Modality Matters: Fasted Individuals Inhibit Food Stimuli Better Than Neutral Stimuli for Words, but Not for Pictures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design and Procedure
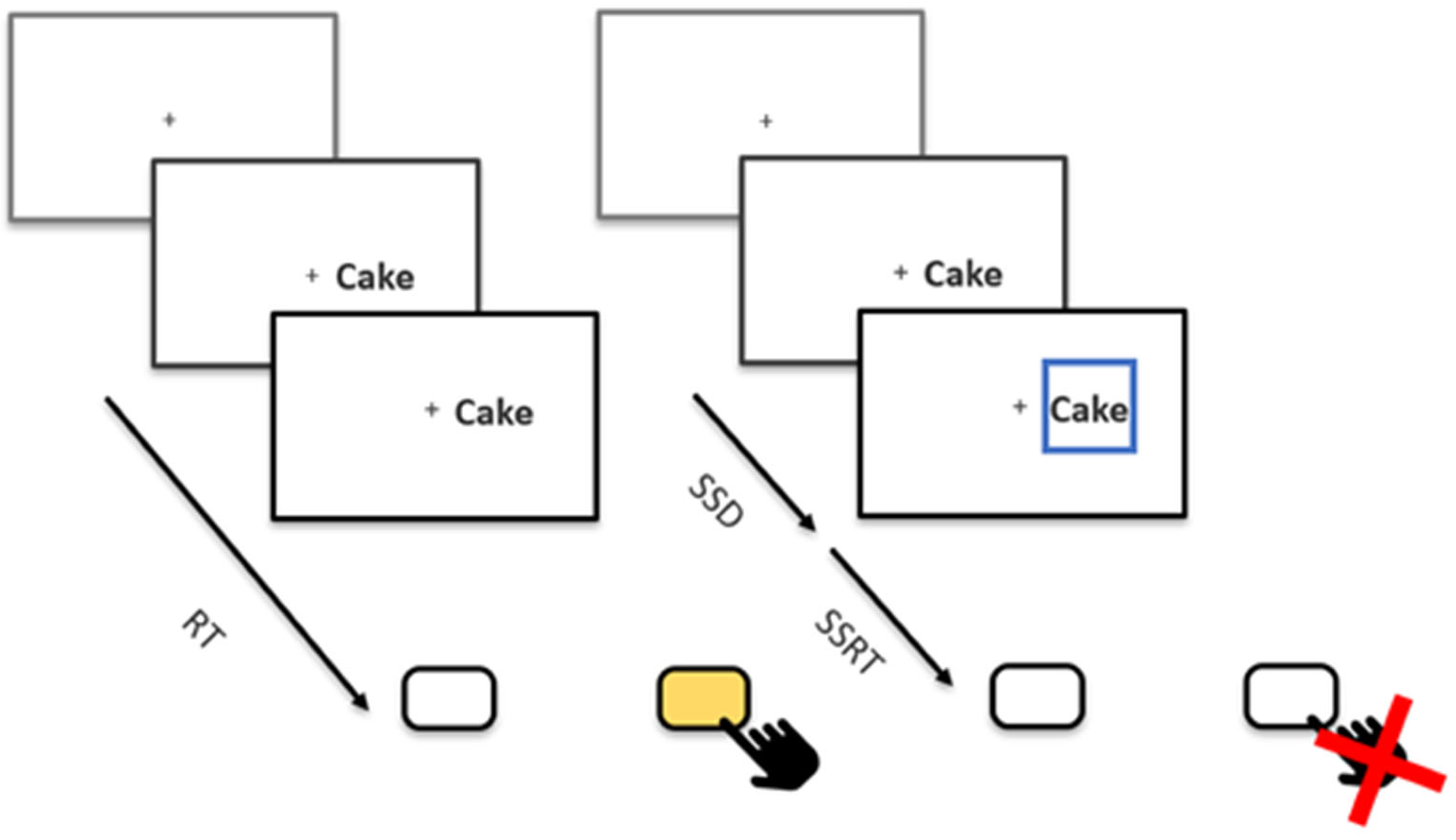
2.3. Stop-Signal Task
2.4. Questionnaires
2.5. Data Analysis
3. Results
3.1. Group Characteristics
3.2. Stop-Signal Reaction Time
3.3. Reaction Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolaidis, S. Environment and obesity. Metabolism 2019, 100, 153942. [Google Scholar] [CrossRef] [PubMed]
- Higgs, S.; Spetter, M.S.; Thomas, J.M.; Rotshtein, P.; Lee, M.; Hallschmid, M.; Dourish, C.T. Interactions between metabolic, reward and cognitive processes in appetite control: Implications for novel weight management therapies. J. Psychopharmacol. 2017, 31, 1460–1474. [Google Scholar] [CrossRef] [PubMed]
- Nederkoorn, C.; Guerrieri, R.; Havermans, R.; Roefs, A.; Jansen, A. The interactive effect of hunger and impulsivity on food intake and purchase in a virtual supermarket. Int. J. Obes. 2009, 33, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Strack, F.; Deutsch, R. Reflective and Impulsive Determinants of Social Behavior. Personal. Soc. Psychol. Rev. 2004, 8, 220–247. [Google Scholar] [CrossRef]
- Hoefling, A.; Strack, F. The tempting effect of forbidden foods. High calorie content evokes conflicting implicit and explicit evaluations in restrained eaters. Appetite 2008, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Burger, K.; Yokum, S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. NeuroImage 2013, 67, 322–330. [Google Scholar] [CrossRef]
- Siep, N.; Roefs, A.; Roebroeck, A.; Havermans, R.; Bonte, M.L.; Jansen, A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav. Brain Res. 2009, 198, 149–158. [Google Scholar] [CrossRef]
- Berthoud, H.-R. Homeostatic and Non-homeostatic Pathways Involved in the Control of Food Intake and Energy Balance. Obesity 2006, 14, 197S–200S. [Google Scholar] [CrossRef]
- Castellanos, E.H.; Charboneau, E.; Dietrich, M.S.; Park, S.; Bradley, B.P.; Mogg, K.; Cowan, R.L. Obese adults have visual attention bias for food cue images: Evidence for altered reward system function. Int. J. Obes. 2009, 33, 1063–1073. [Google Scholar] [CrossRef]
- Levitan, R.D.; Rivera, J.; Silveira, P.P.; Steiner, M.; Gaudreau, H.; Hamilton, J.; Kennedy, J.L.; Davis, C.; Dube, L.; Fellows, L.; et al. Gender differences in the association between stop-signal reaction times, body mass indices and/or spontaneous food intake in pre-school children: An early model of compromised inhibitory control and obesity. Int. J. Obes. 2015, 39, 614–619. [Google Scholar] [CrossRef]
- Byrne, M.E.; Shank, L.M.; Altman, D.R.; Swanson, T.N.; Ramirez, E.; Moore, N.A.; Rubin, S.G.; LeMay-Russell, S.; Parker, M.N.; Kaufman, R.E.; et al. Inhibitory control and negative affect in relation to food intake among youth. Appetite 2021, 156, 104858. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Robinson, E.; Duckworth, J.; Kersbergen, I.; Clarke, N.; Field, M. The effects of exposure to appetitive cues on inhibitory control: A meta-analytic investigation. Appetite 2018, 128, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Loeber, S.; Grosshans, M.; Herpertz, S.; Kiefer, F.; Herpertz, S.C. Hunger modulates behavioral disinhibition and attention allocation to food-associated cues in normal-weight controls. Appetite 2013, 71, 32–39. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Huang, X.; Zhang, S.; Turel, O.; Ma, L.; Bechara, A. Dynamic causal modeling of insular, striatal, and prefrontal cortex activities during a food-specific Go/NoGo task. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 1080–1089. [Google Scholar] [CrossRef]
- Bartholdy, S.; Cheng, J.; Schmidt, U.; Campbell, I.C.; O’Daly, O.G. Task-based and questionnaire measures of inhibitory control are differentially affected by acute food restriction and by motivationally salient food stimuli in healthy adults. Front. Psychol. 2016, 7, 1303. [Google Scholar] [CrossRef] [PubMed]
- Logan, G.D. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In Inhibitory Processes in Attention, Memory, and Language; Academic Press: San Diego, CA, USA, 1994; pp. 189–239. [Google Scholar]
- Logan, G.D.; Cowan, W.B. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev. 1984, 91, 295–327. [Google Scholar] [CrossRef]
- Verbruggen, F.; Aron, A.R.; Band, G.P.; Beste, C.; Bissett, P.G.; Brockett, A.T.; Brown, J.W.; Chamberlain, S.R.; Chambers, C.D.; Colonius, H.; et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. Elife 2019, 8, e46323. [Google Scholar] [CrossRef]
- Fedoroff, I.D.C.; Polivy, J.; Herman, C.P. The Effect of Pre-exposure to Food Cues on the Eating Behavior of Restrained and Unrestrained Eaters. Appetite 1997, 28, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.K.; Hermans, R.C.J.; Engels, R.C.M.E. Food intake in response to food-cue exposure. Examining the influence of duration of the cue exposure and trait impulsivity. Appetite 2012, 58, 907–913. [Google Scholar] [CrossRef]
- Jansen, A.; Theunissen, N.; Slechten, K.; Nederkoorn, C.; Boon, B.; Mulkens, S.; Roefs, A. Overweight children overeat after exposure to food cues. Eat. Behav. 2003, 4, 197–209. [Google Scholar] [CrossRef]
- Wang, G.-J.; Volkow, N.D.; Telang, F.; Jayne, M.; Ma, J.; Rao, M.; Zhu, W.; Wong, C.T.; Pappas, N.R.; Geliebter, A.; et al. Exposure to appetitive food stimuli markedly activates the human brain. NeuroImage 2004, 21, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Paivio, A. A dual coding approach to perception and cognition. In Modes of Perceiving and Processing Information; Erlbaum: Hillsdale, NJ, USA, 1978; pp. 39–51. [Google Scholar]
- Glaser, W.R. Picture naming. Cognition 1992, 42, 61–105. [Google Scholar] [CrossRef] [PubMed]
- Kaup, B.; Ulrich, R.; Bausenhart, K.M.; Bryce, D.; Butz, M.V.; Dignath, D.; Dudschig, C.; Franz, V.H.; Friedrich, C.; Gawrilow, C.; et al. Modal and amodal cognition: An overarching principle in various domains of psychology. Psychol. Res. 2024, 88, 307–337. [Google Scholar] [CrossRef] [PubMed]
- Dudschig, C.; Kaup, B. Pictorial vs. linguistic negation: Investigating negation in imperatives across different symbol domains. Acta Psychol. 2021, 214, 103266. [Google Scholar] [CrossRef] [PubMed]
- Houwer, J.D.; Hermans, D. Differences in the affective processing of words and pictures. Cogn. Emot. 1994, 8, 1–20. [Google Scholar] [CrossRef]
- Killgore, W.D.S.; Young, A.D.; Femia, L.A.; Bogorodzki, P.; Rogowska, J.; Yurgelun-Todd, D.A. Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage 2003, 19, 1381–1394. [Google Scholar] [CrossRef]
- Schmitz, F.; Naumann, E.; Trentowska, M.; Svaldi, J. Attentional bias for food cues in binge eating disorder. Appetite 2014, 80, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Gauggel, S.; Heusinger, A.; Forkmann, T.; Boecker, M.; Lindenmeyer, J.; Miles Cox, W.; Staedtgen, M. Effects of alcohol cue exposure on response inhibition in detoxified alcohol-dependent patients. Alcohol. Clin. Exp. Res. 2010, 34, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; De Houwer, J. Do emotional stimuli interfere with response inhibition? Evidence from the stop signal paradigm. Cogn. Emot. 2007, 21, 391–403. [Google Scholar] [CrossRef]
- Stockburger, J.; Weike, A.I.; Hamm, A.O.; Schupp, H.T. Deprivation selectively modulates brain potentials to food pictures. Behav. Neurosci. 2008, 122, 936–942. [Google Scholar] [CrossRef]
- Lakens, D.; Caldwell, A.R. Simulation-Based Power Analysis for Factorial Analysis of Variance Designs. Adv. Methods Pract. Psychol. Sci. 2021, 4, 2515245920951503. [Google Scholar] [CrossRef]
- Leiner, D.J. SoSci Survey, Version 3.3.13; [Computer Software]. 2019. Available online: https://soscisurvey.de(accessed on 2 June 2024).
- Svaldi, J.; Naumann, E.; Trentowska, M.; Schmitz, F. General and food-specific inhibitory deficits in binge eating disorder. Int. J. Eat. Disord. 2014, 47, 534–542. [Google Scholar] [CrossRef]
- Mayer, S. Imagefluency: Image Statistics Based on Processing Fluency. R Package Version 0.2.4. 2024. Available online: https://doi.org/10.5281/zenodo.5614665 (accessed on 3 June 2024).
- Davies, M. The Corpus of Contemporary American English as the first reliable monitor corpus of English. Lit. Linguist. Comput. 2010, 25, 447–464. [Google Scholar] [CrossRef]
- de Leeuw, J.R. jsPsych: A JavaScript library for creating behavioral experiments in a Web browser. Behav. Res. Methods 2015, 47, 1–12. [Google Scholar] [CrossRef]
- Wöstmann, N.M.; Aichert, D.S.; Costa, A.; Rubia, K.; Möller, H.-J.; Ettinger, U. Reliability and plasticity of response inhibition and interference control. Brain Cogn. 2013, 81, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.; Mumford, J.A.; Cohen, J.R.; Galvan, A.; Canli, T.; Poldrack, R.A. Measurement and Reliability of Response Inhibition. Front. Psychol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soreni, N.; Crosbie, J.; Ickowicz, A.; Schachar, R. Stop Signal and Conners’ Continuous Performance Tasks:Test—Retest Reliability of Two Inhibition Measures in ADHD Children. J. Atten. Disord. 2009, 13, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Fairburn, C.G.; Beglin, S.J. Assessment of eating disorders: Interview or self-report questionnaire? Int. J. Eat. Disord. 1994, 16, 363–370. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Carbin, M.G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Hilbert, A.; Tuschen-Caffier, B.; Karwautz, A.; Niederhofer, H.; Munsch, S. Eating Disorder Examination-Questionnaire. Diagnostica 2007, 53, 144–154. [Google Scholar] [CrossRef]
- Kühner, C.; Bürger, C.; Keller, F.; Hautzinger, M. Reliability and validity of the Revised Beck Depression Inventory (BDI-II). Results from German samples. Nervenarzt 2007, 78, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Herman, C.P.; Polivy, J. Restraint Eating. In A.J. Standard (Ed.), Obesity; W.B. Saunders Company: London, UK, 1980. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Houben, K.; Jansen, A. Training inhibitory control. A recipe for resisting sweet temptations. Appetite 2011, 56, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Oomen, D.; Grol, M.; Spronk, D.; Booth, C.; Fox, E. Beating uncontrolled eating: Training inhibitory control to reduce food intake and food cue sensitivity. Appetite 2018, 131, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Nijs, I.M.T.; Muris, P.; Euser, A.S.; Franken, I.H.A. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite 2010, 54, 243–254. [Google Scholar] [CrossRef]
- Freijy, T.; Mullan, B.; Sharpe, L. Food-related attentional bias. Word versus pictorial stimuli and the importance of stimuli calorific value in the dot probe task. Appetite 2014, 83, 202–208. [Google Scholar] [CrossRef]
- Martens, M.J.; Born, J.M.; Lemmens, S.G.; Karhunen, L.; Heinecke, A.; Goebel, R.; Adam, T.C.; Westerterp-Plantenga, M.S. Increased sensitivity to food cues in the fasted state and decreased inhibitory control in the satiated state in the overweight. Am. J. Clin. Nutr. 2013, 97, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Lutz, A.; Krawietz, V.; Stützer, J.; Vögele, C.; Kübler, A. Food-cue affected motor response inhibition and self-reported dieting success: A pictorial affective shifting task. Front. Psychol. 2014, 5. [Google Scholar] [CrossRef]
- Biswas, D.; Labrecque, L.I.; Lehmann, D.R. Effects of Sequential Sensory Cues on Food Taste Perception: Cross-Modal Interplay Between Visual and Olfactory Stimuli. J. Consum. Psychol. 2021, 31, 746–764. [Google Scholar] [CrossRef]
- Houben, K.; Nederkoorn, C.; Jansen, A. Eating on impulse: The relation between overweight and food-specific inhibitory control. Obesity 2014, 22, E6–E8. [Google Scholar] [CrossRef]
- Schroeder, P.A.; Farshad, M.; Svaldi, J. Anodal stimulation of inhibitory control and craving in satiated restrained eaters. Nutr. Neurosci. 2022, 26, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qi, L.; Morys, F.; Wu, Q.; Chen, H. Food-Specific Inhibition Training for Food Devaluation: A Meta-Analysis. Nutrients 2022, 14, 1363. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.S.; Verbruggen, F.; Morrison, S.; Adams, R.C.; Chambers, C.D. Stopping to food can reduce intake. Effects of stimulus-specificity and individual differences in dietary restraint. Appetite 2015, 85, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Yokum, S.; Veling, H.; Kemps, E.; Lawrence, N.S. Pilot test of a novel food response and attention training treatment for obesity: Brain imaging data suggest actions shape valuation. Behav. Res. Ther. 2017, 94, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Best, M.; Lawrence, N.S.; Logan, G.D.; McLaren, I.P.L.; Verbruggen, F. Should I stop or should I go? The role of associations and expectancies. J. Exp. Psychol. Hum. Percept. Perform. 2016, 42, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Pool, E.; Brosch, T.; Delplanque, S.; Sander, D. Attentional bias for positive emotional stimuli: A meta-analytic investigation. Psychol. Bull. 2016, 142, 79. [Google Scholar] [CrossRef]
- Enriquez-Geppert, S.; Konrad, C.; Pantev, C.; Huster, R.J. Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. NeuroImage 2010, 51, 877–887. [Google Scholar] [CrossRef]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data12. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef]

| Fasted Mean (SD) | Satiated Mean (SD) | Statistics | p-Value | |
|---|---|---|---|---|
| n | 67 | 76 | ||
| Sex (n women) | 43 | 55 | χ2(1) = 1.59 | 0.21 |
| Age | 24.3 (3.9) | 24.9 (3.4) | t(141) = −1.05 | 0.30 |
| Hunger | 72.1 (23.3) | 16.3 (16.3) | t(141) = 18.3 | <0.001 |
| BMI | 21.9 (1.7) | 21.6 (1.9) | t(141) = 0.97 | 0.34 |
| RS | 11.9 (6.2) | 11.6 (5.9) | t(141) = 0.25 | 0.81 |
| EDE-Q total | 1.4 (1.2) | 1.3 (1.2) | t(141) = 0.42 | 0.68 |
| BDI-II | 13.9 (11.1) | 14.9 (11.4) | t(141) = −0.51 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Hoek Ostende, M.M.; Schwarz, U.; Gawrilow, C.; Kaup, B.; Svaldi, J. Modality Matters: Fasted Individuals Inhibit Food Stimuli Better Than Neutral Stimuli for Words, but Not for Pictures. Nutrients 2024, 16, 2190. https://doi.org/10.3390/nu16142190
van den Hoek Ostende MM, Schwarz U, Gawrilow C, Kaup B, Svaldi J. Modality Matters: Fasted Individuals Inhibit Food Stimuli Better Than Neutral Stimuli for Words, but Not for Pictures. Nutrients. 2024; 16(14):2190. https://doi.org/10.3390/nu16142190
Chicago/Turabian Stylevan den Hoek Ostende, Mechteld M., Ulrike Schwarz, Caterina Gawrilow, Barbara Kaup, and Jennifer Svaldi. 2024. "Modality Matters: Fasted Individuals Inhibit Food Stimuli Better Than Neutral Stimuli for Words, but Not for Pictures" Nutrients 16, no. 14: 2190. https://doi.org/10.3390/nu16142190
APA Stylevan den Hoek Ostende, M. M., Schwarz, U., Gawrilow, C., Kaup, B., & Svaldi, J. (2024). Modality Matters: Fasted Individuals Inhibit Food Stimuli Better Than Neutral Stimuli for Words, but Not for Pictures. Nutrients, 16(14), 2190. https://doi.org/10.3390/nu16142190






