Postbiotics as Molecules Targeting Cellular Events of Aging Brain—The Role in Pathogenesis, Prophylaxis and Treatment of Neurodegenerative Diseases
Abstract
:1. Introduction
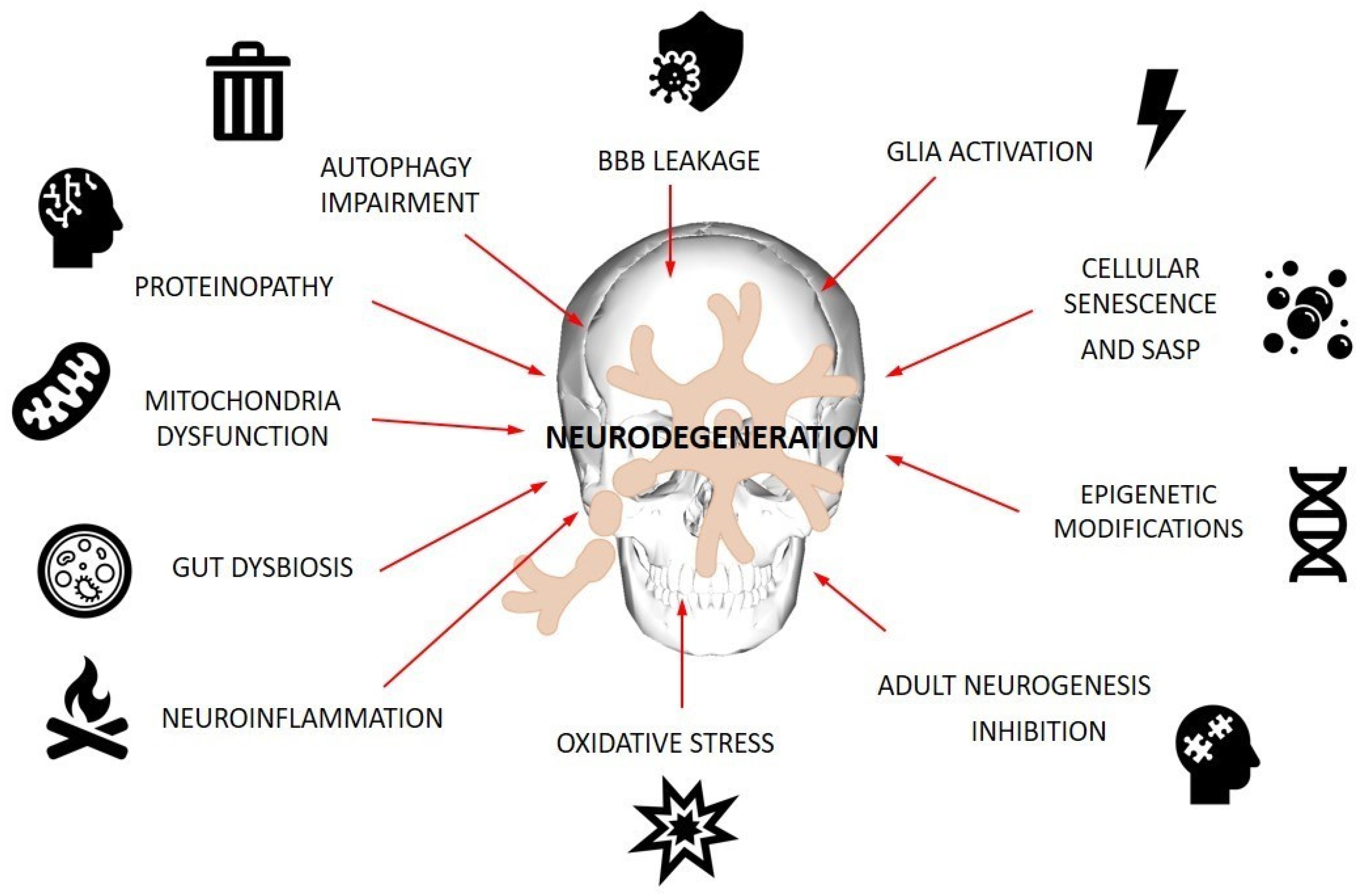
2. Cellular Events Involved in Pathogenesis of ND
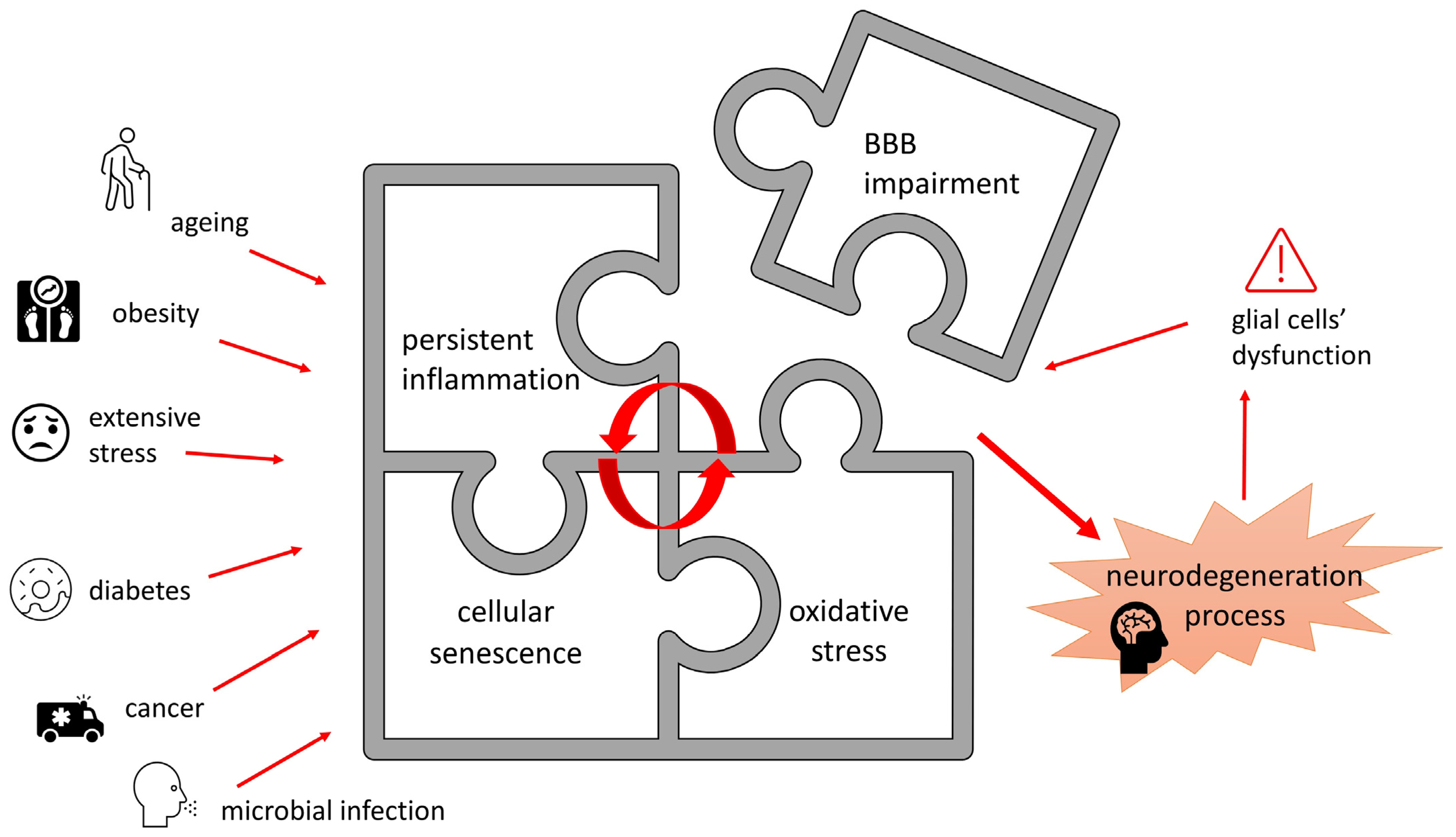
2.1. Oxidative Stress and Neuroinflammation
2.2. Autophagy
2.3. Cellular Senescence
2.4. Neurogenesis
2.5. Blood–Brain Barrier Dysfunction
3. Probiotic By-Products as Tools Supporting CNS Function
3.1. Postbiotics as Mixture of Molecules Targeting Cellular Machinery of ND Pathogenesis
3.1.1. Short-Chain Fatty Acids
3.1.2. Lactate
3.1.3. Polyamines
3.1.4. Tryptophan Derivates
3.1.5. Polyphenol Metabolites
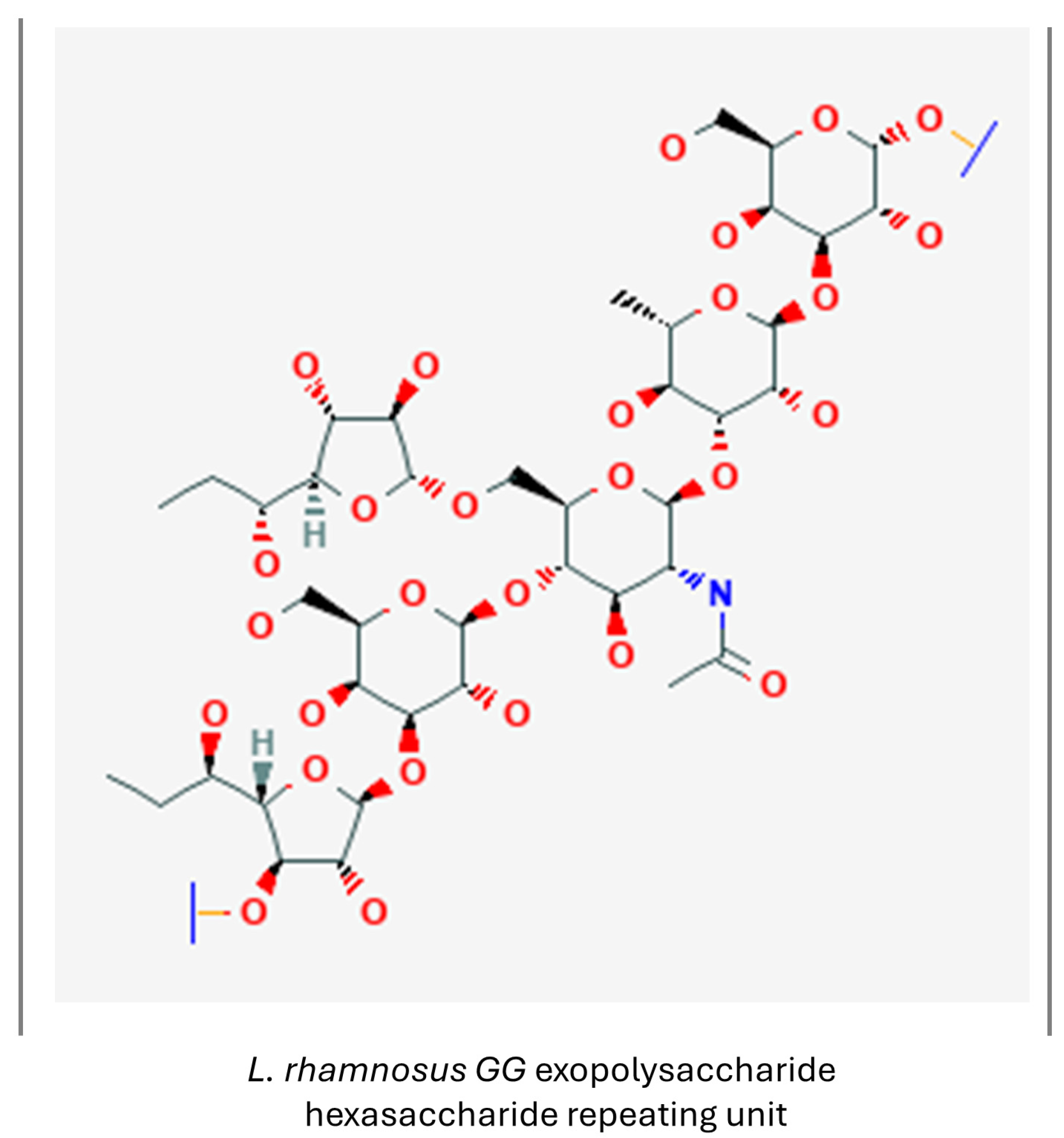
3.1.6. Exopolysaccharides
3.1.7. Extracellular Vesicles
3.1.8. Other Metabolites

3.2. Brief Overview of Postbiotics’ Impact on Processes Involved in ND Pathogenesis
4. Future Perspectives and Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| α-syn | synuclein α |
| Aβ | amyloid β |
| AD | Alzheimer’s disease |
| ATG | autophagy-related gene |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BV-2 | murine microglia cell line |
| CEBPβ | CCAA T/enhancer-binding protein β |
| CMA | chaperone-mediated autophagy |
| CNS | central nervous system |
| COX-2 | cyclooxygenase 2 |
| CREB | cAMP-response element-binding protein |
| DA | dopamine |
| EPS | exopolysaccharide |
| ERK | extracellular signal-regulated kinase |
| ETC | electron transport chain |
| EVs | extracellular vesicles |
| FOXO3a | Forkhead Transcription Factor O Subfamily Member 3a |
| GFAP | glial fibrillary acidic protein |
| GR | glucocorticoid receptor |
| GSK-3β | glycogen synthase kinase-3β |
| HDAC | NAD+-dependent histone deacetylase |
| HDACi | histone deacetylase inhibitor |
| HSV | Herpes simplex virus |
| IFN | interferon |
| IGF-1 | insulin-like growth factor 1 |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LAB | lactic acid bacteria |
| LPS | lipopolysaccharide |
| LRRK2 | leucine-rich repeat kinase 2 |
| LTA | lipoteichoic acids |
| MAO | monoamine oxidase |
| MCT1 | monocarboxylate transporter 1 |
| MIP | macrophage inflammatory protein |
| MMP-3 | metalloproteinase 3 |
| MPP+ | 1-methyl-4-phenylpyridinium |
| mTOR | mammalian target of rapamycin |
| NAMO | nicotinamide N-oxide |
| ND | neurodegenerative disease |
| Neurog2 | neurogenin 2 |
| NF-κB | nuclear factor κB |
| NFT | neurofibrillary tangle |
| NO | nitric oxide |
| NQO1 | quinone oxidoreductase 1 |
| NRF-1 | nuclear respiratory factor 1 |
| NSC | neural stem cells |
| OPC | oligodendrocyte precursor cells |
| OS | oxidative stress |
| PD | Parkinson’s disease |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| QSP | quorum sensing peptides |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SASP | senescence-associated secretory phenotype |
| SCFA | short-chain fatty acid |
| SGK1 | serum- and glucocorticoid kinase 1 |
| SH-SY5Y | human neuroblastoma cel line |
| SIRT | sirtuin |
| SK-MEL-28 | human melanoma cell line |
| TFAM | mitochondrial transcription factor A |
| TH | tyrosine hydroxylase |
| TGF-β | transforming growth factor β |
| TLR | Toll-like receptor |
| TNF-α | tumor necrosis factor α |
| UPS | ubiquitin-proteasome system |
| VEGF | vascular endothelial growth factor |
References
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights into Pathophysiology. Front. Aging Neurosci. 2022, 14, 742408. [Google Scholar] [CrossRef] [PubMed]
- Kulcsarova, K.; Skorvanek, M.; Postuma, R.B.; Berg, D. Defining Parkinson’s Disease: Past and Future. J. Park. Dis. 2024, 1–15. [CrossRef] [PubMed]
- Ahmad, S.S.; Waheed, T.; Rozeen, S.; Mahmood, S.; Kamal, M.A. Therapeutic Study of Phytochemicals Against Cancer and Alzheimer’s Disease Management. Curr. Drug Metab. 2019, 20, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Petersen, R.C. Cellular Senescence in Brain Aging and Neurodegenerative Diseases: Evidence and Perspectives. J. Clin. Invest. 2018, 128, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Bashir, B.; Alam, S.; Khandale, N.; Birla, D.; Vishwas, S.; Pandey, N.K.; Gupta, G.; Paudel, K.R.; Dureja, H.; Kumar, P.; et al. Opening Avenues for Treatment of Neurodegenerative Disease Using Post-Biotics: Breakthroughs and Bottlenecks in Clinical Translation. Ageing Res. Rev. 2024, 95, 102236. [Google Scholar] [CrossRef]
- Taghizadeh Ghassab, F.; Shamlou Mahmoudi, F.; Taheri Tinjani, R.; Emami Meibodi, A.; Zali, M.R.; Yadegar, A. Probiotics and the Microbiota-Gut-Brain Axis in Neurodegeneration: Beneficial Effects and Mechanistic Insights. Life Sci. 2024, 350, 122748. [Google Scholar] [CrossRef] [PubMed]
- Bulacios, G.A.; Cataldo, P.G.; Naja, J.R.; de Chaves, E.P.; Taranto, M.P.; Minahk, C.J.; Hebert, E.M.; Saavedra, M.L. Improvement of Key Molecular Events Linked to Alzheimer’s Disease Pathology Using Postbiotics. ACS Omega 2023, 8, 48042–48049. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.-K.; Paik, H.-D. A Narrative Review on the Advance of Probiotics to Metabiotics. J. Microbiol. Biotechnol. 2024, 34, 487–494. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.-S.; Lee, K.-J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Rao, A.V.; Balachandran, B. Role of Oxidative Stress and Antioxidants in Neurodegenerative Diseases. Nutr. Neurosci. 2002, 5, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative Stress: The Core Pathogenesis and Mechanism of Alzheimer’s Disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative Stress in the Aging Substantia Nigra and the Etiology of Parkinson’s Disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed]
- Hemmati-Dinarvand, M.; Saedi, S.; Valilo, M.; Kalantary-Charvadeh, A.; Alizadeh Sani, M.; Kargar, R.; Safari, H.; Samadi, N. Oxidative Stress and Parkinson’s Disease: Conflict of Oxidant-Antioxidant Systems. Neurosci. Lett. 2019, 709, 134296. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao Chakole, R.; Nemade, L.S.; Kishor Kale, N.; Borah, S.; Shrikant Deokar, S.; et al. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease, and Parkinson’s Disease, Huntington’s Disease and Amyotrophic Lateral Sclerosis—An Updated Review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Tzoneva, R. Oxidative Stress and Aging as Risk Factors for Alzheimer’s Disease and Parkinson’s Disease: The Role of the Antioxidant Melatonin. Int. J. Mol. Sci. 2023, 24, 3022. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative Stress and Mitochondrial Dysfunction-Linked Neurodegenerative Disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Percário, S.; da Silva Barbosa, A.; Varela, E.L.P.; Gomes, A.R.Q.; Ferreira, M.E.S.; de Nazaré Araújo Moreira, T.; Dolabela, M.F. Oxidative Stress in Parkinson’s Disease: Potential Benefits of Antioxidant Supplementation. Oxidative Med. Cell. Longev. 2020, 2020, e2360872. [Google Scholar] [CrossRef]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Kono, R.; Ikegaya, Y.; Koyama, R. Phagocytic Glial Cells in Brain Homeostasis. Cells 2021, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018, 2018, 4784268. [Google Scholar] [CrossRef] [PubMed]
- Çınar, E.; Tel, B.C.; Şahin, G. Neuroinflammation in Parkinson’s Disease and Its Treatment Opportunities. Balk. Med. J. 2022, 39, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s Disease and Its Potential as Therapeutic Target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Reimer, L.; Vesterager, L.B.; Betzer, C.; Zheng, J.; Nielsen, L.D.; Kofoed, R.H.; Lassen, L.B.; Bølcho, U.; Paludan, S.R.; Fog, K.; et al. Inflammation Kinase PKR Phosphorylates α-Synuclein and Causes α-Synuclein-Dependent Cell Death. Neurobiol. Dis. 2018, 115, 17–28. [Google Scholar] [CrossRef]
- Kawahata, I.; Finkelstein, D.I.; Fukunaga, K. Pathogenic Impact of α-Synuclein Phosphorylation and Its Kinases in α-Synucleinopathies. Int. J. Mol. Sci. 2022, 23, 6216. [Google Scholar] [CrossRef]
- Lin, J.; Ou, R.; Li, C.; Hou, Y.; Zhang, L.; Wei, Q.; Pang, D.; Liu, K.; Jiang, Q.; Yang, T.; et al. Plasma Glial Fibrillary Acidic Protein as a Biomarker of Disease Progression in Parkinson’s Disease: A Prospective Cohort Study. BMC Med. 2023, 21, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, X.; Song, Y.-Q.; Tu, J. Autophagy in Alzheimer’s Disease Pathogenesis: Therapeutic Potential and Future Perspectives. Ageing Res. Rev. 2021, 72, 101464. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Watzlawik, J.O.; Fiesel, F.C.; Springer, W. Autophagy in Parkinson’s Disease. J. Mol. Biol. 2020, 432, 2651–2672. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, D.A. Influence of Normal Aging on Brain Autophagy: A Complex Scenario. Front. Aging Neurosci. 2019, 11, 11–49. [Google Scholar] [CrossRef]
- Liu, G.; Yu, Q.; Tan, B.; Ke, X.; Zhang, C.; Li, H.; Zhang, T.; Lu, Y. Gut Dysbiosis Impairs Hippocampal Plasticity and Behaviors by Remodeling Serum Metabolome. Gut Microbes 2022, 14, 2104089. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gallage, S.; Gil, J. TORn about SASP Regulation. Cell Cycle 2015, 14, 3771–3772. [Google Scholar] [CrossRef] [PubMed]
- Salotti, J.; Johnson, P.F. Regulation of Senescence and the SASP by the Transcription Factor C/EBPβ. Exp. Gerontol. 2019, 128, 110752. [Google Scholar] [CrossRef] [PubMed]
- Jęśko, H.; Wencel, P.; Strosznajder, R.P.; Strosznajder, J.B. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2017, 42, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Zuo, H.; Pei, G.; Huang, S.; Hou, Y. Alzheimer’s Amyloid-β Accelerates Cell Senescence and Suppresses SIRT1 in Human Neural Stem Cells. Biomolecules 2024, 14, 189. [Google Scholar] [CrossRef]
- Lilja, S.; Oldenburg, J.; Pointner, A.; Dewald, L.; Lerch, M.; Hippe, B.; Switzeny, O.; Haslberger, A. Epigallocatechin Gallate Effectively Affects Senescence and Anti-SASP via SIRT3 in 3T3-L1 Preadipocytes in Comparison with Other Bioactive Substances. Oxid. Med. Cell Longev. 2020, 2020, 4793125. [Google Scholar] [CrossRef]
- Cuanalo-Contreras, K.; Schulz, J.; Mukherjee, A.; Park, K.-W.; Armijo, E.; Soto, C. Extensive Accumulation of Misfolded Protein Aggregates during Natural Aging and Senescence. Front. Aging Neurosci. 2022, 14, 1090109. [Google Scholar] [CrossRef]
- Golde, T.E.; Miller, V.M. Proteinopathy-Induced Neuronal Senescence: A Hypothesis for Brain Failure in Alzheimer’s and Other Neurodegenerative Diseases. Alzheimers Res. Ther. 2009, 1, 5. [Google Scholar] [CrossRef]
- Pantelis, P.; Theocharous, G.; Lagopati, N.; Veroutis, D.; Thanos, D.-F.; Lampoglou, G.-P.; Pippa, N.; Gatou, M.-A.; Tremi, I.; Papaspyropoulos, A.; et al. The Dual Role of Oxidative-Stress-Induced Autophagy in Cellular Senescence: Comprehension and Therapeutic Approaches. Antioxidants 2023, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.B.; Sinclair, D.A. When Stem Cells Grow Old: Phenotypes and Mechanisms of Stem Cell Aging. Development 2016, 143, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Culig, L.; Chu, X.; Bohr, V.A. Neurogenesis in Aging and Age-Related Neurodegenerative Diseases. Ageing Res. Rev. 2022, 78, 101636. [Google Scholar] [CrossRef] [PubMed]
- Hagg, T. From Neurotransmitters to Neurotrophic Factors to Neurogenesis. Neuroscientist 2009, 15, 20–27. [Google Scholar] [CrossRef]
- Gould, E.; Reeves, A.J.; Graziano, M.S.A.; Gross, C.G. Neurogenesis in the Neocortex of Adult Primates. Science 1999, 286, 548–552. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the Adult Human Hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Salta, E.; Lazarov, O.; Fitzsimons, C.P.; Tanzi, R.; Lucassen, P.J.; Choi, S.H. Adult Hippocampal Neurogenesis in Alzheimer’s Disease: A Roadmap to Clinical Relevance. Cell Stem Cell 2023, 30, 120–136. [Google Scholar] [CrossRef]
- Kot, M.; Neglur, P.K.; Pietraszewska, A.; Buzanska, L. Boosting Neurogenesis in the Adult Hippocampus Using Antidepressants and Mesenchymal Stem Cells. Cells 2022, 11, 3234. [Google Scholar] [CrossRef]
- Audesse, A.J.; Webb, A.E. Mechanisms of Enhanced Quiescence in Neural Stem Cell Aging. Mech. Ageing Dev. 2020, 191, 111323. [Google Scholar] [CrossRef]
- Anand, K.S.; Dhikav, V. Hippocampus in Health and Disease: An Overview. Ann. Indian. Acad. Neurol. 2012, 15, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Bang, Y.; Choi, H.J. Abnormal Hippocampal Neurogenesis in Parkinson’s Disease: Relevance to a New Therapeutic Target for Depression with Parkinson’s Disease. Arch. Pharm. Res. 2018, 41, 943–954. [Google Scholar] [CrossRef]
- Boldrini, M.; Underwood, M.D.; Hen, R.; Rosoklija, G.B.; Dwork, A.J.; John Mann, J.; Arango, V. Antidepressants Increase Neural Progenitor Cells in the Human Hippocampus. Neuropsychopharmacology 2009, 34, 2376–2389. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Boström, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of Hippocampal Neurogenesis in Adult Humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Hen, R. Adult Hippocampal Neurogenesis and Cognitive Flexibility—Linking Memory and Mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Cattaneo, A.; Luoni, A.; Musaelyan, K.; Zunszain, P.A.; Milanesi, E.; Rybka, J.; Berry, A.; Cirulli, F.; Thuret, S.; et al. Glucocorticoid-Related Molecular Signaling Pathways Regulating Hippocampal Neurogenesis. Neuropsychopharmacology 2013, 38, 872–883. [Google Scholar] [CrossRef]
- Anacker, C.; Cattaneo, A.; Musaelyan, K.; Zunszain, P.A.; Horowitz, M.; Molteni, R.; Luoni, A.; Calabrese, F.; Tansey, K.; Gennarelli, M.; et al. Role for the Kinase SGK1 in Stress, Depression, and Glucocorticoid Effects on Hippocampal Neurogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 8708–8713. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Gamit, N.; Dharmarajan, A.; Sethi, G.; Warrier, S. Want of Wnt in Parkinson’s Disease: Could sFRP Disrupt Interplay between Nurr1 and Wnt Signaling? Biochem. Pharmacol. 2023, 212, 115566. [Google Scholar] [CrossRef]
- Marchetti, B. Nrf2/Wnt Resilience Orchestrates Rejuvenation of Glia-Neuron Dialogue in Parkinson’s Disease. Redox Biol. 2020, 36, 101664. [Google Scholar] [CrossRef]
- Arredondo, S.B.; Valenzuela-Bezanilla, D.; Mardones, M.D.; Varela-Nallar, L. Role of Wnt Signaling in Adult Hippocampal Neurogenesis in Health and Disease. Front. Cell Dev. Biol. 2020, 8, 860. [Google Scholar] [CrossRef]
- Marchetti, B.; Tirolo, C.; L’Episcopo, F.; Caniglia, S.; Testa, N.; Smith, J.A.; Pluchino, S.; Serapide, M.F. Parkinson’s Disease, Aging and Adult Neurogenesis: Wnt/β-Catenin Signalling as the Key to Unlock the Mystery of Endogenous Brain Repair. Aging Cell 2020, 19, e13101. [Google Scholar] [CrossRef] [PubMed]
- L’Episcopo, F.; Tirolo, C.; Serapide, M.F.; Caniglia, S.; Testa, N.; Leggio, L.; Vivarelli, S.; Iraci, N.; Pluchino, S.; Marchetti, B. Microglia Polarization, Gene-Environment Interactions and Wnt/β-Catenin Signaling: Emerging Roles of Glia-Neuron and Glia-Stem/Neuroprogenitor Crosstalk for Dopaminergic Neurorestoration in Aged Parkinsonian Brain. Front. Aging Neurosci. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wang, P.; Gong, J.; Zhang, S. New Insights into the Role of GSK-3β in the Brain: From Neurodegenerative Disease to Tumorigenesis. PeerJ 2023, 11, e16635. [Google Scholar] [CrossRef]
- Cope, E.C.; Gould, E. Adult Neurogenesis, Glia, and the Extracellular Matrix. Cell Stem Cell 2019, 24, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Andromidas, F.; Atashpanjeh, S.; Myers, A.J.; MacKinnon, B.E.; Shaffer, M.M.; Koob, A.O. The Astrogenic Balance in the Aging Brain. Curr. Neuropharmacol. 2021, 19, 1952–1965. [Google Scholar] [CrossRef]
- Bergles, D.E.; Richardson, W.D. Oligodendrocyte Development and Plasticity. Cold Spring Harb. Perspect. Biol. 2015, 8, a020453. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Dynamics of Oligodendrocyte Generation in Multiple Sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef]
- Grabert, K.; Michoel, T.; Karavolos, M.H.; Clohisey, S.; Baillie, J.K.; Stevens, M.P.; Freeman, T.C.; Summers, K.M.; McColl, B.W. Microglial Brain Region−dependent Diversity and Selective Regional Sensitivities to Aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Y.; Zhang, X.; Gu, X.; Mao, Y.; Peng, B. The Origin and Repopulation of Microglia. Dev. Neurobiol. 2022, 82, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, B.; L’Episcopo, F.; Morale, M.C.; Tirolo, C.; Testa, N.; Caniglia, S.; Serapide, M.F.; Pluchino, S. Uncovering Novel Actors in Astrocyte–Neuron Crosstalk in Parkinson’s Disease: The Wnt/β-Catenin Signaling Cascade as the Common Final Pathway for Neuroprotection and Self-Repair. Eur. J. Neurosci. 2013, 37, 1550–1563. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.-K.; Park, Y.; Yoon, B.; Bae, J.-S.; Han, S.-W.; Heo, J.-E.; Kim, D.-E.; Ryu, K.-Y. Reduced Secretion of LCN2 (Lipocalin 2) from Reactive Astrocytes through Autophagic and Proteasomal Regulation Alleviates Inflammatory Stress and Neuronal Damage. Autophagy 2023, 19, 2296–2317. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Santos, T.; Sampaio-Marques, B.; Novais, A.; Mesquita, S.D.; Ludovico, P.; Bernardino, L.; Correia-Neves, M.; Sousa, N.; Palha, J.A.; et al. Lipocalin-2 Regulates Adult Neurogenesis and Contextual Discriminative Behaviours. Mol. Psychiatry 2018, 23, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Aparicio, I.; Paris, I.; Sierra-Torre, V.; Plaza-Zabala, A.; Rodríguez-Iglesias, N.; Márquez-Ropero, M.; Beccari, S.; Huguet, P.; Abiega, O.; Alberdi, E.; et al. Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. J. Neurosci. 2020, 40, 1453–1482. [Google Scholar] [CrossRef] [PubMed]
- Nicola, R.; Okun, E. Adult Hippocampal Neurogenesis: One Lactate to Rule Them All. Neuromol. Med. 2021, 23, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Couillard-Després, S. Neuron and Brain Maturation 2.0. Int. J. Mol. Sci. 2023, 24, 17113. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Akram, R.; Anwar, H.; Sajid, F.; Iman, T.; Han, H.S.; Raza, C.; De Aguilar, J.-L.G. Adult Neurogenesis: A Real Hope or a Delusion? Neural Regen. Res. 2024, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Moyaert, P.; Padrela, B.E.; Morgan, C.A.; Petr, J.; Versijpt, J.; Barkhof, F.; Jurkiewicz, M.T.; Shao, X.; Oyeniran, O.; Manson, T.; et al. Imaging Blood-Brain Barrier Dysfunction: A State-of-the-Art Review from a Clinical Perspective. Front. Aging Neurosci. 2023, 15, 1132077. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood–Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef]
- Kaya, M.; Ahishali, B. Basic Physiology of the Blood-Brain Barrier in Health and Disease: A Brief Overview. Tissue Barriers 2021, 9, 1840913. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, B.; Duran, G.; Hoeks, C.; Hermans, D.; Schepers, M.; Baeten, P.; Poelmans, J.; Coenen, B.; Bekar, K.; Pintelon, I.; et al. Cerebral Microvascular Endothelial Cell-Derived Extracellular Vesicles Regulate Blood-Brain Barrier Function. Fluids Barriers CNS 2023, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- de Rus Jacquet, A.; Alpaugh, M.; Denis, H.L.; Tancredi, J.L.; Boutin, M.; Decaestecker, J.; Beauparlant, C.; Herrmann, L.; Saint-Pierre, M.; Parent, M.; et al. The Contribution of Inflammatory Astrocytes to BBB Impairments in a Brain-Chip Model of Parkinson’s Disease. Nat. Commun. 2023, 14, 3651. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Galea, I. The Blood-Brain Barrier in Systemic Inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fock, E.; Parnova, R. Mechanisms of Blood–Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.L.; Stevenson, R.J. Vulnerability of the Hippocampus to Insults: Links to Blood-Brain Barrier Dysfunction. Int. J. Mol. Sci. 2024, 25, 1991. [Google Scholar] [CrossRef] [PubMed]
- Ivanidze, J.; Mackay, M.; Hoang, A.; Chi, J.M.; Cheng, K.; Aranow, C.; Volpe, B.; Diamond, B.; Sanelli, P.C. Dynamic Contrast-Enhanced MRI Reveals Unique Blood-Brain Barrier Permeability Characteristics in the Hippocampus in the Normal Brain. AJNR Am. J. Neuroradiol. 2019, 40, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.N.; Noble, E.E.; Kanoski, S.E. Regulation of Memory Function by Feeding-Relevant Biological Systems: Following the Breadcrumbs to the Hippocampus. Front. Mol. Neurosci. 2019, 12, 101. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Bogale, T.A.; Koistinaho, J.; Pizzi, M.; Rolova, T.; Bellucci, A. The Contribution of β-Amyloid, Tau and α-Synuclein to Blood–Brain Barrier Damage in Neurodegenerative Disorders. Acta Neuropathol. 2024, 147, 39. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The Blood-Brain Barrier in Aging and Neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, J.; Pettersson, S.; Reynolds, R.; Tan, E.-K. The Link between Neuroinflammation and the Neurovascular Unit in Synucleinopathies. Sci. Adv. 2023, 9, eabq1141. [Google Scholar] [CrossRef]
- Hotel, A. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. In Proceedings of the Joint FAO/WHO Expert Consultation, Córdoba, Argentina, 1–4 October 2001. [Google Scholar]
- Moradi, M.; Molaei, R.; Guimarães, J.T. A Review on Preparation and Chemical Analysis of Postbiotics from Lactic Acid Bacteria. Enzym. Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, K. Anticancer Activity of Lactic Acid Bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef] [PubMed]
- González-Lozano, E.; García-García, J.; Gálvez, J.; Hidalgo-García, L.; Rodríguez-Nogales, A.; Rodríguez-Cabezas, M.E.; Sánchez, M. Novel Horizons in Postbiotics: Lactobacillaceae Extracellular Vesicles and Their Applications in Health and Disease. Nutrients 2022, 14, 5296. [Google Scholar] [CrossRef]
- Lou, X.; Xue, J.; Shao, R.; Mo, C.; Wang, F.; Chen, G. Postbiotics as Potential New Therapeutic Agents for Sepsis. Burn. Trauma. 2023, 11, tkad022. [Google Scholar] [CrossRef]
- Descamps, H.C.; Herrmann, B.; Wiredu, D.; Thaiss, C.A. The Path toward Using Microbial Metabolites as Therapies. eBioMedicine 2019, 44, 747–754. [Google Scholar] [CrossRef]
- Fang, X.; Yue, M.; Wei, J.; Wang, Y.; Hong, D.; Wang, B.; Zhou, X.; Chen, T. Evaluation of the Anti-Aging Effects of a Probiotic Combination Isolated From Centenarians in a SAMP8 Mouse Model. Front. Immunol. 2021, 12, 792746. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Cheng, L.-H.; Liu, Y.-W.; Jeng, O.-J.; Lee, Y.-K. Gerobiotics: Probiotics Targeting Fundamental Aging Processes. Biosci. Microbiota Food Health 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Tran, S.M.-S.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Sharma, R.; Padwad, Y. Probiotic Bacteria as Modulators of Cellular Senescence: Emerging Concepts and Opportunities. Gut Microbes 2020, 11, 335–349. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Bailo, P.S.; Martín, E.L.; Calmarza, P.; Breva, S.M.; Gómez, A.B.; Giráldez, A.P.; Callau, J.J.S.-P.; Santamaría, J.M.V.; Khialani, A.D.; Micó, C.C.; et al. The Role of Oxidative Stress in Neurodegenerative Diseases and Potential Antioxidant Therapies. Adv. Lab. Med./Av. Med. Lab. 2022, 3, 342–350. [Google Scholar] [CrossRef]
- Morén, C.; deSouza, R.M.; Giraldo, D.M.; Uff, C. Antioxidant Therapeutic Strategies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 9328. [Google Scholar] [CrossRef] [PubMed]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Güngören, A.; Koluman, A.; İlhak, O.İ.; Kanmaz, H.; Kaya, B.; Hayaloğlu, A.A. Characterization of Lactic Acid Bacteria Postbiotics, Evaluation in-Vitro Antibacterial Effect, Microbial and Chemical Quality on Chicken Drumsticks. Food Microbiol. 2022, 104, 104001. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Abdelmotilib, N.M.; Darwish, A.M.G.; Zeitoun, A.M. Commercial Probiotic Cell-Free Supernatants for Inhibition of Clostridium Perfringens Poultry Meat Infection in Egypt. Anaerobe 2020, 62, 102181. [Google Scholar] [CrossRef] [PubMed]
- Angelin, J.; Kavitha, M. Exopolysaccharides from Probiotic Bacteria and Their Health Potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Kim, H.S.; Chae, H.S.; Jeong, S.G.; Ham, J.S.; Im, S.K.; Ahn, C.N.; Lee, J.M. In Vitro Antioxidative Properties of Lactobacilli. Asian Australas. Asian Australas. J. Anim. Sci. 2005, 19, 262–265. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Montalbán-Rodríguez, A.; Abalo, R.; López-Gómez, L. From the Gut to the Brain: The Role of Enteric Glial Cells and Their Involvement in the Pathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 1294. [Google Scholar] [CrossRef]
- Kocot, A.M.; Jarocka-Cyrta, E.; Drabińska, N. Overview of the Importance of Biotics in Gut Barrier Integrity. Int. J. Mol. Sci. 2022, 23, 2896. [Google Scholar] [CrossRef] [PubMed]
- Askarova, S.; Umbayev, B.; Masoud, A.-R.; Kaiyrlykyzy, A.; Safarova, Y.; Tsoy, A.; Olzhayev, F.; Kushugulova, A. The Links Between the Gut Microbiome, Aging, Modern Lifestyle and Alzheimer’s Disease. Front. Cell Infect. Microbiol. 2020, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Park, S.; Park, G.; Shin, H.; Park, M.S.; Kim, J. Administration of Bifidobacterium Bifidum BGN4 and Bifidobacterium Longum BORI Improves Cognitive and Memory Function in the Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 709091. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Busetti, A.; Fotiadou, P.; Vincy Jose, N.; Reid, S.; Georgieva, M.; Brown, S.; Dunbar, H.; Beurket-Ascencio, G.; Delday, M.I.; et al. In Vitro Characterization of Gut Microbiota-Derived Bacterial Strains With Neuroprotective Properties. Front. Cell. Neurosci. 2019, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.-H.; Bock, H.-J.; Lee, N.-K.; Paik, H.-D. Soy Yogurt Using Lactobacillus Plantarum 200655 and Fructooligosaccharides: Neuroprotective Effects against Oxidative Stress. J. Food Sci. Technol. 2022, 59, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Michael, D.R.; Davies, T.S.; Loxley, K.E.; Allen, M.D.; Good, M.A.; Hughes, T.R.; Plummer, S.F. In Vitro Neuroprotective Activities of Two Distinct Probiotic Consortia. Benef. Microbes 2019, 10, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K.; Kim, D.H. Lactobacillus Mucosae and Bifidobacterium Longum Synergistically Alleviate Immobilization Stress-Induced Anxiety/Depression in Mice by Suppressing Gut Dysbiosis. J. Microbiol. Biotechnol. 2019, 29, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Hor, Y.-Y.; Ooi, C.-H.; Khoo, B.-Y.; Choi, S.-B.; Seeni, A.; Shamsuddin, S.; Oon, C.-E.; Ong, K.-L.; Jeong, W.-S.; Liong, M.-T. Lactobacillus Strains Alleviated Aging Symptoms and Aging-Induced Metabolic Disorders in Aged Rats. J. Med. Food 2019, 22, 1–13. [Google Scholar] [CrossRef]
- Chakraborty, P.; Gamage, H.K.A.H.; Laird, A.S. Butyrate as a Potential Therapeutic Agent for Neurodegenerative Disorders. Neurochem. Int. 2024, 176, 105745. [Google Scholar] [CrossRef]
- Dugan, B.; Conway, J.; Duggal, N.A. Inflammaging as a Target for Healthy Ageing. Age Ageing 2023, 52, afac328. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, P.V.; Ververis, K.; Karagiannis, T.C. Histone Deacetylase Inhibition and Dietary Short-Chain Fatty Acids. Int. Sch. Res. Not. 2011, 2011, e869647. [Google Scholar] [CrossRef] [PubMed]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human Gut Bacteria as Potent Class I Histone Deacetylase Inhibitors in Vitro through Production of Butyric Acid and Valeric Acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 176, Acetic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acetic-Acid (accessed on 1 July 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1032, Propionic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Propionic-Acid (accessed on 1 July 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 264, Butyric Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Butyric-Acid (accessed on 1 July 2024).
- Flynn, C.M.; Yuan, Q. Probiotic Supplement as a Promising Strategy in Early Tau Pathology Prevention: Focusing on GSK-3β? Front. Neurosci. 2023, 17, 1159314. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Z.; Zhao, Y.; Wang, Z.; Wang, C.; Yang, G.; Li, S. Lactobacillus Plantarum DP189 Prevents Cognitive Dysfunction in D-Galactose/AlCl3 Induced Mouse Model of Alzheimer’s Disease via Modulating Gut Microbiota and PI3K/Akt/GSK-3β Signaling Pathway. Nutr. Neurosci. 2022, 25, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xie, L.; Schröder, J.; Schuster, I.S.; Nakai, M.; Sun, G.; Sun, Y.B.Y.; Mariño, E.; Degli-Esposti, M.A.; Marques, F.Z.; et al. Dietary Fiber and Microbiota Metabolite Receptors Enhance Cognition and Alleviate Disease in the 5xFAD Mouse Model of Alzheimer’s Disease. J. Neurosci. 2023, 43, 6460–6475. [Google Scholar] [CrossRef] [PubMed]
- Bull-Larsen, S.; Mohajeri, M.H. The Potential Influence of the Bacterial Microbiome on the Development and Progression of ADHD. Nutrients 2019, 11, 2805. [Google Scholar] [CrossRef] [PubMed]
- Feitelson, M.A.; Arzumanyan, A.; Medhat, A.; Spector, I. Short-Chain Fatty Acids in Cancer Pathogenesis. Cancer Metastasis Rev. 2023, 42, 677–698. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xie, D.; Wang, Y.; Niu, L.; Jiang, H. Short-Chain Fatty Acids Reduce Oligodendrocyte Precursor Cells Loss by Inhibiting the Activation of Astrocytes via the SGK1/IL-6 Signalling Pathway. Neurochem. Res. 2022, 47, 3476–3489. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Zhang, X.; Wang, W.; Chen, Y.; Cai, Z.; Wang, Q.; Wang, J.; Shi, Y. Promotion of Astrocyte-Neuron Glutamate-Glutamine Shuttle by SCFA Contributes to the Alleviation of Alzheimer’s Disease. Redox Biol. 2023, 62, 102690. [Google Scholar] [CrossRef]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, T.; Guo, H.; Cui, H.; Li, P.; Feng, D.; Hu, E.; Huang, Q.; Yang, A.; Zhou, J.; et al. Lactate Potentiates Angiogenesis and Neurogenesis in Experimental Intracerebral Hemorrhage. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Ichihara, Y.; Doi, T.; Ryu, Y.; Nagao, M.; Sawada, Y.; Ogata, T. Oligodendrocyte Progenitor Cells Directly Utilize Lactate for Promoting Cell Cycling and Differentiation. J. Cell Physiol. 2017, 232, 986–995. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 612, Lactic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lactic-Acid (accessed on 1 July 2024).
- Scandella, V.; Knobloch, M. Sensing the Environment: Extracellular Lactate Levels Control Adult Neurogenesis. Cell Stem Cell 2019, 25, 729–731. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Xu, J.; Pan, H.; Wang, W.; Yu, X.; Shi, S. Histone Lactylation: From Tumor Lactate Metabolism to Epigenetic Regulation. Int. J. Biol. Sci. 2024, 20, 1833–1854. [Google Scholar] [CrossRef]
- Pan, R.-Y.; He, L.; Zhang, J.; Liu, X.; Liao, Y.; Gao, J.; Liao, Y.; Yan, Y.; Li, Q.; Zhou, X.; et al. Positive Feedback Regulation of Microglial Glucose Metabolism by Histone H4 Lysine 12 Lactylation in Alzheimer’s Disease. Cell Metab. 2022, 34, 634–648.e6. [Google Scholar] [CrossRef]
- Wei, L.; Yang, X.; Wang, J.; Wang, Z.; Wang, Q.; Ding, Y.; Yu, A. H3K18 Lactylation of Senescent Microglia Potentiates Brain Aging and Alzheimer’s Disease through the NFκB Signaling Pathway. J. Neuroinflamm. 2023, 20, 208. [Google Scholar] [CrossRef]
- Han, H.; Zhao, Y.; Du, J.; Wang, S.; Yang, X.; Li, W.; Song, J.; Zhang, S.; Zhang, Z.; Tan, Y.; et al. Exercise Improves Cognitive Dysfunction and Neuroinflammation in Mice through Histone H3 Lactylation in Microglia. Immun. Ageing 2023, 20, 63. [Google Scholar] [CrossRef]
- Ji, X.; Yu, W.; Jin, M.; Lu, L.; Yin, H.; Wang, H. Possible Role of Cellular Polyamine Metabolism in Neuronal Apoptosis. Curr. Med. Sci. 2024, 44, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Sandusky-Beltran, L.A.; Kovalenko, A.; Ma, C.; Calahatian, J.I.T.; Placides, D.S.; Watler, M.D.; Hunt, J.B.; Darling, A.L.; Baker, J.D.; Blair, L.J.; et al. Spermidine/Spermine-N1-Acetyltransferase Ablation Impacts Tauopathy-Induced Polyamine Stress Response. Alzheimers Res. Ther. 2019, 11, 58. [Google Scholar] [CrossRef]
- Hirano, R.; Shirasawa, H.; Kurihara, S. Health-Promoting Effects of Dietary Polyamines. Med. Sci. 2021, 9, 8. [Google Scholar] [CrossRef]
- Noack, J.; Dongowski, G.; Hartmann, L.; Blaut, M. The Human Gut Bacteria Bacteroides Thetaiotaomicron and Fusobacterium Varium Produce Putrescine and Spermidine in Cecum of Pectin-Fed Gnotobiotic Rats. J. Nutr. 2000, 130, 1225–1231. [Google Scholar] [CrossRef]
- Arena, M.E.; Manca de Nadra, M.C. Biogenic Amine Production by Lactobacillus. J. Appl. Microbiol. 2001, 90, 158–162. [Google Scholar] [CrossRef]
- Xu, T.-T.; Li, H.; Dai, Z.; Lau, G.K.; Li, B.-Y.; Zhu, W.-L.; Liu, X.-Q.; Liu, H.-F.; Cai, W.-W.; Huang, S.-Q.; et al. Spermidine and Spermine Delay Brain Aging by Inducing Autophagy in SAMP8 Mice. Aging 2020, 12, 6401–6414. [Google Scholar] [CrossRef]
- Vijayan, B.; Raj, V.; Nandakumar, S.; Kishore, A.; Thekkuveettil, A. Spermine Protects Alpha-Synuclein Expressing Dopaminergic Neurons from Manganese-Induced Degeneration. Cell Biol. Toxicol. 2019, 35, 147–159. [Google Scholar] [CrossRef]
- Schwarz, C.; Benson, G.S.; Horn, N.; Wurdack, K.; Grittner, U.; Schilling, R.; Märschenz, S.; Köbe, T.; Hofer, S.J.; Magnes, C.; et al. Effects of Spermidine Supplementation on Cognition and Biomarkers in Older Adults With Subjective Cognitive Decline: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2213875. [Google Scholar] [CrossRef]
- Pekar, T.; Bruckner, K.; Pauschenwein-Frantsich, S.; Gschaider, A.; Oppliger, M.; Willesberger, J.; Ungersbäck, P.; Wendzel, A.; Kremer, A.; Flak, W.; et al. The Positive Effect of Spermidine in Older Adults Suffering from Dementia. Wien. Klin. Wochenschr. 2021, 133, 484–491. [Google Scholar] [CrossRef]
- Pekar, T.; Wendzel, A.; Jarisch, R. The Positive Effect of Spermidine in Older Adults Suffering from Dementia after 1 Year. Wien. Klin. Wochenschr. 2024, 136, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.; Hess, M.; Weimer, B.C. Microbial-Derived Tryptophan Metabolites and Their Role in Neurological Disease: Anthranilic Acid and Anthranilic Acid Derivatives. Microorganisms 2023, 11, 1825. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Y.; Liu, X.; Li, W.; Hu, Y.; Zhang, B.; Wang, S. Gut Microbiota-Derived Indole Derivatives Alleviate Neurodegeneration in Aging through Activating GPR30/AMPK/SIRT1 Pathway. Mol. Nutr. Food Res. 2023, 67, 2200739. [Google Scholar] [CrossRef]
- Wei, G.Z.; Martin, K.A.; Xing, P.Y.; Agrawal, R.; Whiley, L.; Wood, T.K.; Hejndorf, S.; Ng, Y.Z.; Low, J.Z.Y.; Rossant, J.; et al. Tryptophan-Metabolizing Gut Microbes Regulate Adult Neurogenesis via the Aryl Hydrocarbon Receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2021091118. [Google Scholar] [CrossRef]
- Maitre, M.; Klein, C.; Patte-Mensah, C.; Mensah-Nyagan, A.-G. Tryptophan Metabolites Modify Brain Aβ Peptide Degradation: A Role in Alzheimer’s Disease? Prog. Neurobiol. 2020, 190, 101800. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Song, X.; Wang, Z.; Jia, J.; Qing, S.; Huang, L.; Wang, Y.; Wang, S.; Ren, Z.; et al. The Intestinal Microbial Metabolite Nicotinamide N-Oxide Prevents Herpes Simplex Encephalitis via Activating Mitophagy in Microglia. Gut Microbes 2022, 14, 2096989. [Google Scholar] [CrossRef]
- Song, X.; Cao, W.; Wang, Z.; Li, F.; Xiao, J.; Zeng, Q.; Wang, Y.; Li, S.; Ye, C.; Wang, Y.; et al. Nicotinamide N-Oxide Attenuates HSV-1-Induced Microglial Inflammation through Sirtuin-1/NF-κB Signaling. Int. J. Mol. Sci. 2022, 23, 16085. [Google Scholar] [CrossRef]
- Schwarcz, R.; Foo, A.; Sathyasaikumar, K.V.; Notarangelo, F.M. The Probiotic Lactobacillus Reuteri Preferentially Synthesizes Kynurenic Acid from Kynurenine. Int. J. Mol. Sci. 2024, 25, 3679. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3744, 3-Indolepropionic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3-Indolepropionic-acid (accessed on 1 July 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 8617, Indole-3-butyric acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Indole-3-butyric-acid (accessed on 1 July 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3845, Kynurenic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Kynurenic-acid (accessed on 1 July 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 72661, Nicotinamide N-oxide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nicotinamide-N-oxide (accessed on 1 July 2024).
- Láng, L.; McArthur, S.; Lazar, A.S.; Pourtau, L.; Gaudout, D.; Pontifex, M.G.; Müller, M.; Vauzour, D. Dietary (Poly)Phenols and the Gut–Brain Axis in Ageing. Nutrients 2024, 16, 1500. [Google Scholar] [CrossRef]
- Sharma, R. Emerging Interrelationship Between the Gut Microbiome and Cellular Senescence in the Context of Aging and Disease: Perspectives and Therapeutic Opportunities. Probiotics Antimicro. Prot. 2022, 14, 648–663. [Google Scholar] [CrossRef]
- Lin, X.-H.; Ye, X.-J.; Li, Q.-F.; Gong, Z.; Cao, X.; Li, J.-H.; Zhao, S.-T.; Sun, X.-D.; He, X.-S.; Xuan, A.-G. Urolithin A Prevents Focal Cerebral Ischemic Injury via Attenuating Apoptosis and Neuroinflammation in Mice. Neuroscience 2020, 448, 94–106. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. Neuroprotective Effects of Bioavailable Polyphenol-Derived Metabolites against Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef]
- Leyrolle, Q.; Prado-Perez, L.; Layé, S. The Gut-Derived Metabolites as Mediators of the Effect of Healthy Nutrition on the Brain. Front. Nutr. 2023, 10, 1155533. [Google Scholar] [CrossRef]
- Ruotolo, R.; Minato, I.; La Vitola, P.; Artioli, L.; Curti, C.; Franceschi, V.; Brindani, N.; Amidani, D.; Colombo, L.; Salmona, M.; et al. Flavonoid-Derived Human Phenyl-γ-Valerolactone Metabolites Selectively Detoxify Amyloid-β Oligomers and Prevent Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2020, 64, e1900890. [Google Scholar] [CrossRef]
- Johnson, S.L.; Park, H.Y.; Vattem, D.A.; Grammas, P.; Ma, H.; Seeram, N.P. Equol, a Blood-Brain Barrier Permeable Gut Microbial Metabolite of Dietary Isoflavone Daidzein, Exhibits Neuroprotective Effects against Neurotoxins Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells and Caenorhabditis Elegans. Plant Foods Hum. Nutr. 2020, 75, 512–517. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5488186, Urolithin A. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/urolithin-A (accessed on 1 July 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 91469, Equol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Equol (accessed on 1 July 2024).
- National Center for Biotechnology Information. PubChem Substance Record for SID 433987232, Lactobacillus Rhamnosus GG Exopolysaccharide Hexasaccharide Repeating Unit, Source: BioCyc. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/433987232. (accessed on 7 July 2024).
- Sirin, S.; Aslim, B. Protective Effect of Exopolysaccharides from Lactic Acid Bacteria against Amyloid Beta1-42induced Oxidative Stress in SH-SY5Y Cells: Involvement of the AKT, MAPK, and NF-κB Signaling Pathway. Process Biochem. 2021, 106, 50–59. [Google Scholar] [CrossRef]
- Li, J.-Y.; Jin, M.-M.; Meng, J.; Gao, S.-M.; Lu, R.-R. Exopolysaccharide from Lactobacillus Planterum LP6: Antioxidation and the Effect on Oxidative Stress. Carbohydr. Polym. 2013, 98, 1147–1152. [Google Scholar] [CrossRef]
- Kumari, M.; Dasriya, V.L.; Nataraj, B.H.; Nagpal, R.; Behare, P.V. Lacticaseibacillus Rhamnosus-Derived Exopolysaccharide Attenuates D-Galactose-Induced Oxidative Stress and Inflammatory Brain Injury and Modulates Gut Microbiota in a Mouse Model. Microorganisms 2022, 10, 2046. [Google Scholar] [CrossRef]
- Singh, H.; Chopra, C.; Singh, H.; Malgotra, V.; Khurshid Wani, A.; Singh Dhanjal, D.; Sharma, I.; Nepovimova, E.; Alomar, S.; Singh, R.; et al. Gut-Brain Axis and Alzheimer’s Disease: Therapeutic Interventions and Strategies. J. Funct. Foods 2024, 112, 105915. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Kalluri, R. Emerging Role of Bacterial Extracellular Vesicles in Cancer. Oncogene 2020, 39, 6951–6960. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Wang, S.; Weng, W.; Jing, Y.; Su, J. Bacterial Extracellular Vesicles as Bioactive Nanocarriers for Drug Delivery: Advances and Perspectives. Bioact. Mater. 2021, 14, 169–181. [Google Scholar] [CrossRef]
- Xie, J.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. Bacterial Extracellular Vesicles: An Emerging Avenue to Tackle Diseases. Trends Microbiol. 2023, 31, 1206–1224. [Google Scholar] [CrossRef]
- Modasia, A.A.; Jones, E.J.; Martel, L.M.-P.; Louvel, H.; Couraud, P.-O.; Blackshaw, L.A.; Carding, S.R. The Use of a Multicellular in Vitro Model to Investigate Uptake and Migration of Bacterial Extracellular Vesicles Derived from the Human Gut Commensal Bacteroides Thetaiotaomicron. J. Extracell. Biol. 2023, 2, e93. [Google Scholar] [CrossRef]
- Li, M.; Lee, K.; Hsu, M.; Nau, G.; Mylonakis, E.; Ramratnam, B. Lactobacillus-Derived Extracellular Vesicles Enhance Host Immune Responses against Vancomycin-Resistant Enterococci. BMC Microbiol. 2017, 17, 66. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Z.; Lv, A.; Fan, C. How Toll-like Receptors Influence Parkinson’s Disease in the Microbiome–Gut–Brain Axis. Front. Immunol. 2023, 14, 1154626. [Google Scholar] [CrossRef]
- Heidari, A.; Yazdanpanah, N.; Rezaei, N. The Role of Toll-like Receptors and Neuroinflammation in Parkinson’s Disease. J. Neuroinflamm. 2022, 19, 135. [Google Scholar] [CrossRef]
- Frederiksen, H.R.; Haukedal, H.; Freude, K. Cell Type Specific Expression of Toll-Like Receptors in Human Brains and Implications in Alzheimer’s Disease. BioMed Res. Int. 2019, 2019, e7420189. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.-K.; Han, P.-L. Extracellular Vesicles Derived from Lactobacillus Plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice. Exp. Neurobiol. 2019, 28, 158–171. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Gao, Y.; Xu, F.; Chen, H.; Zhang, C.; Ni, X. The Activation Impact of Lactobacillus-Derived Extracellular Vesicles on Lipopolysaccharide-Induced Microglial Cell. BMC Microbiol. 2024, 24, 70. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, E.-H.; Park, S.-Y.; Park, J.-Y.; Hong, J.-H.; Kim, E.-K.; Shin, T.-S.; Kim, Y.-K.; Han, P.-L. Lactobacillus-Derived Extracellular Vesicles Counteract Aβ42-Induced Abnormal Transcriptional Changes through the Upregulation of MeCP2 and Sirt1 and Improve Aβ Pathology in Tg-APP/PS1 Mice. Exp. Mol. Med. 2023, 55, 2067–2082. [Google Scholar] [CrossRef]
- Ha, J.Y.; Seok, J.; Kim, S.-J.; Jung, H.-J.; Ryu, K.-Y.; Nakamura, M.; Jang, I.-S.; Hong, S.-H.; Lee, Y.; Lee, H.-J. Periodontitis Promotes Bacterial Extracellular Vesicle-Induced Neuroinflammation in the Brain and Trigeminal Ganglion. PLoS Pathog. 2023, 19, e1011743. [Google Scholar] [CrossRef]
- Ma, X.; Yoo, J.-W.; Shin, Y.-J.; Park, H.-S.; Son, Y.-H.; Kim, D.-H. Alleviation of Porphyromonas Gingivalis or Its Extracellular Vesicles Provoked Periodontitis and Cognitive Impairment by Lactobacillus Pentosus NK357 and Bifidobacterium Bifidum NK391. Nutrients 2023, 15, 1068. [Google Scholar] [CrossRef]
- Shao, Z.; Lu, Y.; Xing, A.; He, X.; Xie, H.; Hu, M. Effect of Outer Membrane Vesicles of Lactobacillus Pentosus on Tau Phosphorylation and CDK5-Calpain Pathway in Mice. Exp. Gerontol. 2024, 189, 112400. [Google Scholar] [CrossRef]
- Wen, X.; Dong, H.; Zou, W. The Role of Gut Microorganisms and Metabolites in Intracerebral Hemorrhagic Stroke: A Comprehensive Review. Front. Neurosci. 2024, 18, 1346184. [Google Scholar] [CrossRef]
- Ikeguchi, S.; Izumi, Y.; Kitamura, N.; Kishino, S.; Ogawa, J.; Akaike, A.; Kume, T. Inhibitory Effect of the Gut Microbial Linoleic Acid Metabolites, 10-Oxo-Trans-11-Octadecenoic Acid and 10-Hydroxy-Cis-12-Octadecenoic Acid, on BV-2 Microglial Cell Activation. J. Pharmacol. Sci. 2018, 138, 9–15. [Google Scholar] [CrossRef]
- Stachulski, A.V.; Knausenberger, T.B.-A.; Shah, S.N.; Hoyles, L.; McArthur, S. A Host–Gut Microbial Amino Acid Co-Metabolite, p-Cresol Glucuronide, Promotes Blood–Brain Barrier Integrity in Vivo. Tissue Barriers 2023, 11, 2073175. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10308378, 10-Oxo-11-octadecenoic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10-Oxo-11-octadecenoic-acid (accessed on 1 July 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 129725103, Hydroxy-cis-12-octadecenoic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydroxy-cis-12-octadecenoic-acid (accessed on 1 July 2024).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 154035, p-Cresol Glucuronide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/p-Cresol-glucuronide (accessed on 7 July 2024).
- Olesen, S.V.; Rajabi, N.; Svensson, B.; Olsen, C.A.; Madsen, A.S. An NAD+-Dependent Sirtuin Depropionylase and Deacetylase (Sir2La) from the Probiotic Bacterium Lactobacillus Acidophilus NCFM. Biochemistry 2018, 57, 3903–3915. [Google Scholar] [CrossRef]
- Sharma, R. Bioactive Food Components for Managing Cellular Senescence in Aging and Disease: A Critical Appraisal and Perspectives. PharmaNutrition 2021, 18, 100281. [Google Scholar] [CrossRef]
- Janssens, Y.; Wynendaele, E.; Verbeke, F.; Debunne, N.; Gevaert, B.; Audenaert, K.; Van DeWiele, C.; De Spiegeleer, B. Screening of Quorum Sensing Peptides for Biological Effects in Neuronal Cells. Peptides 2018, 101, 150–156. [Google Scholar] [CrossRef]
- Janssens, Y.; Debunne, N.; De Spiegeleer, A.; Wynendaele, E.; Planas, M.; Feliu, L.; Quarta, A.; Claes, C.; Van Dam, D.; De Deyn, P.P.; et al. PapRIV, a BV-2 Microglial Cell Activating Quorum Sensing Peptide. Sci. Rep. 2021, 11, 10723. [Google Scholar] [CrossRef]
- Dicks, L.M.T. How Does Quorum Sensing of Intestinal Bacteria Affect Our Health and Mental Status? Microorganisms 2022, 10, 1969. [Google Scholar] [CrossRef]
- Salman, M.K.; Abuqwider, J.; Mauriello, G. Anti-Quorum Sensing Activity of Probiotics: The Mechanism and Role in Food and Gut Health. Microorganisms 2023, 11, 793. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in Their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicro. Prot. 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M. do C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef]
- Microbiome Targeted Oral Butyrate Therapy in Gulf War Multisymptom Illness, (NCT05367245), Posted 10 May 2022. Available online: https://clinicaltrials.gov/study/NCT05367245 (accessed on 7 July 2024).
- Dalile, B.; Vervliet, B.; Bergonzelli, G.; Verbeke, K.; Van Oudenhove, L. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: A randomized, placebo-controlled trial. Neuropsychopharmacology 2020, 45, 2257–2266. [Google Scholar] [CrossRef]
- A Randomized, Double-blinded, Placebo-controlled, Parallel Pilot Study, to Assess the Effect of a Postbiotic Blend on Symptoms of Anxiety in Healthy Adults With Self-Reported Mild to Moderate Anxiety, (NCT05562739), Posted 3 October 2022. Available online: https://clinicaltrials.gov/study/NCT05562739 (accessed on 7 July 2024).
- PhytoSERM for Menopausal Hot Flashes and Sustained Brain Health: A Double-Blind, Randomized, Placebo-Controlled Phase 2 Clinical Trial, (NCT06186531), posted 2 January 2024. Available online: https://clinicaltrials.gov/study/NCT06186531 (accessed on 7 July 2024).
- PhytoSERM Efficacy to Prevent Menopause Associated Decline in Brain Metabolism and Cognition: A Double-Blind, Randomized, Placebo-Controlled Phase 2 Clinical Trial, (NCT05664477), Posted 23 December 2022. Available online: https://clinicaltrials.gov/study/NCT05664477 (accessed on 7 July 2024).
- Wilkins, H.M.; Mahnken, J.D.; Welch, P.; Bothwell, R.; Koppel, S.; Jackson, R.L.; Burns, J.M.; Swerdlow, R.H. A Mitochondrial Biomarker-Based Study of S-Equol in Alzheimer’s Disease Subjects: Results of a Single-Arm, Pilot Trial. J. Alzheimer’s Dis. 2017, 59, 291–300. [Google Scholar] [CrossRef]




| Compound | Action | References | |
|---|---|---|---|
| Postculture mixture | Postculture mixture | ||
| Cell-free supernatant from P. distasonis and M. massiliensis | neuroprotective properties, antioxidant activity, ↑ differentiation of neural cells, ↓ pro-inflammatory cytokines, preferential protection of differentiated neurons | [116] | |
| L. plantarum fermented soy milk | ↓ H2O2 cytotoxicity towards neural cells, ↑ viability, ↑ BDNF and TH, ↓ proapoptotic gene expression | [117] | |
| LAB-derived mixture | ↓ rotenone and MPP+ cytotoxicity towards neural cells, ↑ BBB restoration, inhibition of pathogenic QSP spread | [85,118,206] | |
| B. longum derived | ↑ BDNF in corticosterone-stimulated SH-SY5Y cells | [119] | |
| Short-chain fatty acids | SCFA | ||
| SCFA | antioxidant and anti-inflammatory properties, modification of intestinal microbiota composition, ↑ neural cells’ maturation and function, ↓ GSK-3β activity, ↓ Aβ and tau proteinopathies, ↓ ND pathology, ↓ cognitive deficits, ↑ adult neurogenesis, ↑ BDNF, ↑ epigenetic modificaitons, ↑ glutamate-glutamine shuttle, ↑ astrocytes’ functioning, ↑ astrocyte-neuron communication, ↓ oxidative stress damage to neurons | [122,128,129,130,131,132,134] | |
| Acetate | histone deacetylase inhibitors (HDACis), inducing chromatin remodeling change | [122,124] | |
| Propionate | histone deacetylase inhibitors (HDACis) protective action toward BBB cells, ↑ BBB integrity and homeostasis | [90,123,124] | |
| Butyrate | histone deacetylase inhibitors (HDACis) protective action toward BBB cells, ↑ BBB integrity and homeostasis, ↑ BBB restoration | [85,90,123,124] | |
| Lactate | Lactate | ||
| Lactate | neuroprotective effect, ↑ neurogenesis, but excessive accumulation ↓ neurogenesis, ↑ OPCs differentiation, ↑ histone lactylation, ↑ epigenetic modifications, metabolic pathways alterations, ↑ inflammation, ↑ NF-κB, ↑ IL-6 and IL-8, regulates the mechanism of microglia anti- or pro-inflammatory phenotype switch | [135,136,137,139,140,141,142,143,144] | |
| Polyamines | Polyamines | ||
| Acetylated amines | ↑ cognitive decline, ↑ tau fibrilization | [146] | |
| Spermidine | antioxidant, ↑ proliferation, ↑ differentiation, ↑ autophagy, ↑ neurotrophic factors, ↑ mitochondrial function, ↓ proinflammatory proteins, ↑ dementia patients’ memory performance | [147,150,154] | |
| Spermine | antioxidant, ↑ autophagy, ↑ neurotrophic factors, ↑ mitochondrial function, ↓ proinflammatory proteins, ↓ α-syn cytotoxicity towards dopaminergic neurons, ↓ manganese cytotoxicity | [150,151] | |
| Trypthophan metabolites | Trypthophan metabolites | ||
| Indoles | ↓ oxidative stress, ↓ neuroinflammation, ↓ apoptosis in neurons, ↑ neurogenesis | [157,158] | |
| indole acetic acid and indole propionic acid | ↑ GPR30/AMPK/SIRT1 pathway, ↓ neurodegeneration process, ↑ β-catenin, ↑ Neurog2, ↑ VEGF-alpha expression | [157] | |
| 5-hydroxyindole acetic acid and kynurenic acid | ↑ neprilisin, ↑ Aβ degradation, ↓ Aβ cytotoxicity | [159] | |
| Nicotinamide N-oxide | ↓ neuroinflammation, ↓ microglia polarization, ↑ anti-inflammatory microglia phenotype, ↑ mitophagy, ↓ glial pro-inflammatory cytokine release, ↑ sirtuin 1 expression | [160,161] | |
| Anthranilic acid, Hydroxykynurenine, Quinolic acid and Picolinic acid | ↑ oxidative stress, ↑ neuroinflammation, ↑ neuronal apoptosis | [156] | |
| Polyphenols | Polyphenols | ||
| Urolithin A | ↑ BBB integrity, ↓ neuroinflammation, ↑neurogenesis, ↑ microglial phagocytosis ↑ mitophagy | [90,169,170,171] | |
| Dihydroxyphenyl-γ-valerolactones | ↑ neuritogenesis | [171,172] | |
| Aryl-γ-valerolactones | ↓ amyloid-β | [171,172] | |
| Equol | ↓ microglia inflammation, ↓ LPS, 6-OHDA- and MPP+-induced cytotoxicity | [173] | |
| Exopolysaccharides | Exopolysaccharides | ||
| LAB-derived | Inhibitors of pathogenic QSP | [206] | |
| L. plantarum-derived | ↓ oxidative stress | [177] | |
| L. delbrueckii ssp. Bulgaricus B3- and L. plantarum GD2-derived | ↑ viability, ↓ oxidative stress, ↓ Aβ1-42 cytotoxicity | [178] | |
| Ram12 from L. rhamnosus | ↓ oxidative stress and pro-inflammatory markers, ↑ anti-inflammatory cytokine IL-10 | [179] | |
| Extracellular vesicles | Extracellular vesicles | ||
| L. plantarum-derived | ↑ BDNF expression, ↓ stress-induced depressive behavior | [189] | |
| L. rhamnosus-derived | ↓ microglia pro-inflammatory polarization, ↓ inflammatory response | [190] | |
| L. paracasei-derived | ↓ Aβ-induced pathology ↑ neurotrophic factors, ↑ Aβ-degrading proteases, ↑ sirtuin 1, ↓ astrogliosis, ↑ hippocampal neurogenesis, ↓ cognitive decline | [191] | |
| A. actinomycetemcomitans | ↑ pro-inflammatory cytokines, ↑ TLR4 and TLR8 | [192] | |
| P. gingivalis | ↑ neuroinflammation, implied in dementia pathogenesis | [193] | |
| AD patients’ microbiota-derived | ↑ neuroinflammation, ↑ hyperphosphorylated tau, ↑ cognitive decline | [194] | |
| Other metabolites | Other metabolites | ||
| methylamine trimethylamine N-oxide | ↑ BBB unity, ↓ inflammation, results not conclusive | [134,195] | |
| 10-oxo-trans-11-octadecenoic acid, hydroxy-cis-12-octadecenoic acid | anti-inflammatory action, ↓ nitric oxide production, ↓ ERK phosphorylation | [196] | |
| glucuronide form of p-cresol | ↑ BBB integrity, an antagonist of Toll-like receptor 4 complex | [197] | |
| QSP | various effects on CNS cells including cytotoxic, varied effect on neurite growth and differentiation, ↑ IL-6, ↑ NO, ↑ AD pathogenesis | [203,205] | |
| PapRIV | ↑ microglial cytotoxicity towards neurons | [204] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowacka, P.; Oszajca, K.; Pudlarz, A.; Szemraj, J.; Witusik-Perkowska, M. Postbiotics as Molecules Targeting Cellular Events of Aging Brain—The Role in Pathogenesis, Prophylaxis and Treatment of Neurodegenerative Diseases. Nutrients 2024, 16, 2244. https://doi.org/10.3390/nu16142244
Głowacka P, Oszajca K, Pudlarz A, Szemraj J, Witusik-Perkowska M. Postbiotics as Molecules Targeting Cellular Events of Aging Brain—The Role in Pathogenesis, Prophylaxis and Treatment of Neurodegenerative Diseases. Nutrients. 2024; 16(14):2244. https://doi.org/10.3390/nu16142244
Chicago/Turabian StyleGłowacka, Pola, Katarzyna Oszajca, Agnieszka Pudlarz, Janusz Szemraj, and Monika Witusik-Perkowska. 2024. "Postbiotics as Molecules Targeting Cellular Events of Aging Brain—The Role in Pathogenesis, Prophylaxis and Treatment of Neurodegenerative Diseases" Nutrients 16, no. 14: 2244. https://doi.org/10.3390/nu16142244







