The Combined Effects of the Most Important Dietary Patterns on the Incidence and Prevalence of Chronic Renal Failure: Results from the US National Health and Nutrition Examination Survey and Mendelian Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Dietary Scores
2.2.1. Healthy Eating Index 2020 (HEI-2020)
2.2.2. Dietary Inflammatory Index (DII)
2.2.3. Alternative Mediterranean Diet (aMed)
2.2.4. Dietary Approaches to Stop Hypertension (DASH)
2.3. The Diagnosis of CKD Staging and Kidney Dialysis
2.4. Statistical Analysis
2.5. MR Design
2.5.1. Data Source and Selection of IVs
2.5.2. MR Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Association between Dietary Scores and CKD and CKD—Very High Risk
3.3. Subgroup Analysis of Age and Gender
3.4. Associations between Various Nutrient Intakes and CKD and CKD—Very High Risk
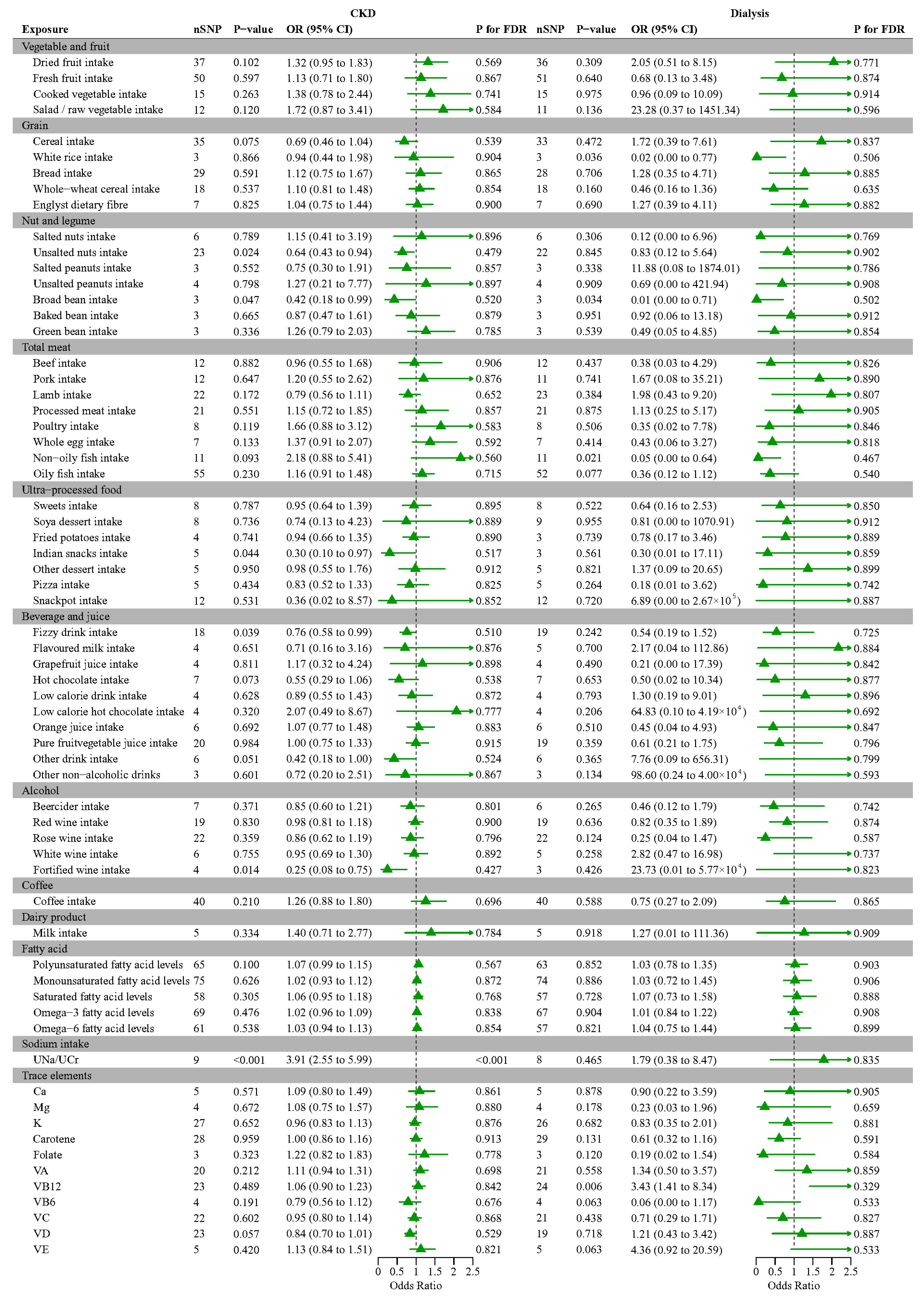
3.5. Mendelian Randomization Analysis of Various Foods and Nutrients with CKD, Dialysis
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef]
- Yamagata, K.; Ishida, K.; Sairenchi, T.; Takahashi, H.; Ohba, S.; Shiigai, T.; Narita, M.; Koyama, A. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int. 2007, 71, 159–166. [Google Scholar] [CrossRef]
- McClellan, W.M.; Flanders, W.D. Risk factors for progressive chronic kidney disease. J. Am. Soc. Nephrol. 2003, 14 (Suppl S2), S65–S70. [Google Scholar] [CrossRef]
- Rico-Fontalvo, J.; Aroca, G.; Cabrales, J.; Daza-Arnedo, R.; Yánez-Rodríguez, T.; Martínez-Ávila, M.C.; Uparella-Gulfo, I.; Raad-Sarabia, M. Molecular Mechanisms of Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 8668. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mandal, J.; Yang, T.; Cheng, X.; Yeo, J.Y.; McCarthy, C.G.; Wenceslau, C.F.; Koch, L.G.; Hill, J.W.; Vijay-Kumar, M.; et al. Metabolites and Hypertension: Insights into Hypertension as a Metabolic Disorder: 2019 Harriet Dustan Award. Hypertension 2020, 75, 1386–1396. [Google Scholar] [CrossRef]
- Chakraborty, S.; Galla, S.; Cheng, X.; Yeo, J.Y.; Mell, B.; Singh, V.; Yeoh, B.; Saha, P.; Mathew, A.V.; Vijay-Kumar, M.; et al. Salt-Responsive Metabolite, β-Hydroxybutyrate, Attenuates Hypertension. Cell Rep. 2018, 25, 677–689.e4. [Google Scholar] [CrossRef] [PubMed]
- Rinschen, M.M.; Palygin, O.; Guijas, C.; Palermo, A.; Palacio-Escat, N.; Domingo-Almenara, X.; Montenegro-Burke, R.; Saez-Rodriguez, J.; Staruschenko, A.; Siuzdak, G. Metabolic rewiring of the hypertensive kidney. Sci. Signal. 2019, 12, eaax9760. [Google Scholar] [CrossRef]
- MacLaughlin, H.L.; Friedman, A.N.; Ikizler, T.A. Nutrition in Kidney Disease: Core Curriculum 2022. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2022, 79, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Borkhanova, E.; Maksudova, A. The Assessment of Nutritional Status and Mortality in Geriatric Patients with CKD 3B-5 Stages. Nephrol. Dial. Transplant. 2021, 36, 357. [Google Scholar] [CrossRef]
- Apetrii, M.; Timofte, D.; Voroneanu, L.; Covic, A. Nutrition in Chronic Kidney Disease-The Role of Proteins and Specific Diets. Nutrients 2021, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Zarraga, I.G.; Schwarz, E.R. Impact of dietary patterns and interventions on cardiovascular health. Circulation 2006, 114, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Liu, Y.; Crews, D.C. Dietary Patterns and CKD Progression. Blood Purif. 2016, 41, 117–122. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.H.; Van Duong, T.; Nien, S.W.; Tseng, I.H.; Wu, Y.M.; Chiang, Y.J.; Wang, H.H.; Chiang, C.Y.; Wang, M.H.; Chiu, C.H.; et al. High diet quality indices associated with lower risk of lipid profile abnormalities in Taiwanese kidney transplant recipients. Sci. Rep. 2023, 13, 19662. [Google Scholar] [CrossRef]
- Zhan, J.J.; Hodge, R.A.; Dunlop, A.L.; Lee, M.M.; Bui, L.; Liang, D.; Ferranti, E.P. Dietaryindex: A User-Friendly and Versatile R Package for Standardizing Dietary Pattern Analysis in Epidemiological and Clinical Studies. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O.; Godin, J.; Cahill, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Park, Y.M.; Fung, T.T.; Rockwood, K. Relationship between diet quality scores and the risk of frailty and mortality in adults across a wide age spectrum. BMC Med. 2021, 19, 64. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Y.; Liu, Y.; Yan, Z.; Peng, W. A J-shaped association between Dietary Inflammatory Index (DII) and depression: A cross-sectional study from NHANES 2007–2018. J. Affect. Disord. 2023, 323, 257–263. [Google Scholar] [CrossRef]
- Oddo, V.M.; Welke, L.; McLeod, A.; Pezley, L.; Xia, Y.; Maki, P.; Koenig, M.D.; Kominiarek, M.A.; Langenecker, S.; Tussing-Humphreys, L. Adherence to a Mediterranean Diet Is Associated with Lower Depressive Symptoms among U.S. Adults. Nutrients 2022, 14, 278. [Google Scholar] [CrossRef]
- Ferguson, C.C.; Knol, L.L.; Ellis, A.C. Visceral adiposity index and its association with Dietary Approaches to Stop Hypertension (DASH) diet scores among older adults: National Health and Nutrition Examination Surveys 2011–2014. Clin. Nutr. 2021, 40, 4085–4089. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, W.; Yang, J.; Mao, G.; Lu, H.; Xing, W. Association between probiotic, prebiotic, and yogurt consumption and chronic kidney disease: The NHANES 2010–2020. Front. Nutr. 2022, 9, 1058238. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Friedenreich, C.M.; Yang, L. Association of Daily Sitting Time and Leisure-Time Physical Activity with Survival among US Cancer Survivors. JAMA Oncol. 2022, 8, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Huang, H.; Liu, Z.; Li, Y.; Yu, C.; Xu, L.; Xu, C. The associations between modifiable risk factors and nonalcoholic fatty liver disease: A comprehensive Mendelian randomization study. Hepatology 2023, 77, 949–964. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Wuttke, M.; Li, Y.; Li, M.; Sieber, K.B.; Feitosa, M.F.; Gorski, M.; Tin, A.; Wang, L.; Chu, A.Y.; Hoppmann, A.; et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat. Genet. 2019, 51, 957–972. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Haycock, P.C.; Sanderson, E.; Gaunt, T.R.; Zheng, J.; Morris, A.P.; Davey Smith, G.; Hemani, G. Exploiting horizontal pleiotropy to search for causal pathways within a Mendelian randomization framework. Nat. Commun. 2020, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.L.; Kuchenbaecker, K.B.; Michailidou, K.; Beesley, J.; Kar, S.; Lindström, S.; Hui, S.; Lemaçon, A.; Soucy, P.; Dennis, J.; et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat. Genet. 2017, 49, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B-Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bach, K.E.; Kelly, J.T.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.M.; Campbell, K.L. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2019, 14, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Coresh, J.; Anderson, C.A.M.; Appel, L.J.; Grams, M.E.; Crews, D.C.; Mills, K.T.; He, J.; Scialla, J.; Rahman, M.; et al. Adherence to Healthy Dietary Patterns and Risk of CKD Progression and All-Cause Mortality: Findings From the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2021, 77, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Robinson-Cohen, C.; Ellis, C.; Headley, S.A.E.; Tuttle, K.; Wood, R.J.; Evans, E.E.; Milch, C.M.; Moody, K.A.; Germain, M.; et al. Metabolic Effects of Diet and Exercise in Patients with Moderate to Severe CKD: A Randomized Clinical Trial. J. Am. Soc. Nephrol. 2018, 29, 250–259. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Sukhato, K.; Akksilp, K.; Dellow, A.; Vathesatogkit PAnothaisintawee, T. Efficacy of different dietary patterns on lowering of blood pressure level: An umbrella review. Am. J. Clin. Nutr. 2020, 112, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- McMahon, E.J.; Bauer, J.D.; Hawley, C.M.; Isbel, N.M.; Stowasser, M.; Johnson, D.W.; Campbell, K.L. A randomized trial of dietary sodium restriction in CKD. J. Am. Soc. Nephrol. 2013, 24, 2096–2103. [Google Scholar] [CrossRef]
- He, J.; Mills, K.T.; Appel, L.J.; Yang, W.; Chen, J.; Lee, B.T.; Rosas, S.E.; Porter, A.; Makos, G.; Weir, M.R.; et al. Urinary Sodium and Potassium Excretion and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutiérrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2014, 99, 320–327. [Google Scholar] [CrossRef]
- Mazzaferro, S.; de Martini, N.; Cannata-Andía, J.; Cozzolino, M.; Messa, P.; Rotondi, S.; Tartaglione, L.; Pasquali, M.; ERA-EDTA CKD-MBD Working Group. Focus on the possible role of dietary sodium, potassium, phosphate, magnesium, and calcium on CKD progression. J. Clin. Med. 2021, 10, 958. [Google Scholar] [CrossRef]
- Qu, S.; Fang, J.; Zhao, S.; Wang, Y.; Gao, W.; Li, Z.; Xu, H.; Zhang, Y.; Shi, S.; Cheng, X.; et al. Associations of dietary inflammatory index with low estimated glomerular filtration rate, albuminuria and chronic kidney disease in U.S adults: Results from the NHANES 2011–2018. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Kalantar-Zadeh, K.; Chauveau, P.; Carrero, J.J. Risks and Benefits of Different Dietary Patterns in CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2023, 81, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, P.; Aparicio, M.; Bellizzi, V.; Campbell, K.; Hong, X.; Johansson, L.; Kolko, A.; Molina, P.; Sezer, S.; Wanner, C.; et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2018, 33, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease—Potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Zanetti, M.; Gortan Cappellari, G.; Barbetta, D.; Semolic, A.; Barazzoni, R. Omega 3 polyunsaturated fatty acids improve endothelial dysfunction in chronic renal failure: Role of eNOS activation and of oxidative stress. Nutrients 2017, 9, 895. [Google Scholar] [CrossRef]
- Gupta, K.L.; Sahni, N. Dietary antioxidents and oxidative stress in predialysis chronic kidney disease patients. J. Nephropathol. 2012, 1, 134. [Google Scholar]
- Yu, M.K.; Lyles, C.R.; Bent-Shaw, L.A.; Young, B.A. Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: The pathways study. Am. J. Nephrol. 2012, 36, 245–251. [Google Scholar] [CrossRef]
- Giandalia, A.; Giuffrida, A.E.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G.T. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int. J. Mol. Sci. 2021, 22, 5808. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Kim, Y.; Lee, Y.; Kang, M.W.; Kim, K.; Kim, Y.C.; Han, S.S.; Lee, H.; Lee, J.P.; et al. Causal effects of physical activity or sedentary behaviors on kidney function: An integrated population-scale observational analysis and Mendelian randomization study. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2022, 37, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rutkowski, B.; Dębska-Ślizień, A. Vitamins and Microelement Bioavailability in Different Stages of Chronic Kidney Disease. Nutrients 2017, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Zununi Vahed, S.; Hejazian, S.M.; Ardalan, M.; Anagnostou, F.; Pavon-Djavid, G.; Barzegari, A. The impacts of dietary antioxidants on cardiovascular events in hemodialysis patients: An update on the cellular and molecular mechanisms. Nutr. Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Xie, C.; Wang, S.; Xiao, X. Retinol intake is associated with the risk of chronic kidney disease in individuals with type 2 diabetes mellitus: Results from NHANES. Sci. Rep. 2023, 13, 11567. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Tanaka, S.; Tsuruya, K.; Kitazono, T. Low intake of β carotene and dietary fiber from vegetables and fruits in patients with chronic kidney disease. Sci. Rep. 2022, 12, 19953. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Mondragon, E.; Molina-Jijon, E.; Namorado-Tonix, C.; Rodríguez-Muñoz, R.; Pedraza-Chaverri, J.; Reyes, J.L. All-trans retinoic acid ameliorates inflammatory response mediated by TLR4/NF-κB during initiation of diabetic nephropathy. J. Nutr. Biochem. 2018, 60, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Koay, Y.Y.; Tan, G.C.J.; Phang, S.C.W.; Ho, J.I.; Chuar, P.F.; Ho, L.S.; Ahmad, B.; Abdul Kadir, K. A phase IIb randomized controlled trial investigating the effects of tocotrienol-rich vitamin E on diabetic kidney disease. Nutrients 2021, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Huo, Y.; Langman, C.B.; Hou, F.; Chen, Y.; Matossian, D.; Xu, X.; Wang, X. Folic acid therapy and cardiovascular disease in ESRD or advanced chronic kidney disease: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2011, 6, 482–488. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, W.C.; Hsiao, Y.H.; Huang, S.C.; Huang, Y.C. High homocysteine, low vitamin B-6, and increased oxidative stress are independently associated with the risk of chronic kidney disease. Nutrition 2016, 32, 236–241. [Google Scholar] [CrossRef]
- Xu, X.; Qin, X.; Li, Y.; Sun, D.; Wang, J.; Liang, M.; Wang, B.; Huo, Y.; Hou, F.F. Efficacy of folic acid therapy on the progression of chronic kidney disease: The renal substudy of the China Stroke Primary Prevention Trial. JAMA Intern. Med. 2016, 176, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- House, A.A.; Eliasziw, M.; Cattran, D.C.; Churchill, D.N.; Oliver, M.J.; Fine, A.; Dresser, G.K.; Spence, J.D. Effect of B-Vitamin Therapy on Progression of Diabetic Nephropathy: A Randomized Controlled Trial. JAMA 2010, 303, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Felsenfeld, A.J.; Levine, B.S.; Rodriguez, M. Pathophysiology of calcium, phosphorus, and magnesium dysregulation in chronic kidney disease. Semin. Dial. 2015, 28, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Trivedi, B.K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2006, 1, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Potassium and health. Adv. Nutr. 2013, 4, 368S–377S. [Google Scholar] [CrossRef] [PubMed]
- Shamseddin MK Parfrey, P.S. Mechanisms of the cardiorenal syndromes. Nat. Rev. Nephrol. 2009, 5, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Rossi, M.; Johnson, D.W.; Campbell, K.L. Beyond Sodium, Phosphate and Potassium: Potential Dietary Interventions in Kidney Disease. Semin. Dial. 2017, 30, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Crews, D.C.; Grams, M.E.; Steffen, L.M.; Levey, A.S.; Miller, E.R., 3rd; Appel, L.J.; Coresh, J. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2016, 68, 853–861. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Cordaro, M.; Fusco, R.; Peritore, A.F.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Mandalari, G.; Caccamo, D.; Cuzzocrea, S.; et al. Consumption of Cashew (Anacardium occidentale L.) Nuts Counteracts Oxidative Stress and Tissue Inflammation in Mild Hyperhomocysteinemia in Rats. Nutrients 2022, 14, 1474. [Google Scholar] [CrossRef]
- Aukema, H.M.; Gauthier, J.; Roy, M.; Jia, Y.; Li, H.; Aluko, R.E. Distinctive effects of plant protein sources on renal disease progression and associated cardiac hypertrophy in experimental kidney disease. Mol. Nutr. Food Res. 2011, 55, 1044–1051. [Google Scholar] [CrossRef]
- Gallieni, M.; Cupisti, A. DASH and Mediterranean diets as nutritional interventions for CKD patients. Am. J. Kidney Dis. 2016, 68, 828–830. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Total (N = 25,167) | Non-CKD (N = 21,006) | CKD (N = 4161) | p-Value |
|---|---|---|---|---|
| Age, years, mean (SD) | 49.2 (17.8) | 46.6 (16.9) | 62.3 (16.6) | <0.001 |
| Alcohol consumption, gram, mean (SD) | 25.1 (34.9) | 26.4 (35.4) | 18.6 (31.5) | <0.001 |
| Body Mass Index, kg/m2, mean (SD) | 29.4 (7.1) | 29.2 (7.0) | 30.5 (7.5) | <0.001 |
| eGFR, mL/min/1.73 m2, mean (SD) | 96.2 (24.3) | 100.6 (20.0) | 73.5 (30.6) | <0.001 |
| Albumin creatinine ratio, mg/g, mean (SD) | 42.9 (338.9) | 8.1 (5.6) | 810.9 (810.9) | <0.001 |
| Gender, n (%) | 0.025 | |||
| Male | 12,150 (48.3) | 10,207 (48.6) | 1943 (46.7) | |
| Female | 13,017 (51.7) | 10,799 (51.4) | 2218 (53.3) | |
| Ethnicity, n (%) | <0.001 | |||
| Mexican American | 3549 (14.1) | 3034 (14.4) | 515 (12.4) | |
| Other Hispanic | 2509 (10.0) | 2178 (10.4) | 331 (8.0) | |
| Non-Hispanic White | 11,035 (43.9) | 9018 (42.9) | 2017 (48.5) | |
| Non-Hispanic Black | 5309 (21.1) | 4352 (20.7) | 957 (23.0) | |
| Other Race—Including Multi-Racial | 2765 (11.0) | 2424 (11.5) | 341 (8.2) | |
| Education level, n (%) | <0.001 | |||
| Less than high school | 5476 (21.8) | 4323 (20.6) | 1153 (27.7) | |
| High school or above | 19,691 (78.2) | 16,683 (79.4) | 3008 (72.3) | |
| Poverty status, n (%) | <0.001 | |||
| ≤1.30 | 7797 (31.0) | 6402 (30.5) | 1395 (33.5) | |
| 1.30–3.50 | 9441 (37.5) | 7692 (36.6) | 1749 (42.0) | |
| >3.50 | 7929 (31.5) | 6912 (32.9) | 1017 (24.4) | |
| Smoking status, n (%) | <0.001 | |||
| Yes | 10,915 (43.4) | 8857 (42.2) | 2058 (49.5) | |
| No | 14,252 (56.63) | 12,149 (57.8) | 2103 (50.5) | |
| Leisure time physical activity, n (%) | <0.001 | |||
| Adequate | 8606 (34.2) | 7697 (36.6) | 909 (21.9) | |
| Inadequate | 16,561 (65.8) | 13,309 (63.4) | 3252 (78.2) | |
| History of diabetes, n (%) | <0.001 | |||
| Yes | 3267 (13.0) | 1911 (9.1) | 1356 (32.6) | |
| No | 21,900 (87.0) | 19,095 (90.9) | 2805 (67.4) | |
| History of hypertension, n (%) | <0.001 | |||
| Yes | 7011 (27.9) | 4983 (23.7) | 2028 (48.7) | |
| No | 12,581 (50.0) | 11,422 (54.4) | 1159 (28.9) | |
| Unknown or Missing | 5575 (22.2) | 4601 (21.9) | 974 (23.4) | |
| History of cardiovascular disease, n (%) | <0.001 | |||
| Yes | 2048 (8.1) | 1164 (5.5) | 884 (21.2) | |
| No | 17,181 (68.3) | 14,901 (70.9) | 2280 (54.8) | |
| Unknown or Missing | 5938 (23.6) | 4941 (23.5) | 997 (24.0) |
| Dietary Patterns | CKD | CKD—Very High Risk | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| HEI-2020 | ||||||||||||
| Q1 (<42.523) | Reference | Reference | Reference | Reference | ||||||||
| Q2 (42.523–50.483) | 0.88 | (0.76, 1.02) | 0.082 | 0.93 | (0.80, 1.08) | 0.359 | 0.74 | (0.53, 1.03) | 0.070 | 0.76 | (0.54, 1.05) | 0.098 |
| Q3 (50.483–59.475) | 0.79 | (0.69, 0.91) | 0.002 | 0.91 | (0.78, 1.07) | 0.248 | 0.58 | (0.41, 0.80) | 0.001 | 0.66 | (0.47, 0.93) | 0.019 |
| Q4 (≥59.475) | 0.64 | (0.55, 0.74) | <0.001 | 0.78 | (0.67, 0.91) | 0.002 | 0.37 | (0.26, 0.55) | <0.001 | 0.46 | (0.30, 0.71) | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| DII | ||||||||||||
| Q1 (<−0.019) | Reference | Reference | Reference | Reference | ||||||||
| Q2 (−0.019–1.337) | 1.24 | (1.06, 1.44) | 0.008 | 1.14 | (0.97, 1.34) | 0.111 | 1.45 | (0.96, 2.20) | 0.077 | 1.25 | (0.83, 1.88) | 0.293 |
| Q3 (1.337–2.482) | 1.45 | (1.26, 1.66) | <0.001 | 1.26 | (1.08, 1.46) | 0.003 | 2.45 | (1.69, 3.57) | <0.001 | 2.04 | (1.38, 3.03) | <0.001 |
| Q4 (≥2.482) | 1.91 | (1.66, 2.19) | <0.001 | 1.56 | (1.34, 1.82) | <0.001 | 2.98 | (2.07, 4.28) | <0.001 | 2.28 | (1.56, 3.33) | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| aMed | ||||||||||||
| Q1 (<5) | Reference | Reference | Reference | Reference | ||||||||
| Q2 (5–5.5) | 0.94 | (0.82, 1.08) | 0.385 | 1.01 | (0.88, 1.17) | 0.869 | 0.89 | (0.61, 1.30) | 0.547 | 0.92 | (0.62, 1.38) | 0.690 |
| Q3 (5.5–6.5) | 0.85 | (0.74, 0.97) | 0.018 | 0.94 | (0.83, 1.07) | 0.361 | 0.58 | (0.43, 0.79) | <0.001 | 0.61 | (0.44, 0.85) | 0.004 |
| Q4 (≥6.5) | 0.64 | (0.56, 0.74) | <0.001 | 0.81 | (0.70, 0.93) | 0.004 | 0.33 | (0.22, 0.49) | <0.001 | 0.41 | (0.26, 0.64) | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| DASH | ||||||||||||
| Q1 (<2.633) | Reference | Reference | Reference | Reference | ||||||||
| Q2 (2.633–3.405) | 0.98 | (0.83, 1.15) | 0.784 | 1.03 | (0.87, 1.22) | 0.726 | 0.99 | (0.72, 1.36) | 0.938 | 1.01 | (0.711.43) | 0.964 |
| Q3 (3.405–4.303) | 0.89 | (0.79, 1.00) | 0.048 | 0.99 | (0.87, 1.12) | 0.840 | 0.88 | (0.65, 1.21) | 0.435 | 1.01 | (0.71, 1.44) | 0.940 |
| Q4 (≥4.303) | 0.82 | (0.72, 0.94) | 0.004 | 0.94 | (0.81, 1.08) | 0.366 | 0.63 | (0.44, 0.88) | 0.008 | 0.73 | (0.50, 1.06) | 0.096 |
| p for trend | <0.001 | 0.163 | 0.012 | 0.170 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Xu, S.; Wan, T.; Wang, X.; Jiang, S.; Shi, W.; Ma, S.; Wang, H. The Combined Effects of the Most Important Dietary Patterns on the Incidence and Prevalence of Chronic Renal Failure: Results from the US National Health and Nutrition Examination Survey and Mendelian Analyses. Nutrients 2024, 16, 2248. https://doi.org/10.3390/nu16142248
Huang Y, Xu S, Wan T, Wang X, Jiang S, Shi W, Ma S, Wang H. The Combined Effects of the Most Important Dietary Patterns on the Incidence and Prevalence of Chronic Renal Failure: Results from the US National Health and Nutrition Examination Survey and Mendelian Analyses. Nutrients. 2024; 16(14):2248. https://doi.org/10.3390/nu16142248
Chicago/Turabian StyleHuang, Yanqiu, Shiyu Xu, Tingya Wan, Xiaoyu Wang, Shuo Jiang, Wentao Shi, Shuai Ma, and Hui Wang. 2024. "The Combined Effects of the Most Important Dietary Patterns on the Incidence and Prevalence of Chronic Renal Failure: Results from the US National Health and Nutrition Examination Survey and Mendelian Analyses" Nutrients 16, no. 14: 2248. https://doi.org/10.3390/nu16142248
APA StyleHuang, Y., Xu, S., Wan, T., Wang, X., Jiang, S., Shi, W., Ma, S., & Wang, H. (2024). The Combined Effects of the Most Important Dietary Patterns on the Incidence and Prevalence of Chronic Renal Failure: Results from the US National Health and Nutrition Examination Survey and Mendelian Analyses. Nutrients, 16(14), 2248. https://doi.org/10.3390/nu16142248







