Effects of Short-Term Gluten-Free Diet on Cardiovascular Biomarkers and Quality of Life in Healthy Individuals: A Prospective Interventional Study
Abstract
:1. Introduction
2. Materials and Methods
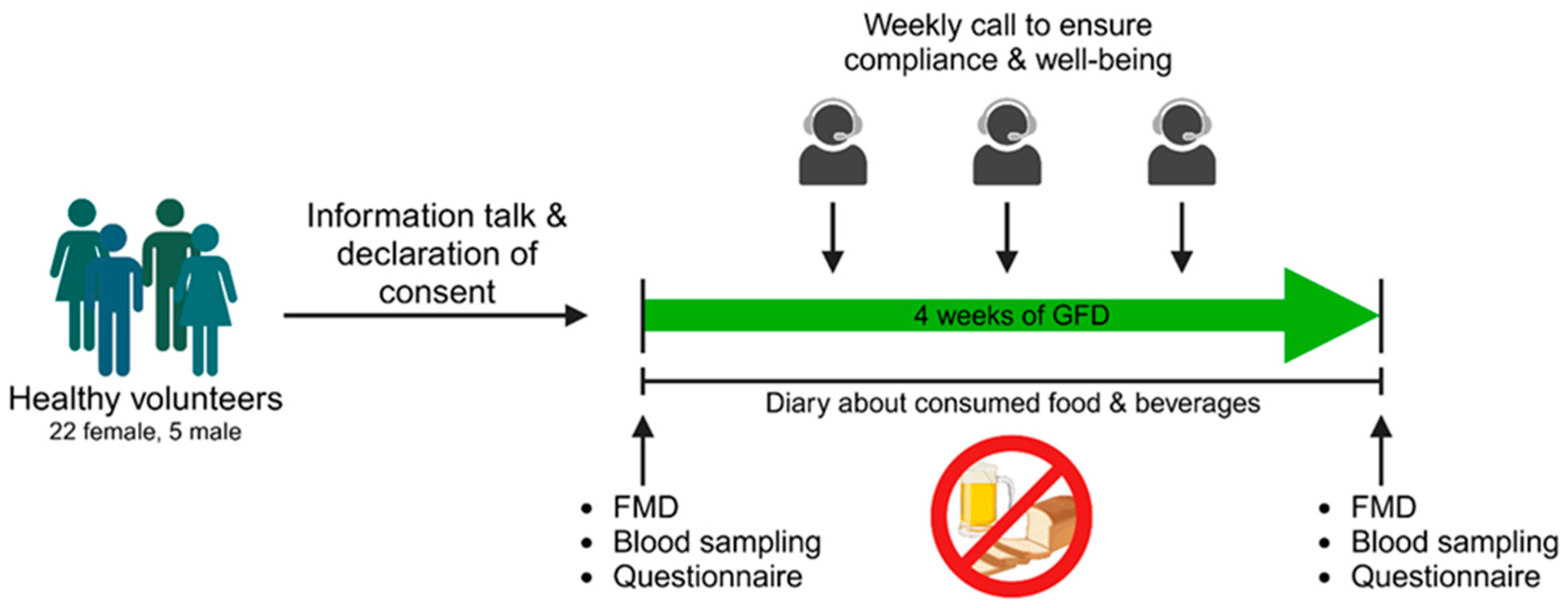
2.1. Study Procedures
2.2. Ethical Approval and Subjects
2.3. Functional, Biochemical, and Clinical Chemistry Parameters
2.4. Plasma Proteomics
2.5. Statistical Analysis
3. Results
3.1. Functional Parameters
3.2. Biochemical and Clinical Chemistry Parameters
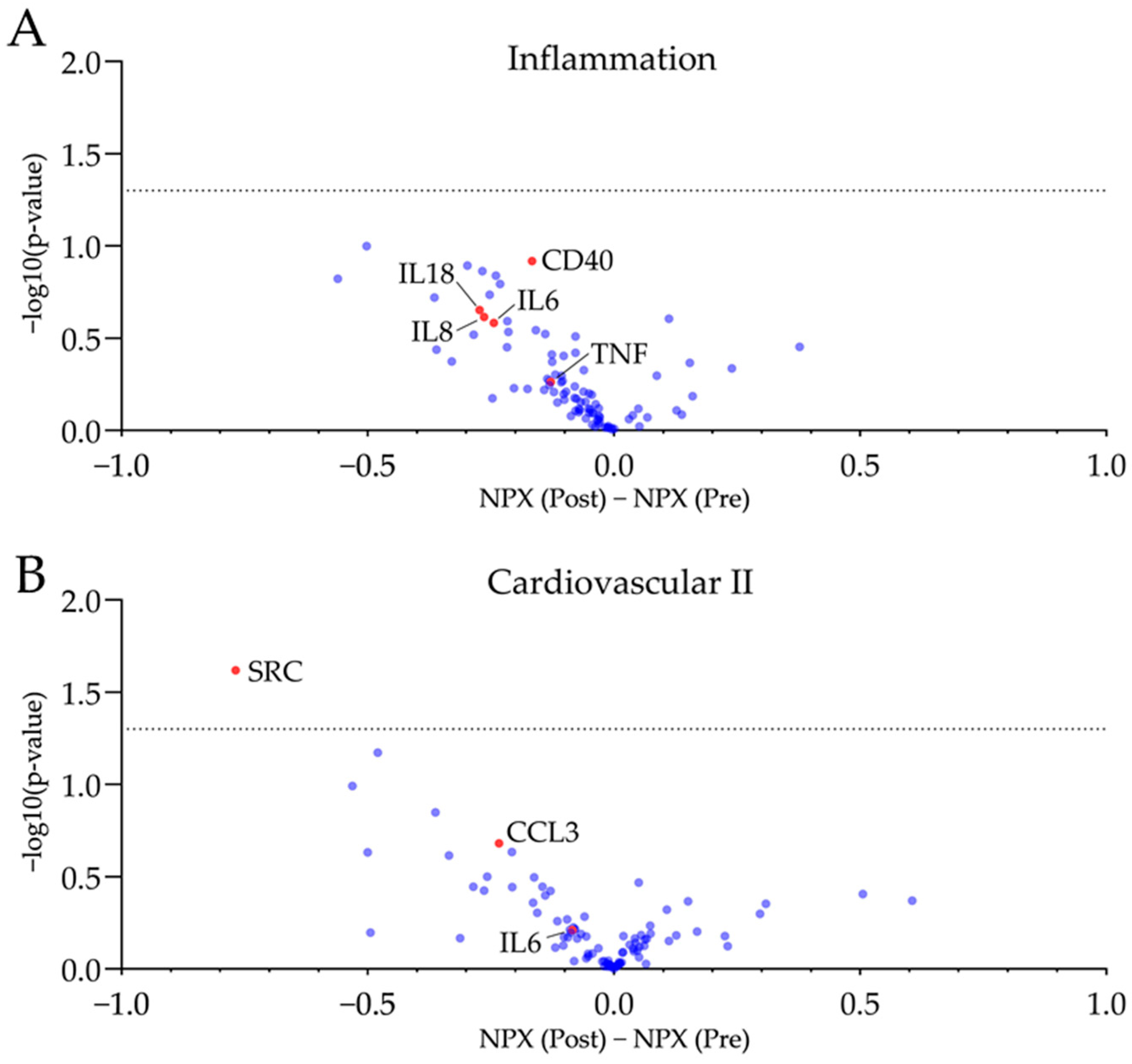
3.3. Plasma Proteomics
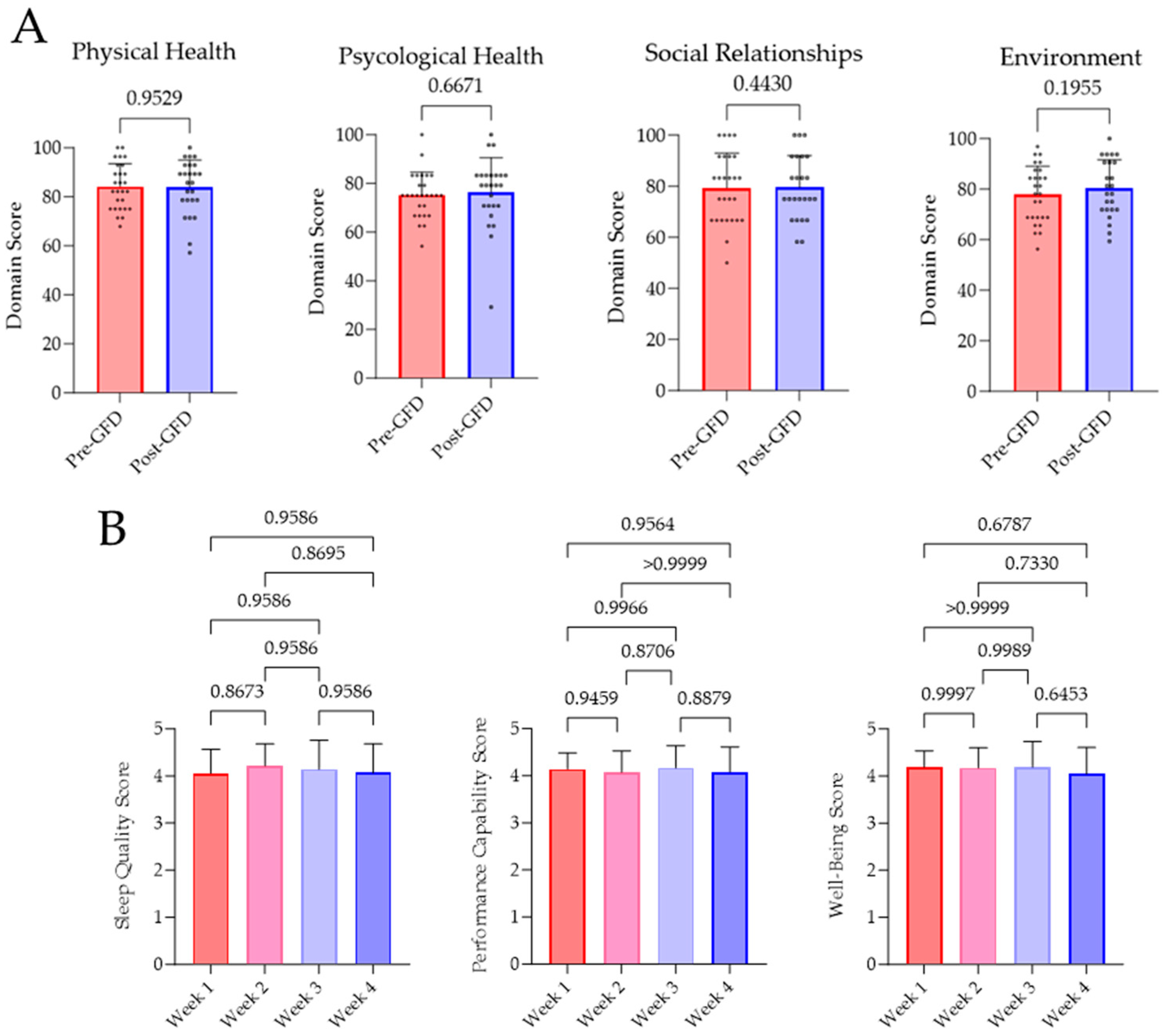
3.4. Quality of Life Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hahad, O.; Al-Kindi, S.; Lelieveld, J.; Munzel, T.; Daiber, A. Supporting and implementing the beneficial parts of the exposome: The environment can be the problem, but it can also be the solution. Int. J. Hyg. Environ. Health 2024, 255, 114290. [Google Scholar] [CrossRef]
- Munzel, T.; Sorensen, M.; Hahad, O.; Nieuwenhuijsen, M.; Daiber, A. The contribution of the exposome to the burden of cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 651–669. [Google Scholar] [CrossRef]
- Vermeulen, R.; Schymanski, E.L.; Barabasi, A.L.; Miller, G.W. The exposome and health: Where chemistry meets biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef]
- Barber, C.; Mego, M.; Sabater, C.; Vallejo, F.; Bendezu, R.A.; Masihy, M.; Guarner, F.; Espin, J.C.; Margolles, A.; Azpiroz, F. Differential Effects of Western and Mediterranean-Type Diets on Gut Microbiota: A Metagenomics and Metabolomics Approach. Nutrients 2021, 13, 2638. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Gueant, J.L.; Gueant-Rodriguez, R.M.; Alpers, D.H. Vitamin B12 absorption and malabsorption. Vitam. Horm. 2022, 119, 241–274. [Google Scholar] [CrossRef]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; James, S.; Askling, J.; Stenestrand, U.; Ingelsson, E. Nationwide cohort study of risk of ischemic heart disease in patients with celiac disease. Circulation 2011, 123, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Sari, C.; Bayram, N.A.; Dogan, F.E.; Bastug, S.; Bolat, A.D.; Sari, S.O.; Ersoy, O.; Bozkurt, E. The evaluation of endothelial functions in patients with celiac disease. Echocardiography 2012, 29, 471–477. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The structure and properties of gluten: An elastic protein from wheat grain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 133–142. [Google Scholar] [CrossRef]
- El Hassouni, K.; Sielaff, M.; Curella, V.; Neerukonda, M.; Leiser, W.; Wurschum, T.; Schuppan, D.; Tenzer, S.; Longin, C.F.H. Genetic architecture underlying the expression of eight alpha-amylase trypsin inhibitors. Theor. Appl. Genet. 2021, 134, 3427–3441. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Schuppan, D.; Rojas Tovar, L.E.; Zevallos, V.F.; Loponen, J.; Ganzle, M. Sourdough Fermentation Degrades Wheat Alpha-Amylase/Trypsin Inhibitor (ATI) and Reduces Pro-Inflammatory Activity. Foods 2020, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq-Khan, M.; Aslam, M.; Qureshi, M.A.; Senkowski, M.S.; Yen-Weng, S.; Strand, S.; Kim, Y.O.; Pickert, G.; Schattenberg, J.M.; Schuppan, D. Dietary wheat amylase trypsin inhibitors promote features of murine non-alcoholic fatty liver disease. Sci. Rep. 2019, 9, 17463. [Google Scholar] [CrossRef] [PubMed]
- Pickert, G.; Wirtz, S.; Matzner, J.; Ashfaq-Khan, M.; Heck, R.; Rosigkeit, S.; Thies, D.; Surabattula, R.; Ehmann, D.; Wehkamp, J.; et al. Wheat Consumption Aggravates Colitis in Mice via Amylase Trypsin Inhibitor-mediated Dysbiosis. Gastroenterology 2020, 159, 257–272.e217. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Russel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113.e1112. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Vegh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75.e65. [Google Scholar] [CrossRef]
- Algera, J.P.; Magnusson, M.K.; Ohman, L.; Storsrud, S.; Simren, M.; Tornblom, H. Randomised controlled trial: Effects of gluten-free diet on symptoms and the gut microenvironment in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2022, 56, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Perrin, L.; Alles, B.; Buscail, C.; Ravel, C.; Hercberg, S.; Julia, C.; Kesse-Guyot, E. Gluten-free diet in French adults without coeliac disease: Sociodemographic characteristics, motives and dietary profile. Br. J. Nutr. 2019, 122, 231–239. [Google Scholar] [CrossRef]
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public Health Nutr. 2015, 18, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Sinning, C.; Post, F.; Warnholtz, A.; Schulz, E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Ostad, M.A.; Eggeling, S.; Tschentscher, P.; Schwedhelm, E.; Boger, R.; Wenzel, P.; Meinertz, T.; Munzel, T.; Warnholtz, A. Flow-mediated dilation in patients with coronary artery disease is enhanced by high dose atorvastatin compared to combined low dose atorvastatin and ezetimibe: Results of the CEZAR study. Atherosclerosis 2009, 205, 227–232. [Google Scholar] [CrossRef] [PubMed]
- The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, E.J.; Lewis, S.K.; Biviano, A.B.; Iyer, V.; Garan, H.; Green, P.H. Cardiovascular involvement in celiac disease. World J. Cardiol. 2017, 9, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Green, P.H.R.; Soderling, J.; Roelstraete, B.; Ludvigsson, J.F. Association Between Celiac Disease and Mortality Risk in a Swedish Population. JAMA 2020, 323, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Gastroenterol Hepatol (N Y) 2018, 14, 82–91. [Google Scholar]
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: Prospective cohort study. BMJ 2017, 357, j1892. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Rimm, E.; Manson, J.E.; Hennekens, C.H.; Willett, W.C. Whole-grain consumption and risk of coronary heart disease: Results from the Nurses’ Health Study. Am. J. Clin. Nutr. 1999, 70, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Mellen, P.B.; Walsh, T.F.; Herrington, D.M. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 283–290. [Google Scholar] [CrossRef] [PubMed]
- de Munter, J.S.; Hu, F.B.; Spiegelman, D.; Franz, M.; van Dam, R.M. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007, 4, e261. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Van Hee, V.F.; Piront, N.; De Backer, F.; Toussaint, O.; Cani, P.D.; Delzenne, N.M. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr. Diabetes 2012, 2, e28. [Google Scholar] [CrossRef] [PubMed]
- Tojo, R.; Suarez, A.; Clemente, M.G.; de los Reyes-Gavilan, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H.M.; Renaula, T.L.; Garioch, J.J.; Leonard, J.N.; Fry, J.S.; Collin, P.; Evans, D.; Fry, L. Protective effect of gluten-free diet against development of lymphoma in dermatitis herpetiformis. Br. J. Dermatol. 1996, 135, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Elkan, A.C.; Sjoberg, B.; Kolsrud, B.; Ringertz, B.; Hafstrom, I.; Frostegard, J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: A randomized study. Arthritis Res. Ther. 2008, 10, R34. [Google Scholar] [CrossRef] [PubMed]
- Bosi, E.; Pastore, M.R.; Molteni, L.; Bazzigaluppi, E.; Bonifacio, E.; Piemonti, L. Gluten-free diet in subjects at risk for type 1 diabetes: A tool for delaying progression to clinical disease? Adv. Exp. Med. Biol. 2005, 569, 157–158. [Google Scholar] [CrossRef]
- Martin, J.; Geisel, T.; Maresch, C.; Krieger, K.; Stein, J. Inadequate nutrient intake in patients with celiac disease: Results from a German dietary survey. Digestion 2013, 87, 240–246. [Google Scholar] [CrossRef]
- Babio, N.; Alcazar, M.; Castillejo, G.; Recasens, M.; Martinez-Cerezo, F.; Gutierrez-Pensado, V.; Masip, G.; Vaque, C.; Vila-Marti, A.; Torres-Moreno, M.; et al. Patients With Celiac Disease Reported Higher Consumption of Added Sugar and Total Fat Than Healthy Individuals. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 63–69. [Google Scholar] [CrossRef]
- Larretxi, I.; Simon, E.; Benjumea, L.; Miranda, J.; Bustamante, M.A.; Lasa, A.; Eizaguirre, F.J.; Churruca, I. Gluten-free-rendered products contribute to imbalanced diets in children and adolescents with celiac disease. Eur. J. Nutr. 2019, 58, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Hopman, E.G.; le Cessie, S.; von Blomberg, B.M.; Mearin, M.L. Nutritional management of the gluten-free diet in young people with celiac disease in The Netherlands. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 102–108. [Google Scholar] [CrossRef]
- Partula, V.; Deschasaux, M.; Druesne-Pecollo, N.; Latino-Martel, P.; Desmetz, E.; Chazelas, E.; Kesse-Guyot, E.; Julia, C.; Fezeu, L.K.; Galan, P.; et al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020, 112, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Lindsay, J.O.; Fromentin, S.; Stagg, A.J.; McCarthy, N.E.; Galleron, N.; Ibraim, S.B.; Roume, H.; Levenez, F.; Pons, N.; et al. Effects of Low FODMAP Diet on Symptoms, Fecal Microbiome, and Markers of Inflammation in Patients With Quiescent Inflammatory Bowel Disease in a Randomized Trial. Gastroenterology 2020, 158, 176–188.e177. [Google Scholar] [CrossRef] [PubMed]
- Nordin, E.; Hellstrom, P.M.; Dicksved, J.; Pelve, E.; Landberg, R.; Brunius, C. Effects of FODMAPs and Gluten on Gut Microbiota and Their Association with the Metabolome in Irritable Bowel Syndrome: A Double-Blind, Randomized, Cross-Over Intervention Study. Nutrients 2023, 15, 3045. [Google Scholar] [CrossRef]
- Algera, J.P.; Demir, D.; Tornblom, H.; Nybacka, S.; Simren, M.; Storsrud, S. Low FODMAP diet reduces gastrointestinal symptoms in irritable bowel syndrome and clinical response could be predicted by symptom severity: A randomized crossover trial. Clin. Nutr. 2022, 41, 2792–2800. [Google Scholar] [CrossRef]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Yogev, N.; Hauptmann, J.; Nikolaev, A.; Pickert, G.; Heib, V.; Fittler, N.; Steven, S.; Luessi, F.; Neerukonda, M.; et al. Dietary wheat amylase trypsin inhibitors exacerbate CNS inflammation in experimental multiple sclerosis. Gut 2023, 73, 92–104. [Google Scholar] [CrossRef]
| Pre GFD (t0) | Post GFD (t1) | Significance Level | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | n.s. p > 0.05 * p < 0.05 | |
| Age (years) | 26.97 ± 4.99 | ||
| Gender | 22 female. 5 male | ||
| Height (cm) | 170.74 ± 7.40 | ||
| BMI (kg/m2) | 22.60 ± 2.60 | 22.20 ± 2.33 | n.s., p = 0.3019 |
| Heart Rate (bpm) | 64.07 ± 7.24 | 68.44 ± 9.72 | n.s., p = 0.1046 |
| Blood pressure | |||
| Systolic BP (mmHg) | 121.52 ± 9.60 | 124.00 ± 10.30 | n.s., p = 0.3118 |
| Diastolic BP (mmHg) | 77.55 ± 6.95 | 78.00 ± 7.63 | n.s., p = 0.7966 |
| Complete Blood Count | |||
| Erythrocytes (/pl) | 4.45 ± 0.38 | 4.45 ± 0.35 | n.s., p = 0.7182 |
| Haematocrit (%) | 39.08 ± 2.81 | 39.25 ± 2.37 | n.s., p = 0.8470 |
| Haemoglobin (g/L) | 13.13 ± 1.01 | 13.21 ± 0.85 | n.s., p = 0.9195 |
| MCV (fl) | 87.95 ± 3.27 | 88.52 ± 3.12 | n.s., p = 0.6196 |
| MCH (pg) | 29.55 ± 1.33 | 29.78 ± 1.20 | n.s., p = 0.4450 |
| MCHC (g/dL) | 33.58 ± 0.84 | 33.66 ± 0.83 | n.s., p = 0.7030 |
| Thrombocytes (/nL) | 224.89 ± 50.33 | 210.65 ± 41.22 | n.s., p = 0.0505 |
| Leucocytes (/nL) | 5.24 ± 1.18 | 5.00 ± 1.25 | *, p = 0.0135 |
| Serum vitamins | |||
| Folic acid (ng/mL) | 6.40 ± 2.36 | 7.06 ± 2.07 | n.s., p = 0.2449 |
| Vitamin B12 (ng/L) | 361.28 ± 103.93 | 382.80 ± 99.04 | n.s., p = 0.6470 |
| Lipid profile | |||
| Triglycerides (mg/dL) | 70.03 ± 22.18 | 62.42 ± 15.81 | n.s., p = 0.7973 |
| Cholesterol (total) (mg/dL) | 172.62 ± 29.39 | 168.12 ± 28.75 | n.s., p = 0.9871 |
| HDL (mg/dL) | 62.52 ± 8.88 | 62.62 ± 9.83 | n.s., p = 0.4032 |
| LDL (mg/dL) | 96.00 ± 21.59 | 92.96 ± 21.35 | n.s., p = 0.7073 |
| LDL/HDL-ratio | 1.56 ± 0.33 | 1.52 ± 0.36 | n.s., p = 0.3512 |
| Hepatic profile | |||
| Albumin (g/L) | 43.45 ± 2.43 | 42.88 ± 2.59 | n.s., p = 0.2947 |
| GOT (U/L) | 23.83 ± 5.38 | 23.31 ± 4.22 | n.s., p = 0.5871 |
| GPT (U/L) | 19.31 ± 5.10 | 22.12 ± 7.91 | n.s., p = 0.4663 |
| Inflammatory profile | |||
| CRP (mg/L) | 0.89 ± 0.66 | 0.62 ± 0.31 | n.s., p = 0.0689 |
| Interleukin 6 (ng/L) | 2.56 ± 0.61 | 2.54 ± 0.84 | n.s., p = 0.7976 |
| Hormone profile | |||
| Adrenalin (pg/mL) | 66.23 ± 35.65 | 64.55 ± 34.72 | n.s., p = 0.4556 |
| Noradrenalin (pg/mL) | 473.13 ± 189.77 | 517.48 ± 278.03 | n.s., p = 0.6385 |
| Dopamine (pg/mL) | 62.50 ± 28.75 | 63.26 ± 28.71 | n.s., p = 0.9220 |
| Vasodilation profile | |||
| FMD (% of baseline diameter) | 9.45 ± 5.39 | 11.61 ± 3.41 | n.s., p = 0.0897 |
| FMD baseline | 3.51 ± 0.51 | 3.49 ± 0.45 | n.s., p = 0.6241 |
| FMD recovery | 3.74 ± 0.47 | 3.86 ± 0.54 | n.s., p = 0.2509 |
| Others | |||
| Glucose (mg/dL) | 83.62 ± 5.61 | 85.35 ± 5.42 | n.s., p = 0.4890 |
| HBA1C (%) | 5.14 ± 0.17 | 5.08 ± 0.19 | n.s., p = 0.2970 |
| Homocysteine (µmol/L) | 8.72 ± 2.17 | 8.99 ± 2.12 | n.s., p = 0.9368 |
| TSH (mU/L) | 1.46 ± 0.55 | 1.42 ± 0.65 | n.s., p = 0.9358 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, S.; Tsohataridis, S.; Boland, N.; Ngo, L.; Hahad, O.; Münzel, T.; Wild, P.; Daiber, A.; Schuppan, D.; Lurz, P.; et al. Effects of Short-Term Gluten-Free Diet on Cardiovascular Biomarkers and Quality of Life in Healthy Individuals: A Prospective Interventional Study. Nutrients 2024, 16, 2265. https://doi.org/10.3390/nu16142265
Lange S, Tsohataridis S, Boland N, Ngo L, Hahad O, Münzel T, Wild P, Daiber A, Schuppan D, Lurz P, et al. Effects of Short-Term Gluten-Free Diet on Cardiovascular Biomarkers and Quality of Life in Healthy Individuals: A Prospective Interventional Study. Nutrients. 2024; 16(14):2265. https://doi.org/10.3390/nu16142265
Chicago/Turabian StyleLange, Simon, Simeon Tsohataridis, Niklas Boland, Lisa Ngo, Omar Hahad, Thomas Münzel, Philipp Wild, Andreas Daiber, Detlef Schuppan, Philipp Lurz, and et al. 2024. "Effects of Short-Term Gluten-Free Diet on Cardiovascular Biomarkers and Quality of Life in Healthy Individuals: A Prospective Interventional Study" Nutrients 16, no. 14: 2265. https://doi.org/10.3390/nu16142265








