The Effect of Protein Nutritional Support on Inflammatory Bowel Disease and Its Potential Mechanisms
Abstract
1. Introduction
2. Pathogenesis
2.1. Genetics
2.2. Immune Response
2.3. Intestinal Microbiota
2.4. Environment
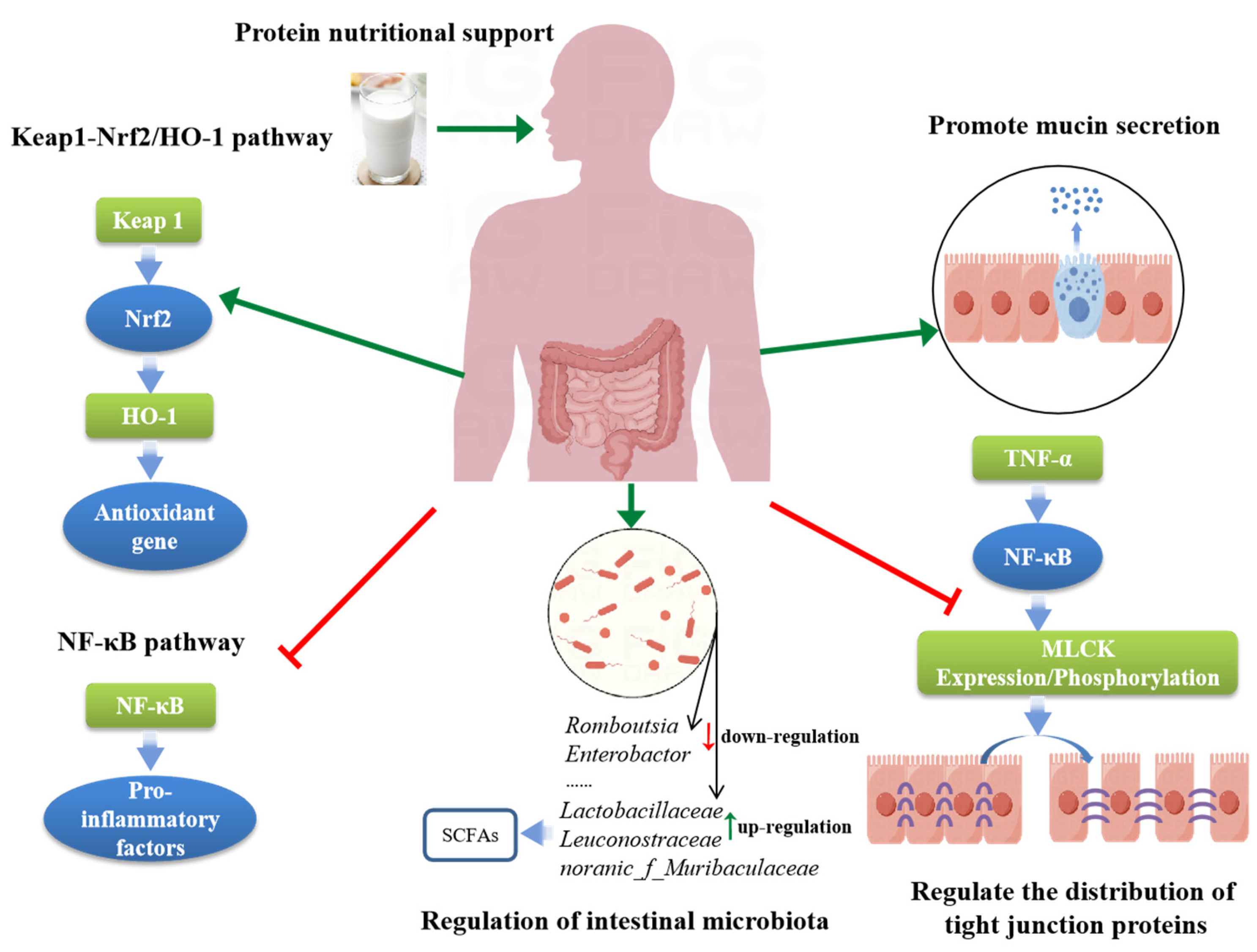
3. Mechanisms of Protein Nutritional Support in Alleviating IBD
3.1. Promote Mucin Secretion
3.2. Regulate Intestinal Tight Junction Proteins
3.3. Regulation of Intestinal Microbiota
3.4. Regulation of Key Signaling Pathways
3.4.1. NF-κB
3.4.2. Keap1/Nrf2/HO-1
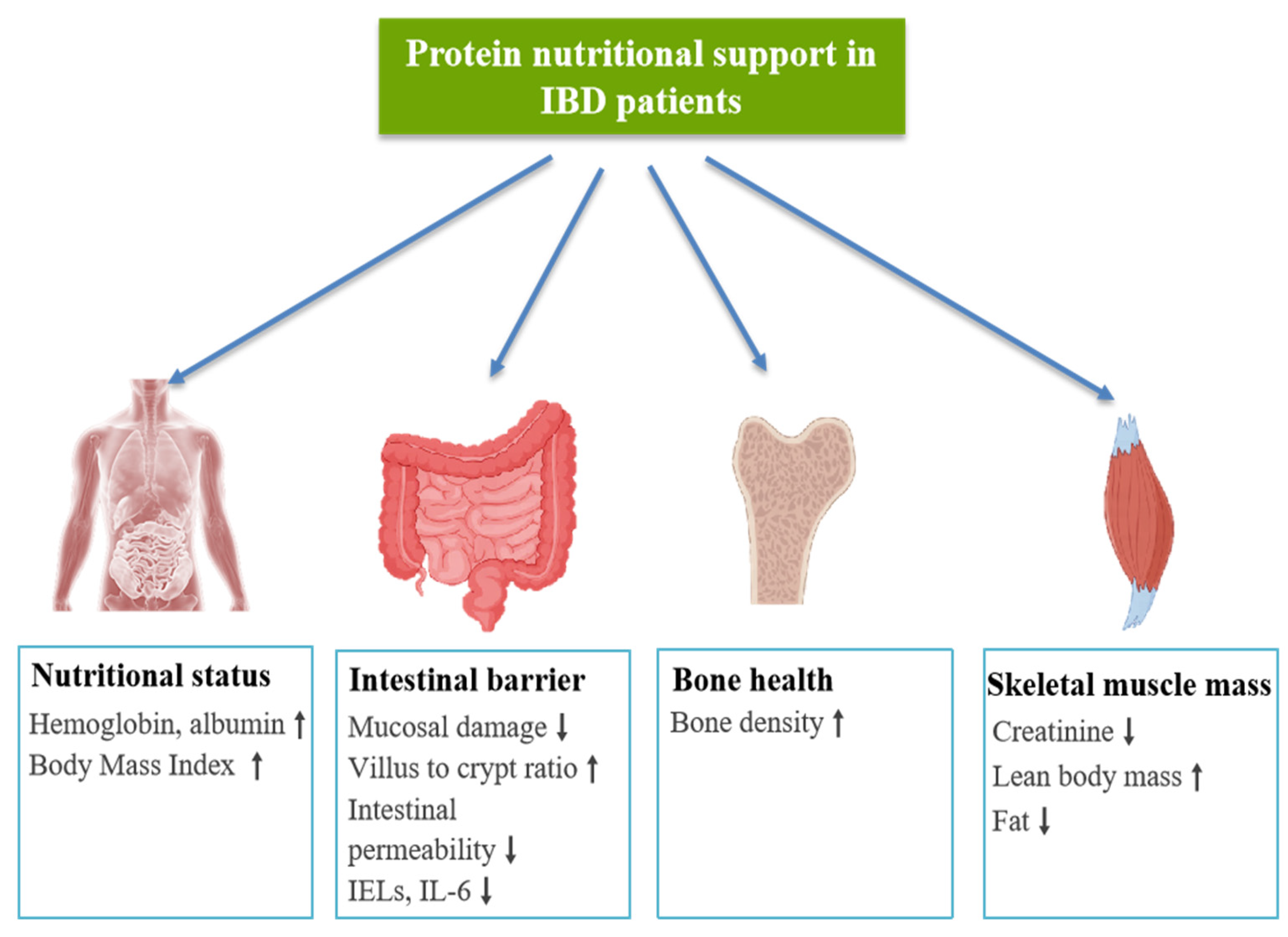
4. The Clinical Application of Protein Nutritional Support in IBD
5. Protein Nutritional Support on IBD Complications
5.1. Malnutrition Associated with IBD
5.2. Sarcopenia Associated with IBD
5.3. Osteoporosis Associated with IBD
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Dayimu, A.; Guan, X.; Duan, M.; Zeng, S.; Wang, H.; Zong, J.; Sun, C.; Yang, X.; Yang, X. Global evolving patterns and cross-country inequalities of inflammatory bowel disease burden from 1990 to 2019: A worldwide report. Inflamm. Res. 2024, 73, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Han, X.; Jiang, H.; Zhang, L.; Hu, J.; Shi, L.; Li, J. Long-term trends in the burden of inflammatory bowel disease in China over three decades: A join point regression and age-period-cohort analysis based on GBD 2019. Front. Public Health 2022, 7, 994619. [Google Scholar]
- Guan, Q.A. Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Shouval, D.S.; Rufo, P.A. The Role of Environmental Factors in the Pathogenesis of Inflammatory Bowel Diseases: A Review. JAMA Pediatr. 2017, 171, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Abreu, M.T.; Cohen, R.; Tremaine, W. American gastroenterological association institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006, 130, 940–987. [Google Scholar] [CrossRef]
- Chande, N.; Patton, P.H.; Tsoulis, D.J.; Thomas, B.S.; MacDonald, J.K. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2015, 10, CD000067. [Google Scholar] [CrossRef]
- Timmer, A.; Patton, P.H.; Chande, N.; McDonald, J.W.; MacDonald, J.K. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2016, 5, CD000478. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Rodrigues-Pinto, E.; Coelho, R.; Andrade, P.; Santos-Antunes, J.; Lopes, S.; Camila-Dias, C.; Macedo, G. Is it possible to change phenotype progression in Crohn’s disease in the era of immunomodulators? Predictive factors of phenotype progression. Am. J. Gastroenterol. 2014, 109, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; AGA Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Amiot, A.; Bouguen, G.; Bonnaud, G.; Bouhnik, Y.; Hagege, H.; Peyrin-Biroulet, L.; French National Consensus Clinical Guidelines for the Management of IBD Study Group. Clinical guidelines for the management of inflammatory bowel disease: Update of a French national consensus. Dig. Liver Dis. 2021, 53, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, B.; Ferrante, M.; Vermeire, S.; Van Assche, G. New Treatment Options for Inflammatory Bowel Diseases. J. Gastroenterol. 2018, 53, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Downer, S.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food is medicine: Actions to integrate food and nutrition into healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S. The Role of Nutrition in Chronic Disease. Nutrients 2023, 15, 664. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Frank, G.; De Lorenzo, A. Nutrition for Prevention and Control of Chronic Degenerative Diseases and COVID-19. Nutrients 2023, 15, 2253. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Rau, S.; Fasulo, C. Preventative and therapeutic potential of nutrition for inflammatory bowel diseases: A narrative review. JPEN J. Parenter Enteral Nutr. 2024, 48, 258–266. [Google Scholar] [CrossRef]
- Wu, X.; Hou, Q.; Zhao, Z.; Wang, J.; Guo, Y.; Lu, L.; Han, J. Effects of Soy-Whey Protein Nutritional Supplementation on Hematopoiesis and Immune Reconstitution in an Allogeneic Transplanted Mice. Nutrients 2022, 14, 3014. [Google Scholar] [CrossRef]
- Yiğit, A.; Bielska, P.; Cais-Sokolińska, D.; Samur, G. Whey proteins as a functional food: Health effects, functional properties, and applications in food. J. Am. Nutr. Assoc. 2023, 42, 758–768. [Google Scholar] [CrossRef]
- Sousa, G.T.; Lira, F.S.; Rosa, J.C.; de Oliveira, E.P.; Oyama, L.M.; Santos, R.V.; Pimentel, G.D. Dietary whey protein lessens several risk factors for metabolic diseases: A review. Lipids Health Dis. 2012, 11, 67. [Google Scholar] [CrossRef]
- Babajafari, S.; Akhlaghi, M.; Mazloomi, S.M.; Ayaz, M.; Noorafshan, A.; Jafari, P.; Hojhabrimanesh, A. The effect of isolated soy protein adjunctive with flaxseed oil on markers of inflammation, oxidative stress, acute phase proteins, and wound healing of burn patients; a randomized clinical trial. Burns 2018, 44, 140–149. [Google Scholar] [CrossRef]
- Ahn, E.; Jeong, H.; Kim, E. Differential effects of various dietary proteins on dextran sulfate sodium-induced colitis in mice. Nutr. Res. Pract. 2022, 16, 700–715. [Google Scholar] [CrossRef]
- Zou, W.; Fu, Z.; Guo, X.; Yao, L.; Hong, H.; Luo, Y.; Tan, Y. Whey Protein Hydrolysate Exerts Anti-Inflammatory Effects to Alleviate Dextran Sodium Sulfate (DSS)-Induced Colitis via Microbiome Restoration. Nutrients 2023, 15, 4393. [Google Scholar] [CrossRef]
- Raffner Basson, A.; Gomez-Nguyen, A.; LaSalla, A.; Buttó, L.; Kulpins, D.; Warner, A.; Di Martino, L.; Ponzani, G.; Osme, A.; Rodriguez-Palacios, A.; et al. Replacing Animal Protein with Soy-Pea Protein in an “American Diet” Controls Murine Crohn Disease-Like Ileitis Regardless of Firmicutes: Bacteroidetes Ratio. J. Nutr. 2021, 151, 579–590. [Google Scholar] [CrossRef]
- Machado, J.F.; Oya, V.; Coy, C.S.; Morcillo, A.M.; Severino, S.D.; Wu, C.; Sgarbieri, V.C.; Vilela, M.M. Whey and soy protein supplements changes body composition in patients with Crohn’s disease undergoing azathioprine and anti-TNF-alpha therapy. Nutr. Hosp. 2015, 31, 1603–1610. [Google Scholar]
- Benjamin, J.; Makharia, G.; Ahuja, V.; Anand Rajan, K.D.; Kalaivani, M.; Gupta, S.D.; Joshi, Y.K. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: A randomized controlled trial. Dig. Dis. Sci. 2012, 57, 1000–1012. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; Yu, X. Effects of nutritional supplement and resistance training for sarcopenia in patients with inflammatory bowel disease: A randomized controlled trial. Medicine 2022, 101, e30386. [Google Scholar] [CrossRef]
- Ferreiro, B.; Llopis-Salinero, S.; Lardies, B.; Granados-Colomina, C.; Milà-Villarroel, R. Clinical and Nutritional Impact of a Semi-Elemental Hydrolyzed Whey Protein Diet in Patients with Active Crohn’s Disease: A Prospective Observational Study. Nutrients 2021, 13, 3623. [Google Scholar] [CrossRef]
- Sasson, A.N.; Ingram, R.J.M.; Zhang, Z.; Taylor, L.M.; Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C.; Ghosh, S.; Raman, M. The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2021, 6, 754–769. [Google Scholar] [CrossRef]
- Moller, F.T.; Andersen, V.; Wohlfahrt, J.; Jess, T. Familial risk of inflammatory bowel disease: A population-based cohort study 1977–2011. Am. J. Gastroenterol. 2015, 110, 564–571. [Google Scholar] [CrossRef]
- Tysk, C.; Lindberg, E.; Järnerot, G.; Flodérus-Myrhed, B. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins: A study of heritability and the influence of smoking. Gut 1988, 29, 990–996. [Google Scholar] [CrossRef]
- Orholm, M.; Binder, V.; Sørensen, T.I.; Rasmussen, L.P.; Kyvik, K.O. Concordance of inflammatory bowel disease among Danish twins: Results of a nationwide study. Scand. J. Gastroenterol. 2000, 35, 1075–1081. [Google Scholar]
- Thompson, N.P.; Driscoll, R.; Pounder, R.E.; Wakefield, A.J. Genetics versus environment in inflammatory bowel disease: Results of a British twin study. BMJ 1996, 312, 95–96. [Google Scholar] [CrossRef]
- Halme, L.; Paavola-Sakki, P.; Turunen, U.; Lappalainen, M.; Farkkila, M.; Kontula, K. Family and twin studies in inflammatory bowel disease. World J. Gastroenterol. 2006, 12, 3668–3672. [Google Scholar] [CrossRef]
- Santos, M.P.C.; Gomes, C.; Torres, J. Familial and ethnic risk in inflammatory bowel disease. Ann. Gastroenterol. 2018, 31, 14–23. [Google Scholar] [CrossRef]
- Mirkov, M.U.; Verstockt, B.; Cleynen, I. Genetics of inflammatory bowel disease: Beyond NOD2. Lancet Gastroenterol. Hepatol. 2017, 2, 224–234. [Google Scholar] [CrossRef]
- Huang, H.; Fang, M.; Jostins, L.; Umićević Mirkov, M.; Boucher, G.; Anderson, C.A.; Andersen, V.; Cleynen, I.; Cortes, A.; Crins, F.; et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 2017, 547, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.A.; Perrigoue, J.; Mortha, A.; Iuga, A.; Song, W.M.; Neiman, E.M.; Llewellyn, S.R.; Di Narzo, A.; Kidd, B.A.; Telesco, S.E.; et al. A functional genomics predictive network model identifies regulators of inflammatory bowel disease. Nat. Genet. 2017, 49, 1437–1449. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Ahmed, S.; Dryden, G. Immunopathophysiology of inflammatory bowel disease: How genetics link barrier dysfunction and innate immunity to inflammation. Innate. Immun. 2017, 23, 497–505. [Google Scholar] [CrossRef]
- Liu, T.C.; Stappenbeck, T.S. Genetics and Pathogenesis of Inflammatory Bowel Disease. Annu. Rev. Pathol. 2016, 11, 127–148. [Google Scholar] [CrossRef]
- Chen, G.B.; Lee, S.H.; Brion, M.J.; Montgomery, G.W.; Wray, N.R.; Radford-Smith, G.L.; Visscher, P.M.; International IBD Genetics Consortium. Estimation and partitioning of (co)heritability of inflammatory bowel disease from GWAS and immunochip data. Hum. Mol. Genet. 2014, 23, 4710–4720. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, S.; Chen, X.; Sun, J.; Kalla, R.; Yu, L.; Wang, L.; Zhou, X.; Kong, X.; Hesketh, T.; et al. The Contribution of Genetic Risk and Lifestyle Factors in the Development of Adult-Onset Inflammatory Bowel Disease: A Prospective Cohort Study. Am. J. Gastroenterol. 2023, 118, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Ince, M.N.; Elliott, D.E. Immunologic and molecular mechanisms in inflammatory bowel disease. Surg. Clin. N. Am. 2007, 87, 681–696. [Google Scholar] [CrossRef]
- Sergiu, M.I.; Elena, M.M.; Marilena, M.; Andrada, D.; Emanuela, P.R. Microbiota: The missing link in the etiology of inflammatory bowel disease. J. Mind Med. Sci. 2020, 7, 29–33. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401–407. [Google Scholar] [CrossRef]
- Tamburini, B.; La Manna, M.P.; La Barbera, L.; Mohammadnezhad, L.; Badami, G.D.; Shekarkar Azgomi, M.; Dieli, F.; Caccamo, N. Immunity and Nutrition: The Right Balance in Inflammatory Bowel Disease. Cells 2022, 11, 455. [Google Scholar] [CrossRef]
- Fiocchi, C. Inflammatory bowel disease: Etiology. Gastroenterology 1998, 115, 182–205. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.L.; Sokol, H. The gut mycobiota: Insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Gut microbiota, inflammation, and colorectal cancer. Annu. Rev. Microbiol. 2016, 70, 395–411. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Rapin, A.; Lebon, L.; Dubey, L.K.; Mosconi, I.; Sarter, K.; Piersigilli, A.; Menin, L.; Walker, A.W.; Rougemont, J.; et al. The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity 2015, 43, 998–1010. [Google Scholar] [CrossRef]
- Saleh, M.; Elson, C.O. Experimental inflammatory bowel disease: Insights into the host-microbiota dialog. Immunity 2011, 34, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Nell, S.; Suerbaum, S.; Josenhans, C. The impact of the microbiota on the pathogenesis of IBD: Lessons from mouse infection models. Nat. Rev. Microbiol. 2010, 8, 564–577. [Google Scholar] [CrossRef]
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Peterson, D.A.; Frank, D.N.; Pace, N.R.; Gordon, J.I. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe 2008, 3, 417–427. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Ahmed, I.; Roy, B.C.; Khan, S.A.; Septer, S.; Umar, S. Microbiome, Metabolome and Inflammatory Bowel Disease. Microorganisms 2016, 4, 20. [Google Scholar] [CrossRef]
- Johansson, M.E.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef]
- Van Muijlwijk, G.H.; van Mierlo, G.; Jansen, P.W.T.C.; Vermeulen, M.; Bleumink-Pluym, N.M.C.; Palm, N.W.; van Putten, J.P.M.; de Zoete, M.R. Identification of Allobaculum mucolyticum as a novel human intestinal mucin degrader. Gut Microbes 2021, 13, 1966278. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Ott, S.J.; Kühbacher, T.; Musfeldt, M.; Rosenstiel, P.; Hellmig, S.; Rehman, A.; Drews, O.; Weichert, W.; Timmis, K.N.; Schreiber, S. Fungi and inflammatory bowel diseases: Alterations of composition and diversity. Scand. J. Gastroenterol. 2008, 43, 831–841. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Li, N.; Li, J. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn’s disease. J. Clin. Gastroenterol. 2014, 48, 513–523. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Srour, B.; Kordahi, M.C.; Bonazzi, E.; Deschasaux-Tanguy, M.; Touvier, M.; Chassaing, B. Ultra-processed foods and human health: From epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 2022, 7, 1128–1140. [Google Scholar] [CrossRef]
- Yadav, P.; Ellinghaus, D.; Rémy, G.; Freitag-Wolf, S.; Cesaro, A.; Degenhardt, F.; Boucher, G.; Delacre, M.; International IBD Genetics Consortium; Peyrin-Biroulet, L.; et al. Genetic Factors Interact with Tobacco Smoke to Modify Risk for Inflammatory Bowel Disease in Humans and Mice. Gastroenterology 2017, 153, 550–565. [Google Scholar] [CrossRef]
- Dar, S.H.; Maniya, M.T.; Merza, N.; Musheer, A.; Zahid, M.; Ahmed, F.; Shurjeel, Q.; Qazi, S.; Ahmed, A.; Shah, H.; et al. The association of antibiotic exposure with new-onset inflammatory bowel disease: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102129. [Google Scholar] [CrossRef]
- O’Toole, A.; Korzenik, J. Environmental triggers for IBD. Curr. Gastroenterol. Rep. 2014, 16, 396. [Google Scholar] [CrossRef]
- Ho, S.M.; Lewis, J.D.; Mayer, E.A.; Plevy, S.E.; Chuang, E.; Rappaport, S.M.; Croitoru, K.; Korzenik, J.R.; Krischer, J.; Hyams, J.S.; et al. Challenges in IBD Research: Environmental Triggers. Inflamm. Bowel Dis. 2019, 25, S13–S23. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Nitescu, M.; Istratescu, D.; Preda, C.M.; Manuc, T.E.; Louis, E.; Manuc, M.; Stroie, T.; Catrinoiu, M.; Tieranu, C.G.; Badea, L.E.; et al. Role of an Exclusion Diet (Reduced Disaccharides, Saturated Fats, Emulsifiers, Red and Ultraprocessed Meats) in Maintaining the Remission of Chronic Inflammatory Bowel Diseases in Adults. Medicina 2023, 59, 329. [Google Scholar] [CrossRef]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics 2012, 130, 794–803. [Google Scholar] [CrossRef]
- Agrawal, M.; Poulsen, G.; Colombel, J.F.; Allin, K.H.; Jess, T. Maternal antibiotic exposure during pregnancy and risk of IBD in offspring: A population-based cohort study. Gut 2023, 72, 804–805. [Google Scholar] [CrossRef]
- Shaw, S.Y.; Blanchard, J.F.; Bernstein, C.N. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 2687–2692. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Si, J.; Li, P.; Gao, H.; Li, W.; Chen, Y. What happens to gut microorganisms and potential repair mechanisms when meet heavy metal(loid)s. Environ. Pollut. 2023, 317, 120780. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Mao, X.; Xiang, X.; Ye, S.; Chen, J.; Zhu, A.; Meng, Y.; Yang, X.; Peng, S.; et al. Adverse health effects of emerging contaminants on inflammatory bowel disease. Front. Public Health 2023, 11, 1140786. [Google Scholar] [CrossRef]
- Beamish, L.A.; Osornio-Vargas, A.R.; Wine, E. Air pollution: An environmental factor contributing to intestinal disease. J. Crohns. Colitis 2011, 5, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wu, X.; Li, X.; Zhu, Y. Effects of microplastics in aquatic environments on inflammatory bowel disease. Environ. Res. 2023, 229, 115974. [Google Scholar] [CrossRef] [PubMed]
- Metzger, C.E.; Narayanan, S.A.; Zawieja, D.C.; Bloomfield, S.A. A moderately elevated soy protein diet mitigates inflammatory changes in gut and in bone turnover during chronic TNBS-induced inflammatory bowel disease. Appl. Physiol. Nutr. Metab. 2019, 44, 595–605. [Google Scholar] [PubMed]
- Jiang, H.; Przybyszewski, J.; Mitra, D.; Becker, C.; Brehm-Stecher, B.; Tentinger, A.; MacDonald, R.S. Soy protein diet, but not Lactobacillus rhamnosus GG, decreases mucin-1, trefoil factor-3, and tumor necrosis factor-α in colon of dextran sodium sulfate-treated C57BL/6 mice. J. Nutr. 2011, 141, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, S.; Arai, K.; Kumada, N.; Ishida, Y.; Tanaka, I.; Iwatsuki, S.; Ohwada, T.; Ohnishi, M.; Tokuji, Y.; Kinoshita, M. The Effect of Oral Intake of Low-Temperature-Processed Whey Protein Concentrate on Colitis and Gene Expression Profiles in Mice. Foods 2014, 3, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Sprong, R.C.; Schonewille, A.J.; van der Meer, R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium-induced colitis: Role of mucin and microbiota. J. Dairy Sci. 2010, 93, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Tunc, N.; Sahin, A.; Demirel, U.; Artas, G.; Sahin, K.; Bahcecioglu, İ.H.; Yalniz, M. Favourable effects of whey protein on acetic acid-induced ulcerative colitis in a rat model. Arch. Med. Sci. 2021, 18, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Lletjós, S.; Andriamihaja, M.; Blais, A.; Grauso, M.; Lepage, P.; Davila, A.M.; Viel, R.; Gaudichon, C.; Leclerc, M.; Blachier, F.; et al. Dietary Protein Intake Level Modulates Mucosal Healing and Mucosa-Adherent Microbiota in Mouse Model of Colitis. Nutrients 2019, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Jiraskova Zakostelska, Z.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10, 919. [Google Scholar] [CrossRef]
- Lan, A.; Blais, A.; Coelho, D.; Capron, J.; Maarouf, M.; Benamouzig, R.; Lancha, A.H., Jr.; Walker, F.; Tomé, D.; Blachier, F. Dual effects of a high-protein diet on DSS-treated mice during colitis resolution phase. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G624–G633. [Google Scholar]
- Li, M.; Li, Q.; Abdlla, R.; Chen, J.; Yue, X.; Quek, S.Y. Donkey whey proteins ameliorate dextran sulfate sodium-induced ulcerative colitis in mice by downregulating the S100A8-TRAF6-NF-κB axis-mediated inflammatory response. Food Sci. Hum. Wellness 2023, 12, 1809–1819. [Google Scholar] [CrossRef]
- Vidal-Lletjós, S.; Khodorova, N.V.; Piscuc, M.; Gaudichon, C.; Blachier, F.; Lan, A. Tissue-specific effect of colitis on protein synthesis in mice: Impact of the dietary protein content. Eur. J. Nutr. 2021, 60, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, G.; Hagihara, N.; Hosomi, R.; Shimono, T.; Kanda, S.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Dietary Alaska Pollock Protein Attenuates the Experimental Colitis Induced by Dextran Sulfate Sodium via Regulation of Gut Microbiota and Its Metabolites in Mice. Metabolites 2022, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Tuo, Y.; You, H.; Li, J.; Wang, L.; Liu, X.; Ding, L. Quinoa protein and its hydrolysate ameliorated DSS-induced colitis in mice by modulating intestinal microbiota and inhibiting inflammatory response. Int. J. Biol. Macromol. 2023, 253, 127588. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Rath, E.; Yuan, D.; Waldschmitt, N.; Khaloian, S.; Allgäuer, M.; Staszewski, O.; Lobner, E.M.; Schöttl, T.; Giesbertz, P.; et al. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat. Commun. 2016, 7, 13171. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, X.; Du, H.; Zhao, M.; Zhang, Z.; Zhu, R.; He, B.; Zhang, Y.; Li, X.; Li, J.; et al. Foodborne Carbon Dot Exposure Induces Insulin Resistance through Gut Microbiota Dysbiosis and Damaged Intestinal Mucus Layer. ACS Nano 2023, 17, 6081–6094. [Google Scholar] [CrossRef]
- Rapa, S.F.; Waltenberger, B.; Di Paola, R.; Adesso, S.; Siracusa, R.; Peritore, A.F.; D’Amico, R.; Autore, G.; Cuzzocrea, S.; Stuppner, H.; et al. Prevents Intestinal Inflammation and Oxidative Stress In Vitro and In Vivo. FASEB J. 2020, 34, 1576–1590. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Dorofeyev, A.E.; Vasilenko, I.V.; Rassokhina, O.A.; Kondratiuk, R.B. Mucosal barrier in ulcerative colitis and Crohn’s disease. Gastroenterol Res. Pract. 2013, 2013, 431231. [Google Scholar] [CrossRef]
- Niv, Y. Mucin Genes Expression in the Intestine of Crohn’s Disease Patients: A Systematic Review and Meta-analysis. J. Gastrointestin. Liver Dis. 2016, 25, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nakamura, T.; Hayama, M.; Hosaka, N.; Akamatsu, T.; Ota, H. Altered expression of CDX-2, PDX-1 and mucin core proteins in “Ulcer-associated cell lineage (UACL)” in Crohn’s disease. J. Mol. Histol. 2008, 39, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Maqueda, D.; Miralles, B.; Ramos, M.; Recio, I. Effect of β-lactoglobulin hydrolysate and β-lactorphin on intestinal mucin secretion and gene expression in human goblet cells. Food Res. Int. 2013, 54, 1287–1291. [Google Scholar] [CrossRef]
- Claustre, J.; Toumi, F.; Trompette, A.; Jourdan, G.; Guignard, H.; Chayvialle, J.A.; Plaisancié, P. Effects of peptides derived from dietary proteins on mucus secretion in rat jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G521–G528. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Maqueda, D.; Miralles, B.; De Pascual-Teresa, S.; Reverón, I.; Muñoz, R.; Recio, I. Food-derived peptides stimulate mucin secretion and gene expression in intestinal cells. J. Agric. Food Chem. 2012, 60, 8600–8605. [Google Scholar] [CrossRef] [PubMed]
- Faure, M.; Moënnoz, D.; Montigon, F.; Mettraux, C.; Breuillé, D.; Ballèvre, O. Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J. Nutr. 2005, 135, 486–491. [Google Scholar] [CrossRef]
- Zeng, B.; Wang, D.; Wang, H.; Chen, T.; Luo, J.; Xi, Q.; Sun, J.; Zhang, Y. Dietary Soy Protein Isolate Attenuates Intestinal Immunoglobulin and Mucin Expression in Young Mice Compared with Casein. Nutrients 2020, 12, 2739. [Google Scholar] [CrossRef]
- Drolia, R.; Tenguria, S.; Durkes, A.C.; Turner, J.R.; Bhunia, A.K. Listeria Adhesion Protein Induces Intestinal Epithelial Barrier Dysfunction for Bacterial Translocation. Cell Host Microbe 2018, 23, 470–484. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.A.; Doelle, S.M.; Halpern, M.D.; Saunders, T.A.; Holubec, H.; Dvorak, K.; Boitano, S.A.; Dvorak, B. Intestinal barrier failure during experimental necrotizing enterocolitis: Protective effect of EGF treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G938–G949. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.V.; He, W.; Marchiando, A.M.; Zha, J.; Singh, G.; Li, H.S.; Biswas, A.; Ong, M.L.D.M.; Jiang, Z.H.; Choi, W. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nat. Med. 2019, 25, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Piegholdt, S.; Pallauf, K.; Esatbeyoglu, T.; Speck, N.; Reiss, K.; Ruddigkeit, L.; Stocker, A.; Huebbe, P.; Rimbach, G. Biochanin A and prunetin improve epithelial barrier function in intestinal CaCo-2 cells via downregulation of ERK, NF-κB, and tyrosine phosphorylation. Free Radic. Biol. Med. 2014, 70, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Mashukova, A.; Wald, F.A.; Salas, P.J. Tumor necrosis factor alpha and inflammation disrupt the polarity complex in intestinal epithelial cells by a posttranslational mechanism. Mol. Cell Biol. 2011, 31, 756–765. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Yang, Q.; Lyu, S.; Xu, M.; Li, S.; Du, Z.; Liu, X.; Shang, X.; Yu, Z.; Liu, J.; Zhang, T. Potential Benefits of Egg White Proteins and Their Derived Peptides in the Regulation of the Intestinal Barrier and Gut Microbiota: A Comprehensive Review. J. Agric. Food. Chem. 2023, 71, 13168–13180. [Google Scholar] [CrossRef]
- Sánchez-Moya, T.; López-Nicolás, R.; Planes, D.; González-Bermúdez, C.A.; Ros-Berruezo, G.; Frontela-Saseta, C. In vitro modulation of gut microbiota by whey protein to preserve intestinal health. Food Funct. 2017, 8, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Boscaini, S.; Skuse, P.; Nilaweera, K.N.; Cryan, J.F.; Cotter, P.D. The ‘Whey’ to good health: Whey protein and its beneficial effect on metabolism, gut microbiota and mental health. Trends Food Sci. Technol. 2023, 133, 1–14. [Google Scholar] [CrossRef]
- Sivixay, S.; Bai, G.; Tsuruta, T.; Nishino, N. Cecum microbiota in rats fed soy, milk, meat, fish, and egg proteins with prebiotic oligosaccharides. AIMS Microbiol. 2021, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Farsijani, S.; Cauley, J.A.; Peddada, S.D.; Langsetmo, L.; Shikany, J.M.; Orwoll, E.S.; Ensrud, K.E.; Cawthon, P.M.; Newman, A.B. Relation Between Dietary Protein Intake and Gut Microbiome Composition in Community-Dwelling Older Men: Findings from the Osteoporotic Fractures in Men Study (MrOS). J. Nutr. 2023, 152, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Cheng, Y.; Deng, Y.; Ren, G.; Wang, J. Composition of gut microbiota involved in alleviation of dexamethasone-induced muscle atrophy by whey protein. NPJ Sci. Food. 2023, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, M.L.; Hemalatha, S. The functional roles of short chain fatty acids as postbiotics in human gut: Future perspectives. Food Sci. Biotechnol. 2023, 33, 275–285. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Tamura, K.; Sasaki, H.; Shiga, K.; Miyakawa, H.; Shibata, S. The Timing Effects of Soy Protein Intake on Mice Gut Microbiota. Nutrients 2019, 12, 87. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Santos, A.R.d.O.d.; Carvalho, A.C.A.d.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.d.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and Regulation of NF-κB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, G.; Moshage, H.; Jansen, P.L. Blockade of NF-κB activation and donation of nitric oxide: New treatment options in inflammatory bowel disease? Scand. J. Gastroenterol. Suppl. 2002, 37, 37–41. [Google Scholar] [CrossRef]
- Bajpai, M.; Seril, D.N.; Van Gurp, J.; Geng, X.; Alvarez, J.; Minacapelli, C.D.; Gorin, S.; Das, K.K.; Poplin, E.; Cheng, J.; et al. Effect of Long-Term Mesalamine Therapy on Cancer-Associated Gene Expression in Colonic Mucosa of Patients with Ulcerative Colitis. Dig. Dis. Sci. 2019, 64, 740–750. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wen, S.; Zhong, Z.; Weng, S.; Jiang, Q.; Mi, H.; Liu, F. The Synergistic Effects of 5-Aminosalicylic Acid and Vorinostat in the Treatment of Ulcerative Colitis. Front. Pharmacol. 2021, 12, 625543. [Google Scholar] [CrossRef]
- Yang, N.; Xia, Z.L.; Shao, N.Y.; Li, B.W.; Xue, L.; Peng, Y.; Zhi, F.; Yang, Y.L. Carnosic acid prevents dextran sulfate sodium-induced acute colitis associated with the regulation of the Keap1/Nrf2 pathway. Sci. Rep. 2017, 7, 11036. [Google Scholar] [CrossRef]
- Gu, R.R.; Cui, T.Y.; Guo, Y.N.; Luan, Y.Y.; Wang, X.R.; Liu, R.X.; Yin, C.H. Angiotensin-(1-7) ameliorates intestinal barrier dysfunction by activating the Keap1/Nrf2/HO-1 signaling pathway in acute pancreatitis. Mol. Biol. Rep. 2023, 50, 5991–6003. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Z.Q.; Misra, B.R.; Zhang, L.; Cao, Z.X.; Yamamoto, M.; Trush, M.A.; Misra, H.P.; Li, Y.B. Nuclear factor E2-related factor 2-dependent myocardiac cytoprotection against oxidative and electrophilic stress. Cardiovasc. Toxicol. 2008, 8, 71–85. [Google Scholar] [CrossRef]
- Zhao, X.L.; Gao, J.Y.; Hogenkamp, A.; Knippels, L.M.J.; Garssen, J.; Bai, J.; Yang, A.S.; Wu, Y.; Chen, H.B. Selenium-Enriched Soy Protein Has Antioxidant Potential via Modulation of the NRF2-HO1 Signaling Pathway. Foods 2021, 10, 2542. [Google Scholar] [CrossRef]
- Xiao, W.W.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Zhang, Y.G.; Zhou, X.Q. Effect of dietary enzyme-treated soy protein on the immunity and antioxidant status in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Res. 2019, 50, 1411–1421. [Google Scholar] [CrossRef]
- Ma, Y.L.; Xu, J.Z.; Guo, R.B.; Teng, G.X.; Chen, Y.D.; Xu, X.X. In vitro gastrointestinal model for the elderly: Effect of high hydrostatic pressure on protein structures and antioxidant activities of whey protein isolate. Food Biosci. 2023, 52, 102452. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Kusumah, J.; Bringe, N.A.; Shen, Y.; Mejia, E.G.D. Peptide release, radical scavenging capacity, and antioxidant responses in intestinal cells are determined by soybean variety and gastrointestinal digestion under simulated conditions. Food Chem. 2023, 405, 134929. [Google Scholar] [CrossRef]
- Li, N.; Wen, L.D.; Li, T.G.; Yang, H.J.; Qiao, M.W.; Wang, T.L.; Song, L.J.; Huang, X.Q.; Li, M.M.; Bukyei, E.; et al. Alleviating Effects of Black Soybean Peptide on Oxidative Stress Injury Induced by Lead in PC12 Cells via Keap1/Nrf2/TXNIP Signaling Pathway. Nutrients 2022, 14, 3102. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zheng, L.; Su, G.W.; Zeng, X.A.; Sun, B.G.; Zhao, M.M. Evaluation and Exploration of Potentially Bioactive Peptides in Casein Hydrolysates against Liver Oxidative Damage in STZ/HFD-Induced Diabetic Rats. J. Agric. Food Chem. 2020, 68, 2393–2405. [Google Scholar] [CrossRef] [PubMed]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Chen, X.C.; Liu, W.L.; Zhang, J.; Li, H.; Liu, X.Q. Selenium-enriched peptides identified from selenium-enriched soybean protein hydrolysate: Protective effects against heat damage in Caco-2 cells. Food Funct. 2023, 14, 7882–7896. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Han, Y.N.; Liu, M.Q.; Piao, Z.H.; Zhang, X.; Xue, Y.T.; Zhang, Y.H. Structure-guided discovery of antioxidant peptides bounded to the Keap1 receptor as hunter for potential dietary antioxidants. Food Chem. 2022, 373, 130999. [Google Scholar] [CrossRef]
- Kumar, N.; Devi, S.; Mada, S.B.; Reddi, S.; Kapila, R.; Kapila, S. Anti-apoptotic effect of buffalo milk casein derived bioactive peptide by directing Nrf2 regulation in starving fibroblasts. Food Biosci. 2020, 35, 100566. [Google Scholar] [CrossRef]
- Gallo, V.; Arienzo, A.; Tomassetti, F.; Antonini, G. Milk Bioactive Compounds and Gut Microbiota Modulation: The Role of Whey Proteins and Milk Oligosaccharides. Foods 2024, 13, 907. [Google Scholar] [CrossRef]
- Zare, R.; Devrim-Lanpir, A.; Guazzotti, S.; Ali Redha, A.; Prokopidis, K.; Spadaccini, D.; Cannataro, R.; Cione, E.; Henselmans, M.; Aragon, A.A. Effect of Soy Protein Supplementation on Muscle Adaptations, Metabolic and Antioxidant Status, Hormonal Response, and Exercise Performance of Active Individuals and Athletes: A Systematic Review of Randomised Controlled Trials. Sports Med. 2023, 53, 2417–2446. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Mazidi, M.; Sankaranarayanan, R.; Tajik, B.; McArdle, A.; Isanejad, M. Effects of whey and soy protein supplementation on inflammatory cytokines in older adults: A systematic review and meta-analysis. Br. J. Nutr. 2023, 129, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.X.; Wang, T.R.; Luo, Y.C. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Valvano, M.; Capannolo, A.; Cesaro, N.; Stefanelli, G.; Fabiani, S.; Frassino, S.; Monaco, S.; Magistroni, M.; Viscido, A.; Latella, G. Nutrition, Nutritional Status, Micronutrients Deficiency, and Disease Course of Inflammatory Bowel Disease. Nutrients 2023, 15, 3824. [Google Scholar] [CrossRef] [PubMed]
- Capristo, E.; Mingrone, G.; Addolorato, G.; Greco, A.V.; Gasbarrini, G. Effect of a vegetable-protein-rich polymeric diet treatment on body composition and energy metabolism in inactive Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2000, 12, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Davanço, T.; Oya, V.; Coy, S.R.; Leal, R.F.; Ayrizono, M.L.S.; Sgarbieri, V.C.; dos Santos Vilela, M.M.; Lomazi, E.A. Nutritional supplementation assessment with whey proteins and TGF-β in patients with Crohn’s disease. Nutr. Hosp. 2012, 27, 1286–1292. [Google Scholar] [PubMed]
- Gomes Coqueiro, F.; Rocha, R.; Menezes, C.A.; Brasil Lopes, M.; Rosa Oliveira, V.; Fortes, F.M.L.; Santana, G.O. What nutritional factors influence bone mineral density in Crohn’s disease patients? Intest. Res. 2018, 16, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Dige, A.; Bendix, M.; Wernlund, P.G.; Christensen, L.A.; Dahlerup, J.F.; Agnholt, J. Casein glycomacropeptide for active distal ulcerative colitis: A randomized pilot study. Eur. J. Clin. Investig. 2016, 46, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.B.; Iigo, M.; Abdelgied, M.; Ozeki, K.; Tanida, S.; Joh, T.; Takahashi, S.; Tsuda, H. Bovine lactoferrin and Crohn’s disease: A case study. Biochem. Cell Biol. 2017, 95, 133–141. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D.; Elsawy, A.; Elsheikh, M.; Donia, A.; Nassar, M. Lactoferrin for iron-deficiency anemia in children with inflammatory bowel disease: A clinical trial. Pediatr. Res. 2022, 92, 762–766. [Google Scholar] [CrossRef]
- Yang, Q.F.; Zhang, T.; Diao, N.; Chao, K.; Shu, H.J.; Wu, J.; Guan, D.X.; Wang, L.; Xu, X.W.; Li, Z.H.; et al. Amino acid-based enteral nutrition is effective for pediatric Crohn’s disease: A multicenter prospective study. Gastroenterol. Rep. 2023, 12, goad072. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, M.; Yokoyama, T.; Saigusa, N.; Sato, H.; Yazawa, K.; Tsurita, G.; Kurokawa, T.; Hata, K.; Yokoyama, Y. Elemental diet therapy plays a significant role in preventing surgical recurrence of Crohn’s disease in the era of biologics. Surg. Today 2021, 51, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Tominaga, K.; Hoshino, A.; Sugaya, T.; Kanke, K.; Hiraishi, H. Therapeutic efficacy of an elemental diet for patients with Crohn’s disease and its association with amino acid metabolism. Saudi. J. Gastroenterol. 2017, 23, 20–27. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Zhang, D.; Gordon, M.; MacDonald, J.K. Enteral nutrition for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 8, CD005984. [Google Scholar] [CrossRef]
- Nakahigashi, M.; Yamamoto, T.; Sacco, R.; Hanai, H.; Kobayashi, F. Enteral nutrition for maintaining remission in patients with quiescent Crohn’s disease: Current status and future perspectives. Int. J. Colorectal Dis. 2016, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tsertsvadze, A.; Gurung, T.; Court, R.; Clarke, A.; Sutcliffe, P. Clinical effectiveness and cost-effectiveness of elemental nutrition for the maintenance of remission in Crohn’s disease: A systematic review and meta-analysis. Health Technol. Assess. 2015, 19, 1–138. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns. Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Lin, J.J.; Liu, M.; Huang, M.F.; Ye, X.H.; Xu, Y.; Lv, C.; Liu, Z.R.; Liu, Y.X.; Tong, Z.H.; Li, W.Q.; et al. Intravenous amino acids may mediate the adverse effect of early parenteral nutrition on mortality in critically ill patients requiring mechanical ventilation: A post hoc analysis of the NEED trial. JPEN J. Parenter. Enteral Nutr. 2023, 47, 301–309. [Google Scholar] [CrossRef]
- Nelson, A.D.; Elkins, J.R.; Stocchi, L.; Farraye, F.A.; Hashash, J.G. Use and Misuse of Parenteral Nutrition in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.; O’Morain, C.A. Review article: Nutrition and adult inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 17, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Mrowiec, S. Nutritional Status and Its Detection in Patients with Inflammatory Bowel Diseases. Nutrients 2023, 15, 1991. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.; Prager, M.; Valentini, L.; Büning, C. Inflammation-Driven Malnutrition: A New Screening Tool Predicts Outcome in Crohn’s Disease. Br. J. Nutr. 2016, 116, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Valentini, L. Disease-Related Malnutrition and Sarcopenia as Determinants of Clinical Outcome. Visc. Med. 2019, 35, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Stobaugh, H. Maximizing Recovery and Growth When Treating Moderate Acute Malnutrition with Whey-Containing Supplements. Food Nutr. Bull. 2018, 39, S30–S34. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Huq, S.; Islam, M.M.; Ahmed, T. Acceptability and efficacy of ready-to-use therapeutic food using soy protein isolate in under-5 children suffering from severe acute malnutrition in Bangladesh: A double-blind randomized non-inferiority trial. Eur. J. Nutr. 2020, 59, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Hébuterne, X.; Frin, G.; Lefevere, S.; Eyraud, E.; Dorigny, B.; Schneider, S. Effectiveness and tolerance of an oral nutritional supplement highly concentrated in protein and energy in elderly subjects at risk of malnutrition. Nutr. Clin. Métab. 2020, 34, 156–160. [Google Scholar] [CrossRef]
- Kaipainen, T.; Hartikainen, S.; Tiihonen, M.; Nykänen, I. Effect of individually tailored nutritional counselling on protein and energy intake among older people receiving home care at risk of or having malnutrition: A non-randomised intervention study. BMC Geriatr. 2022, 22, 391. [Google Scholar] [CrossRef]
- Strasser, B.; Grote, V.; Bily, W.; Nics, H.; Riedl, P.; Jira, I.; Fischer, M.J. Short-Term Effects of Dietary Protein Supplementation on Physical Recovery in Older Patients at Risk of Malnutrition during Inpatient Rehabilitation: A Pilot, Randomized, Controlled Trial. Healthcare 2023, 11, 2317. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, J.; Li, M.; Yi, S.; Xie, J.; Zhang, H.; Wang, J. Protein blend ingestion before allogeneic stem cell transplantation improves protein-energy malnutrition in patients with leukemia. Nutr. Res. 2017, 46, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, F.; Roberto, M.; Arrivi, G.; Sarfati, E.; Schipilliti, F.M.; Crimini, E.; Botticelli, A.; Di Girolamo, M.; Muscaritoli, M.; Marchetti, P. Clinical Impact of Highly Purified, Whey Proteins in Patients Affected with Colorectal Cancer Undergoing Chemotherapy: Preliminary Results of a Placebo-Controlled Study. Integr. Cancer Ther. 2019, 18, 1534735419866920. [Google Scholar] [CrossRef] [PubMed]
- Potcovaru, C.G.; Filip, P.V.; Neagu, O.M.; Diaconu, L.S.; Salmen, T.; Cinteză, D.; Pantea Stoian, A.; Bobirca, F.; Berteanu, M.; Pop, C. Diagnostic Criteria and Prognostic Relevance of Sarcopenia in Patients with Inflammatory Bowel Disease—A Systematic Review. J. Clin. Med. 2023, 12, 4713. [Google Scholar] [CrossRef] [PubMed]
- Fatani, H.; Olaru, A.; Stevenson, R.; Alharazi, W.; Jafer, A.; Atherton, P.; Brook, M.; Moran, G. Systematic review of sarcopenia in inflammatory bowel disease. Clin. Nutr. 2023, 42, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Nakamura, S.; Miyazaki, T.; Kakimoto, K.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Inflammatory Bowel Disease and Sarcopenia: Its Mechanism and Clinical Importance. J. Clin. Med. 2021, 10, 4214. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.Q.; Varma, P.; Strauss, B.J.G.; Rajadurai, A.S.; Moore, G.T. Low Muscle Mass at Initiation of Anti-TNF Therapy for Inflammatory Bowel Disease Is Associated with Early Treatment Failure: A Retrospective Analysis. Eur. J. Clin. Nutr. 2017, 71, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Zanker, J.; Sim, M.; Anderson, K.; Balogun, S.; Brennan-Olsen, S.L.; Dent, E.; Duque, G.; Girgis, C.M.; Grossmann, M.; Hayes, A.; et al. Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J. Cachexia Sarcopenia Muscle 2023, 14, 142–156. [Google Scholar] [CrossRef]
- Kaspy, M.S.; Hannaian, S.J.; Bell, Z.W.; Churchward-Venne, T.A. The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: An update. Nutr. Res. Rev. 2023, 1–14. [Google Scholar] [CrossRef]
- Fiorindi, C.; Cuffaro, F.; Piemonte, G.; Cricchio, M.; Addasi, R.; Dragoni, G.; Scaringi, S.; Nannoni, A.; Ficari, F.; Giudici, F. Effect of long-lasting nutritional prehabilitation on postoperative outcome in elective surgery for IBD. Clin. Nutr. 2021, 40, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Quinlan, J.I.; Overthrow, K.; Greig, C.; Lord, J.M.; Armstrong, M.J.; Cooper, S.C. Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients 2021, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Annese, V.; Vavricka, S.R.; Allez, M.; Barreiro-de Acosta, M.; Boberg, K.M.; Burisch, J.; De Vos, M.; De Vries, A.M.; Dick, A.D.; et al. The First European Evidence-Based Consensus on Extra-Intestinal Manifestations in Inflammatory Bowel Disease. J. Crohn’s Colitis 2016, 10, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Scott, F.I.; Brensinger, C.M.; Roy, J.A.; Osterman, M.T.; Mamtani, R.; Bewtra, M.; Chen, L.; Yun, H.; Xie, F.; et al. Increased Mortality Rates with Prolonged Corticosteroid Therapy When Compared with Antitumor Necrosis Factor-α-Directed Therapy for Inflammatory Bowel Disease. Am. J. Gastroenterol. 2018, 113, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhao, Y.; Xiang, L.S. Bone health in inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Ayub, N.; Faraj, M.; Ghatan, S.; Reijers, J.A.A.; Napoli, N.; Oei, L. The treatment gap in osteoporosis. J. Clin. Med. 2021, 10, 3002. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Reducing the treatment gap in osteoporosis. Lancet Diabetes Endocrinol. 2020, 8, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef]
- Ratajczak-Pawłowska, A.E.; Michalak, M.; Szymczak-Tomczak, A.; Rychter, A.M.; Zawada, A.; Skoracka, K.; Dobrowolska, A.; Krela-Kaźmierczak, I. Physical activity, quality of diet and bone mineral density in patients with inflammatory bowel disease. J. Hum. Nutr. Diet. 2023, 36, 1692–1700. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Zheng, M.; Zhou, L.; Zhang, J.; Wang, J.; Sun, B. The potential benefits and mechanisms of protein nutritional intervention on bone health improvement. Crit. Rev. Food Sci. Nutr. 2023, 19, 6380–6394. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Ribaldone, D.G.; Bellini, M.; Mumolo, M.G.; Costa, F. Inflammatory Bowel Diseases: Is There a Role for Nutritional Suggestions? Nutrients 2021, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Krela-Kaźmierczak, I.; Michalak, M.; Szymczak-Tomczak, A.; Czarnywojtek, A.; Wawrzyniak, A.; Łykowska-Szuber, L.; Stawczyk-Eder, K.; Dobrowolska, A.; Eder, P. Milk and dairy product consumption in patients with inflammatory bowel disease: Helpful or harmful to bone mineral density? Nutrition 2020, 79–80, 110830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Liu, H.; Liu, X.; Yu, Y.; Han, D.; He, X.; Zeng, P.; Wang, J. Soy-whey dual-protein alleviates osteoporosis of ovariectomized rats via regulating bone fat metabolism through gut-liver-bone axis. Nutrition 2022, 103–104, 111723. [Google Scholar] [CrossRef] [PubMed]
| Dietary Protein Sources | Dosage | Model | Intervention Time | Conclusion | Reference |
|---|---|---|---|---|---|
| Soy protein | 35% energy-providing protein intake | TNBS-induced colitis in SD rats | 4 weeks | Soy protein improved podoplanin + infiltration of colonic mucosal structure; inhibited the proliferation of colon tumor necrosis factor-α + cells and RANKL expression; suppressed the expression of pro-inflammatory tumor necrosis factor-α and interleukin-6 in bone proteins; and mitigated the high osteoclast surface and depressed bone formation rate in TNBS rats. | [83] |
| Soy–Pea Protein | 17.2% energy-providing protein intake | DSS-induced colitis in SAMP mice | 6 weeks | Fecal myeloperoxidase (MPO) and FITC-glucan permeability scores were significantly decreased; the severity of cobblestone lesions decreased; the abundance of Lactobacillaceae and Leuconostraceae increased; and the concentration of metabolites glutamine and butyric acid increased, while the concentration of plasma linoleic acid decreased. | [28] |
| Soy protein | 20% energy-providing protein intake | DSS-induced colitis in C57BL/6 mice | 12 days | Soy protein reduced the content of mucin MUC1 and trefoil factor TFF-3 in the colon; inhibited the DSS-induced reduction in colon length; lowered the colon inflammation score; and reduced the expression of tumor necrosis factor-α in the colon and cecum. | [84] |
| Casein and whey protein concentrate | 10% energy-providing casein + 10% energy-providing whey protein concentrate | DSS-induced colitis in BALB/c mice | 3 weeks | Whey protein concentration can improve the loss of body mass in mice, and the effect of low-temperature treatment is more significant; low-temperature treatment of concentrated whey protein significantly reduced colonic inflammation and improved mucosal results; both low-temperature and high-temperature treatment of concentrated whey protein can increase the colonic mucin level; the level of myeloperoxidase in the colon of low-temperature whey protein concentration decreased; and low-temperature whey protein concentration down-regulated the expression of Gbp1, Gbp2, Gbp6 and Cxcl9. | [85] |
| Whey protein | 18% energy-providing protein intake | DSS-induced colitis in Wistar rats | 19 days | Whey protein reduced the expression of interleukin-1β, calprotectin, and inducible nitric oxide synthase; alleviated the clinical symptoms of diarrhea and fecal blood loss; increased the secretion of fecal mucin; and increased the expression of Lactobacillus and Bifidobacterium. | [86] |
| Whey protein | 2.39 or 4.78 g/kg·body weight/day | Acetic acid-induced colitis in Wistar rats | 7 days | Whey protein decreased the levels of inflammatory markers AP-1, COX-2, interleukin-6, interleukin-10, NF-κB, and tumor necrosis factor-α; up-regulated Nrf2 and HO-1 expression; and activated Nrf2/HO-1 pathway. | [87] |
| Acid casein and whey protein | 14%, 30%, and 53% energy-providing protein intake (acid casein:whey protein = 5:1) | DSS-induced colitis in C57BL/6 mice | 21 days | Compared with a dietary protein level of 14%, a 30% dietary protein diet increased epithelial repair by accelerating inflammation resolution, reducing colon permeability; 53% dietary protein diet aggravated DSS-induced inflammation. | [88] |
| Casein, whey protein, soy protein, white meat, and red meat | 40% energy-providing protein intake | DSS-induced colitis in BALB/c mice | 28 days | 40% casein and red meat can exacerbate colitis; 40% whey protein can effectively alleviate colitis. | [26] |
| Casein or wheat gluten | 20% and 60% energy-providing protein intake | DSS-induced colitis in BALB/c mice | 35 days | The increase in animal protein resulted in a significant increase in colon Ly-6Chigh monocytes and their activation; intestinal inflammation associated with anti-inflammatory TGF-β, pro-inflammatory cytokines (TNF-α and IL-1β), and inducible NO synthetase increased in mice fed a diet rich in animal protein, while plant-protein-rich diets generally decreased their expression. | [89] |
| Milk protein | 53% energy-providing protein intake | DSS-induced colitis in C57BL/6 mice | 14 days | Compared with the control group of animals that received DSS treatment, a high-protein diet is harmful in the later stages of induction, but it helps with the repair of colonic crypts after acute inflammation. | [90] |
| Whey proteins or donkey Whey proteins | 0.2 g/d whey proteins or donkey whey proteins | DSS-induced colitis in C57BL/6 mice | 21 days | Compared with the control group, both whey protein and donkey whey protein had the ability to inhibit the expression of proinflammatory protein and inflammatory secretion, and significantly decreased the levels of NF-κB and CD86; donkey whey protein is more effective than bovine whey protein in improving DSS induced colitis. | [91] |
| Dietary protein levels | 14%, 30%, and 53% energy-providing protein intake | DSS-induced colitis in C57BL/6 mice | 28 days | Compared with the other two diets, the diet with 30% protein content is associated with a lower protein synthesis rate, which can restore the initial level of the colon; it can restore colitis-induced changes such as body weight, cecal protein content, and spleen and muscle protein synthesis rates earlier; reduce inflammation and bacterial translocation in mice. | [92] |
| Whey protein hydrolysate | 300 or 600 mg/kg·body weight/day | DSS-induced colitis in C57BL/6 mice | 37 days | High doses of whey protein hydrolysate can significantly inhibit weight loss in mice with colitis, protect the colonic mucosal layer, significantly reduce the levels of inflammatory factors TNF-α, IL-6, and IL-1β in colitis mice; upregulate the secretion of short-chain fatty acids in colitis mice, and restore the imbalance of intestinal flora. | [27] |
| Alaska Pollock protein (APP) | 20% energy-providing protein intake | DSS-induced colitis in C57BL/6 mice | 51 days | APP intake inhibited DSS-induced weight loss, increased the disease activity index, increased spleen weight, shortened colon length, alleviated colonic tissue injury, and changed the structure and composition of fecal microbiota and short-chain fatty acids. | [93] |
| Quinoa protein or quinoa peptide | quinoa protein (1 g/kg·body weight/day) or quinoa peptide (500 or 1000 mg/kg·body weight/day) | DSS-induced colitis in C57BL/6 mice | 35 days | Quinoa protein and quinoa peptide effectively relieve colitis symptoms: diarrhea, abdominal pain, bloody stool, weight loss, colon shortening, inflammatory factor release, and intestinal barrier damage. They also regulate gut microbiota, boost short-chain fatty acid production, and inhibit I-κB-α and NF-κB phosphorylation in colon tissues. | [94] |
| Intervention Factors | Dosage | Age (Years) | Model | Intervention Time | Conclusion | Reference |
|---|---|---|---|---|---|---|
| Whey protein concentrate | 0.5 g/kg·body weight/d | 24~46 | CD | 8 weeks | Intestinal permeability and intestinal morphology were significantly improved; the villous crypt ratio was significantly increased. Inflammatory markers (intestinal epithelial lymphocytes, IELs) were reduced. | [30] |
| A diet rich in plant protein | - | 26~41 | Inactive CD | 4 weeks | Significantly improved body composition in inactive and lactose intolerant CD patients; improved the treatment compliance of lactose intolerant patients. | [155] |
| Whey protein + resistance training (3 times weekly) | 10 g/d whey protein + resistance training (3 times weekly) | 30~58 | IBD | 8 weeks | Skeletal muscle mass was significantly higher than that of placebo group. Albumin, hemoglobin, and creatinine were significantly increased. | [31] |
| Whey protein + transforming growth factor(TGF) | - | 16~62 | CD | 16 weeks | Lean body mass increased, while fat decreased. | [156] |
| Whey protein or soy protein isolate | 30 g/d whey protein or 24 g/d soy protein isolate | - | CD | 16 weeks | Both whey protein and soy protein isolate can reduce the triceps skinfold thickness and body fat percentage, while increasing the mid-arm circumference, correcting the arm muscle area, and increasing the percentage of lean body mass. | [29] |
| Hydrolyzed whey protein | Hydrolyzed whey protein (9.3–27.9 g/d) | - | CD | 12 weeks | The improvement of nutritional status was related to the number of nutritional supplements; average albumin levels and body mass index improved; the index of disease activity decreased significantly; the average number of bowel movements per day decreased. | [32] |
| Dietary protein | 20.7–152.5 g/d | 27~45 | CD | - | Protein intake was positively correlated with spinal bone density. | [157] |
| Casein glycomacropeptide | 30 g/d | 23~76 | UC | 8 weeks | A similar proportion of patients receiving casein glycomacropeptide or mesalamide dose escalation had a clinical response; colitis activity index and endoscopic lesion degree decreased; casein glycomacropeptide had good tolerance and acceptability. | [158] |
| Bovine lactoferrin | 1 g/d | 22 | CD | 42 months | After about 9 months, the disease activity index dropped from 50 to 35. After approximately 3 years and 5 months, blood tests showed no signs of disease relapse. The colonoscopy conducted at 3 years and 6 months revealed almost complete mucosal healing. | [159] |
| Lactoferrin | 100 mg/d | 9~14 | IBD | 3 months | Compared to ferrous sulfate, lactoferrin significantly increases hemoglobin, serum iron, and serum ferritin; lactoferrin significantly decreased interleukin 6 (IL-6) and hepcidin levels. | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, J. The Effect of Protein Nutritional Support on Inflammatory Bowel Disease and Its Potential Mechanisms. Nutrients 2024, 16, 2302. https://doi.org/10.3390/nu16142302
Li Q, Wang J. The Effect of Protein Nutritional Support on Inflammatory Bowel Disease and Its Potential Mechanisms. Nutrients. 2024; 16(14):2302. https://doi.org/10.3390/nu16142302
Chicago/Turabian StyleLi, Qingye, and Jing Wang. 2024. "The Effect of Protein Nutritional Support on Inflammatory Bowel Disease and Its Potential Mechanisms" Nutrients 16, no. 14: 2302. https://doi.org/10.3390/nu16142302
APA StyleLi, Q., & Wang, J. (2024). The Effect of Protein Nutritional Support on Inflammatory Bowel Disease and Its Potential Mechanisms. Nutrients, 16(14), 2302. https://doi.org/10.3390/nu16142302





