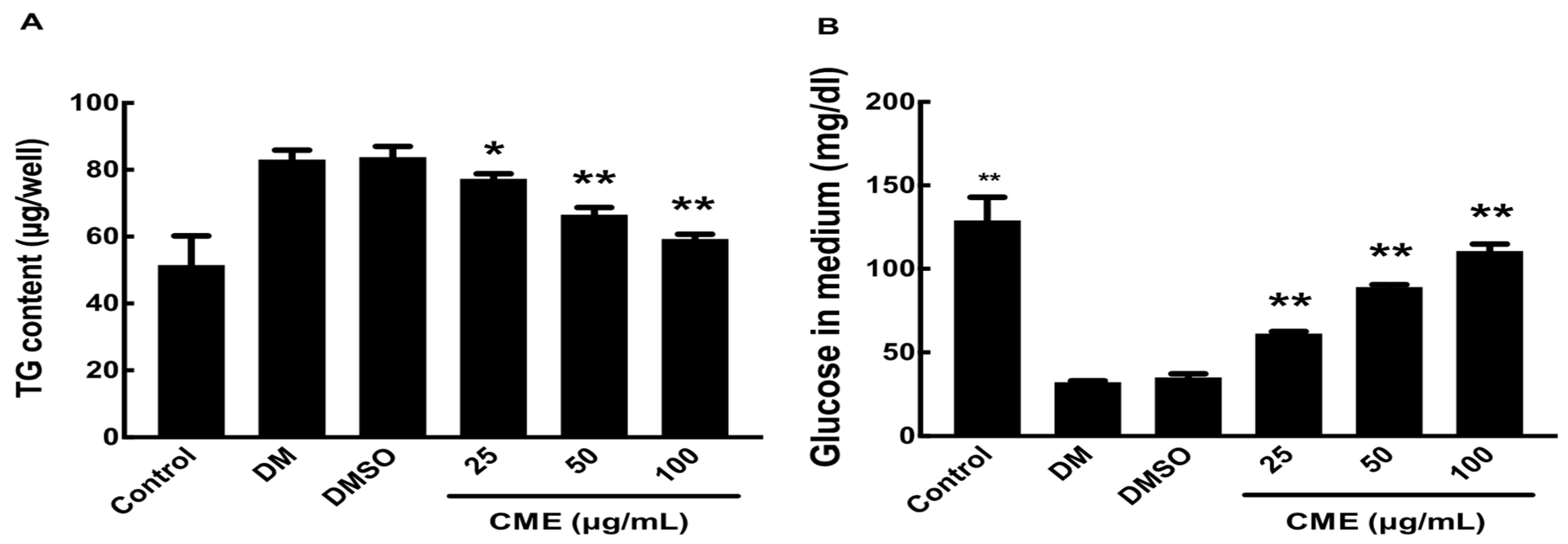
2.7. Glucose Uptake Measurement
2.8. Triglyceride (TG) Measurement
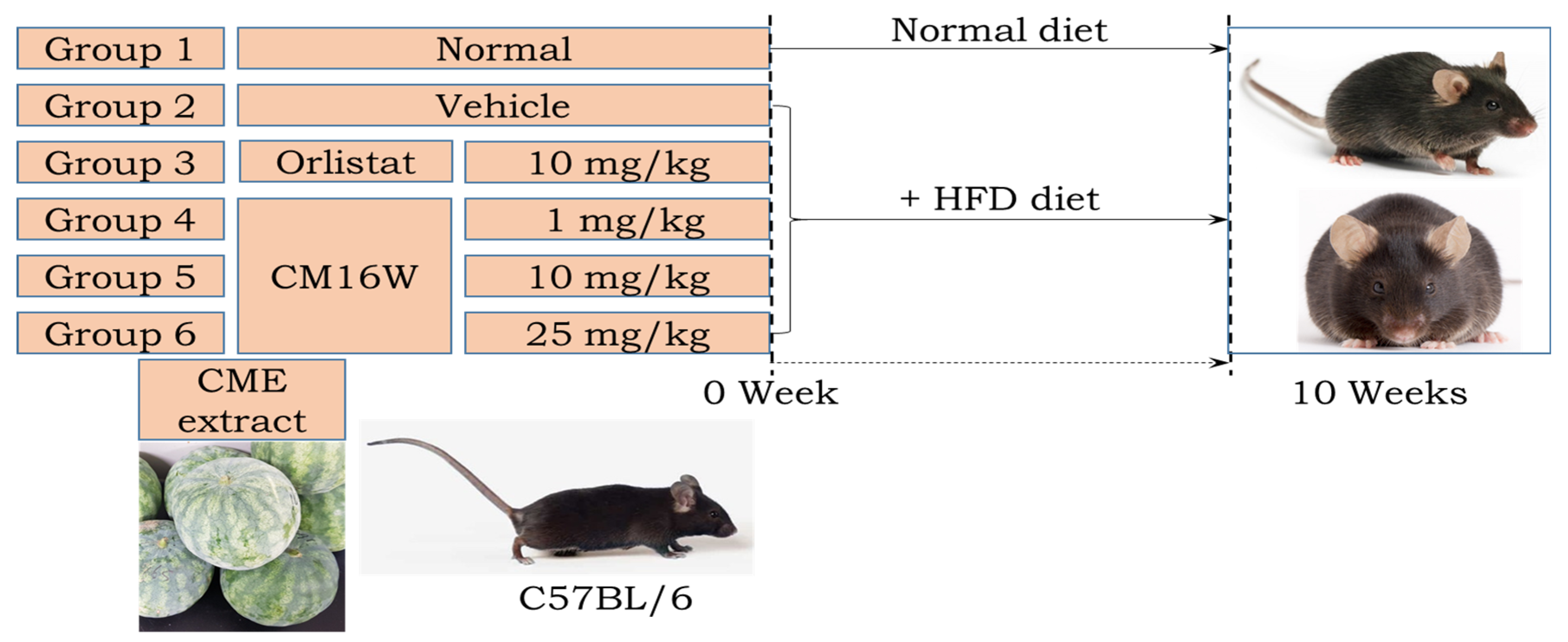
2.9. Animal Anti-Obesity Study
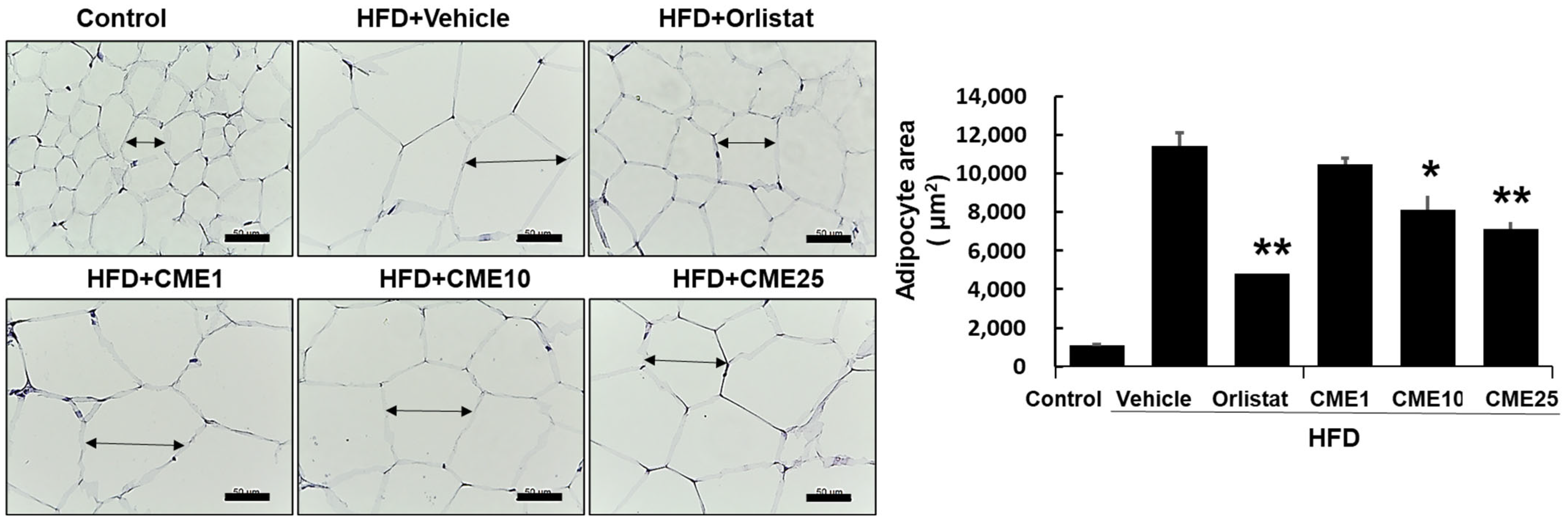
2.10. Histopathological Analysis
2.11. Real-Time Quantitative PCR Analysis
2.12. Statistical Analysis
3. Results
3.1. Inhibition of Adipogenesis by CME in 3T3-L1 Preadipocytes
3.2. CME Mitigates DMI-Induced Metabolic Alterations in 3T3-L1 Adipocytes
3.3. Effect of CME on Body Weight Regulation in HFD-Fed C57BL/6N Mice
3.4. Effect of CME on Hepatic Fat Accumulation in C57BL/6N Mice
3.5. Evaluation of CME’s Impact on Abdominal White Adipose Tissue
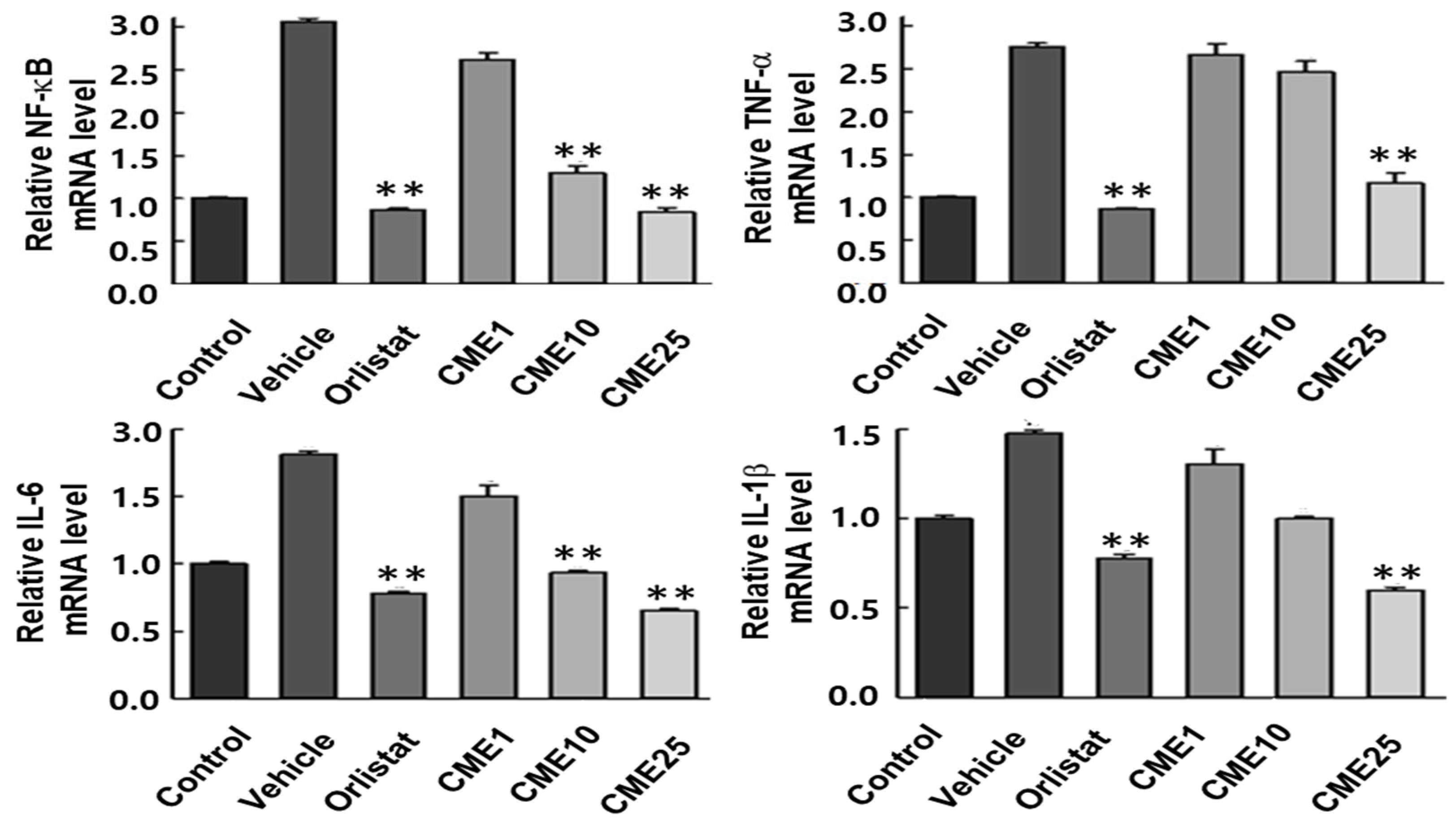
3.6. Impact of CME on HFD-Induced Hepatic Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gortan Cappellari, G.; Brasacchio, C.; Laudisio, D.; Lubrano, C.; Pivari, F.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A.; Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group. Sarcopenic obesity: What about in the cancer setting? Nutrition 2022, 98, 111624. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, Q.; Li, J.; Zhou, Y.; Chu, T. Effects of non-pharmacological interventions on patients with sarcopenic obesity: A meta-analysis. PLoS ONE 2023, 18, e0290085. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Cinkajzlová, A.; Mráz, M.; Haluzík, M. Adipose tissue immune cells in obesity, type 2 diabetes mellitus and cardiovascular diseases. J. Endocrinol. 2021, 252, R1–R22. [Google Scholar] [CrossRef] [PubMed]
- AlZaim, I.; Al-Saidi, A.; Hammoud, S.H.; Darwiche, N.; Al-Dhaheri, Y.; Eid, A.H.; El-Yazbi, A.F. Thromboinflammatory Processes at the Nexus of Metabolic Dysfunction and Prostate Cancer: The Emerging Role of Periprostatic Adipose Tissue. Cancers 2022, 14, 1679. [Google Scholar] [CrossRef] [PubMed]
- AlZaim, I.; El-Nikhely, N.; Al-Saidi, A.; Mougharbil, N.; Darwiche, N.; Abou-Kheir, W.; El-Yazbi, A.F. Periprostatic adipose tissue thromboinflammation triggers prostatic neoplasia in early metabolic impairment: Interruption by rivaroxaban. Life Sci. 2023, 334, 122225. [Google Scholar] [CrossRef] [PubMed]
- Miladinovic, D.; Cusick, T.; Mahon, K.L.; Haynes, A.; Cortie, C.H.; Meyer, B.J.; Stricker, P.D.; Wittert, G.A.; Butler, L.M.; Horvath, L.G.; et al. Assessment of Periprostatic and Subcutaneous Adipose Tissue Lipolysis and Adipocyte Size from Men with Localized Prostate Cancer. Cancers 2020, 12, 1385. [Google Scholar] [CrossRef]
- Altuna-Coy, A.; Ruiz-Plazas, X.; Sánchez-Martin, S.; Ascaso-Til, H.; Prados-Saavedra, M.; Alves-Santiago, M.; Bernal-Escoté, X.; Segarra-Tomás, J.; RChacón, M. The lipidomic profile of the tumoral periprostatic adipose tissue reveals alterations in tumor cell’s metabolic crosstalk. BMC Med. 2022, 20, 255. [Google Scholar] [CrossRef]
- Chen, H.; Yan, X.; Sun, A.; Zhang, L.; Zhang, J.; Yan, Y. High-Fat-Diet-Induced Extracellular Matrix Deposition Regulates Integrin-FAK Signals in Adipose Tissue to Promote Obesity. Mol. Nutr. Food Res. 2022, 66, e2101088. [Google Scholar] [CrossRef]
- Bugler-Lamb, A.R.; Hasib, A.; Weng, X.; Hennayake, C.K.; Lin, C.; McCrimmon, R.J.; Stimson, R.H.; Ashford, M.L.J.; Wasserman, D.H.; Kang, L. Adipocyte integrin-linked kinase plays a key role in the development of diet-induced adipose insulin resistance in male mice. Mol. Metab. 2021, 49, 101197. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Henriques, F.; Bedard, A.H.; Czech, M.P. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Guerra-Cantera, S.; Martín-Rivada, Á.; Canelles, S.; Campillo-Calatayud, A.; Arilla-Ferreiro, E.; Frago, L.M.; Chowen, J.A.; Argente, J. Chronic Central Leptin Infusion Promotes an Anti-Inflammatory Cytokine Profile Related to the Activation of Insulin Signaling in the Gastrocnemius of Male Rats. Biomedicines 2022, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Munkong, N.; Lonan, P.; Mueangchang, W.; Yadyookai, N.; Kanjoo, V.; Yoysungnoen, B. Red Rice Bran Extract Attenuates Adipogenesis and Inflammation on White Adipose Tissues in High-Fat Diet-Induced Obese Mice. Foods 2022, 11, 1865. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kayastha, A.; Singh, V.K.; Kayastha, A.M. In Silico Structural and Functional Insight into the Binding Interactions of the Modeled Structure of Watermelon Urease with Urea. ACS Omega 2023, 9, 2272–2285. [Google Scholar] [CrossRef] [PubMed]
- Mighan, N.M.; Ariaii, P.; Soltani, M.S.; Jafarian, S. Investigating the possibility of increasing the microbial and oxidative stability of silver carp burgers using hydrolyzed protein of watermelon seeds. Food Sci. Biotechnol. 2023, 33, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Feng, M.; Li, X.; Hou, Y.; Wei, C.; Zhang, X. Genome-Wide Identification and Expression Analysis of Chitinase Genes in Watermelon under Abiotic Stimuli and Fusarium oxysporum Infection. Int. J. Mol. Sci. 2024, 25, 638. [Google Scholar] [CrossRef] [PubMed]
- Runde, M.; Ogoko, E.; Aletan, U.; Mohammed Suleiman, H.; Imojara, A.; Hitler, L. Gas chromatography/mass spectrometry analysis and uv absorptivity of bio-oils extracted from some locally grown plant seeds in nothern Nigeria. Heliyon 2023, 9, e20872. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Nimmakayala, P.; Song, Q.; Abburi, V.; Natarajan, P.; Levi, A.; Crosby, K.; Reddy, U.K. Genome-wide association study and population structure analysis of seed-bound amino acids and total protein in watermelon. PeerJ 2021, 9, e12343. [Google Scholar] [CrossRef] [PubMed]
- Olubi, O.; Felix-Minnaar, J.V.; Jideani, V.A. Physicochemical, Mineral and Sensory Characteristics of Instant Citrullus lanatus mucosospermus (Egusi) Soup. Foods 2021, 10, 1817. [Google Scholar] [CrossRef]
- Mandour, Y.M.; Refaat, E.; Hassanein, H.D. Anticancer activity, phytochemical investigation and molecular docking insights of Citrullus colocynthis (L.) fruits. Sci. Rep. 2023, 13, 20038. [Google Scholar] [CrossRef]
- Reddy, C.S.; Natarajan, P.; Nimmakayala, P.; Hankins, G.R.; Reddy, U.K. From Fruit Waste to Medical Insight: The Comprehensive Role of Watermelon Rind Extract on Renal Adenocarcinoma Cellular and Transcriptomic Dynamics. Int. J. Mol. Sci. 2023, 24, 15615. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, N.; Hussain, A.I.; Fatima, T.; Alsuwayt, B.; Althaiban, A.K. Bioactivity-Guided Isolation and Antihypertensive Activity of Citrullus colocynthis Polyphenols in Rats with Genetic Model of Hypertension. Medicina 2023, 59, 1880. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, M.O.; Umer, M.J.; Lu, X.; He, N.; Anees, M.; El-Remaly, E.; Yousef, A.F.; Salama, E.A.A.; Kalaji, H.M.; Liu, W. Comparative physiological and biochemical mechanisms in diploid, triploid, and tetraploid watermelon (Citrullus lanatus L.) grafted by branches. Sci. Rep. 2023, 13, 4993. [Google Scholar] [CrossRef]
- Yan, J.; Xu, P.; Zhang, F.; Huang, X.; Cao, Y.; Zhang, S. The effects of aqueous extract from watermelon (Citrullus lanatus) peel on the growth and physiological characteristics of Dolichospermum flos-aquae. Sci. Rep. 2022, 12, 8086. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, B.O.; Shonibare, M.T.; Oyinloye, B.E. Antidiabetic activity of watermelon (Citrullus lanatus) juice in alloxan-induced diabetic rats. J. Diabetes Metab. Disord. 2020, 19, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.C.; Dudenbostel, T.; Crowe-White, K. Watermelon Juice: A Novel Functional Food to Increase Circulating Lycopene in Older Adult Women. Plant Foods Hum. Nutr. 2019, 74, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Lee, W.; Lin, Y.; Ho, C.; Lu, K.; Lin, S.; Panyod, S.; Chu, Y.; Sheen, L. Ginger Essential Oil Ameliorates Hepatic Injury and Lipid Accumulation in High Fat Diet-Induced Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2016, 64, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Munkong, N.; Somnuk, S.; Jantarach, N.; Ruxsanawet, K.; Nuntaboon, P.; Kanjoo, V.; Yoysungnoen, B. Red Rice Bran Extract Alleviates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease and Dyslipidemia in Mice. Nutrients 2023, 15, 246. [Google Scholar] [CrossRef]
- Yoysungnoen, B.; Srisawat, U.; Piyabhan, P.; Duansak, N.; Sookprasert, N.; Mathuradavong, N.; Poomipark, N.; Munkong, N.; Tingpej, P.; Changtam, C. Short term effect of tetrahydrocurcumin on adipose angiogenesis in very high-fat diet-induced obesity mouse model. Front. Nutr. 2023, 10, 1221935. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Alagarsamy, K.; Thangaleela, S.; Bharathi, M.; Kesika, P.; Chaiyasut, C. Composition, Microbiota, Mechanisms, and Anti-Obesity Properties of Rice Bran. Foods 2023, 12, 1300. [Google Scholar] [CrossRef] [PubMed]

| Group | Control | HFD | Orlistat | CME1 | CME10 | CME25 |
|---|---|---|---|---|---|---|
| Initial body weight (g) | 17.02 ± 0.39 | 17.05 ± 0.23 | 17.13 ± 0.32 | 17.03 ± 0.20 | 16.94 ± 0.21 | 17.07 ± 0.35 |
| Final body weight (g) | 26.9 ± 0.9 | 38.9 ± 1.7 | 30.7 ± 1.3 ** | 36.8 ± 1.4 * | 34.7 ± 1.1 ** | 32.8 ± 1.7 ** |
| Food intake (g·day−1) | 2.25 ± 0.24 | 2.29 ± 0.13 | 1.83 ± 0.10 ** | 2.02 ± 0.11 * | 1.95 ± 0.23 * | 1.85 ± 0.37 ** |
| Liver weight (g·mouse−1) | 0.96 ± 0.03 | 1.39 ± 0.10 | 1.16 ± 0.06 | 1.33 ± 0.09 | 1.19 ± 0.04 | 1.21 ± 0.08 |
| Kidney weight (g·mouse−1) | 0.34 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.0 | 0.40 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.01 |
| Fat weight (g·mouse−1) | 0.82 ± 0.12 | 3.12 ± 0.21 | 2.13 ± 0.31 ** | 2.78 ± 0.22 * | 2.47 ± 0.25 * | 2.37 ± 0.22 * |
| Abdominal fat weight (g·mouse−1) | 0.54 ± 0.10 | 2.27 ± 0.15 | 1.49 ± 0.23 ** | 2.01 ± 0.16 * | 1.88 ± 0.22 * | 1.75 ± 0.18 ** |
| Retroperitoneal fat weight (g·mouse−1) | 0.22 ± 0.06 | 0.85 ± 0.07 | 0.64 ± 0.09 ** | 0.77 ± 0.07 * | 0.60 ± 0.07 * | 0.62 ± 0.08 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.M.; Park, S.Y.; Kim, J.E.; Lee, K.W.; Hwang, D.Y.; Choi, Y.-W. Citrullus mucosospermus Extract Reduces Weight Gain in Mice Fed a High-Fat Diet. Nutrients 2024, 16, 2171. https://doi.org/10.3390/nu16132171
Kang HM, Park SY, Kim JE, Lee KW, Hwang DY, Choi Y-W. Citrullus mucosospermus Extract Reduces Weight Gain in Mice Fed a High-Fat Diet. Nutrients. 2024; 16(13):2171. https://doi.org/10.3390/nu16132171
Chicago/Turabian StyleKang, He Mi, Sun Young Park, Ji Eun Kim, Ki Won Lee, Dae Youn Hwang, and Young-Whan Choi. 2024. "Citrullus mucosospermus Extract Reduces Weight Gain in Mice Fed a High-Fat Diet" Nutrients 16, no. 13: 2171. https://doi.org/10.3390/nu16132171








