Enhancing Vascular Health and Lowering Blood Pressure in Spontaneously Hypertensive Rats through Syrah Grape (Vitis vinifera) Pomace: The Role of Phenolic Compounds
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collection and Storage
2.2. Ultrasound-Assisted Extraction of Samples
2.3. Bioactive Compounds
2.3.1. Determination of Total Phenolic Compounds
2.3.2. Determination of Total Monomeric Anthocyanins
2.3.3. Determination of Condensed Tannins
2.3.4. Determination of Total Carotenoids
2.4. Phytochemical Analysis
2.4.1. Extraction of Non-Anthocyanin Phenolic Compounds for Analysis by HPLC-DAD-ESI-MSn
2.4.2. Identification and Quantification of Non-Anthocyanin Phenolic Compounds by HPLC–DAD-ESI–MSn
2.5. Methodology for Biological Tests
2.5.1. Production and Characterization of Experimental Chow
2.5.2. Determination of Chow Total Phenolic Content
2.5.3. Animals
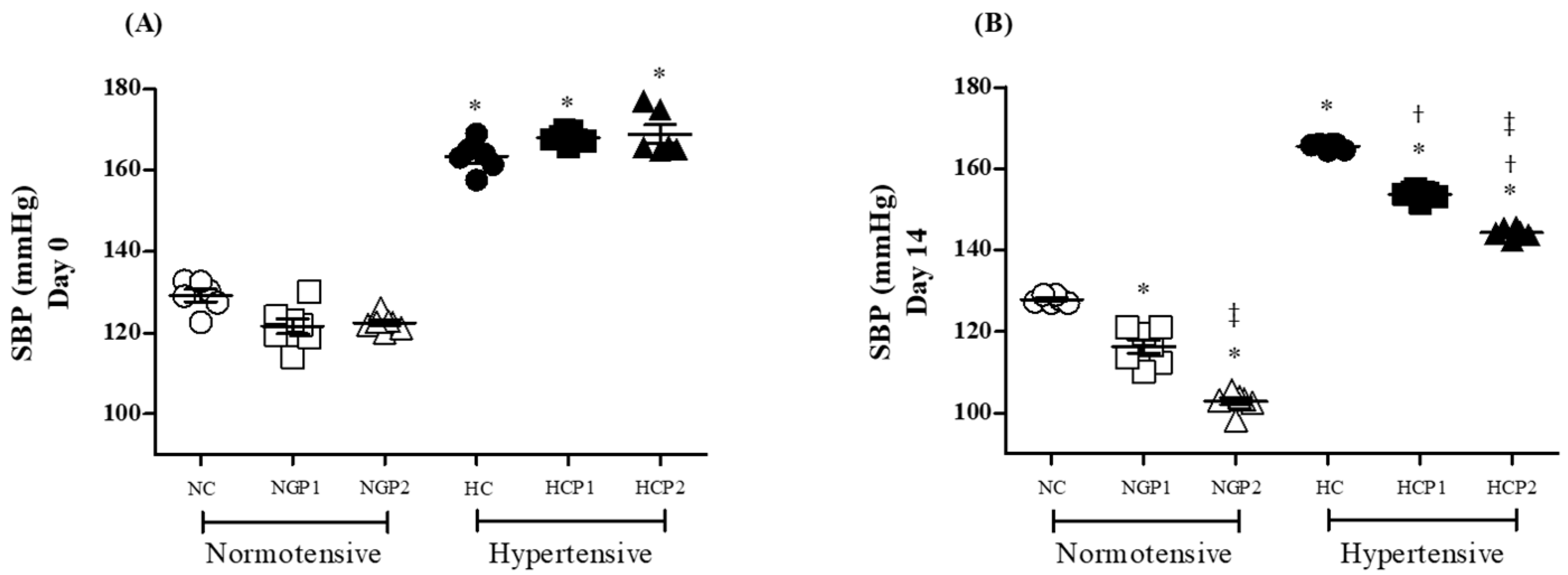
2.5.4. Assessment of Blood Pressure Levels
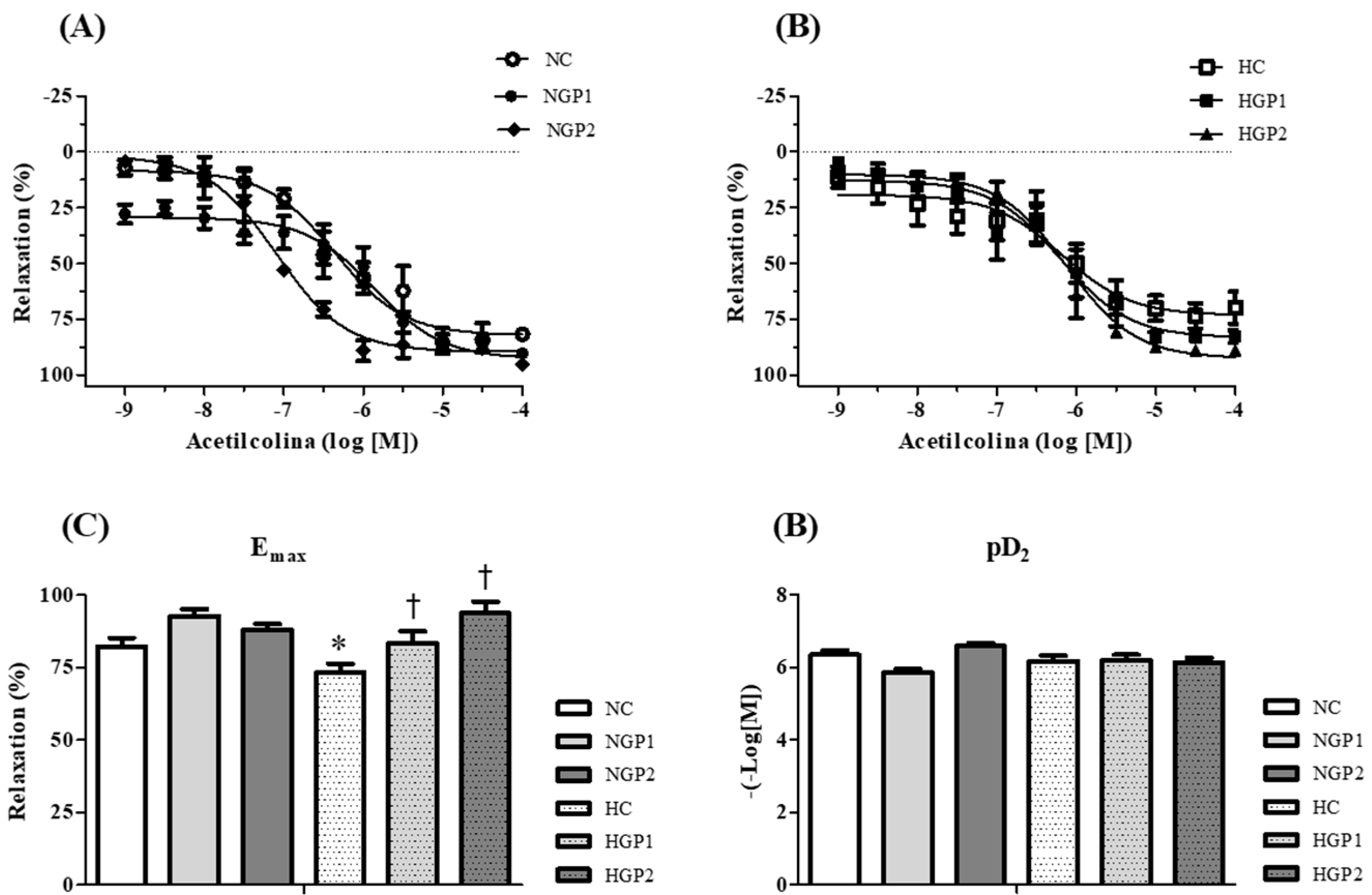
2.5.5. Vascular Reactivity
2.6. Statistical Analysis
3. Results
3.1. Spectrophotometric Analysis of Bioactive Compounds
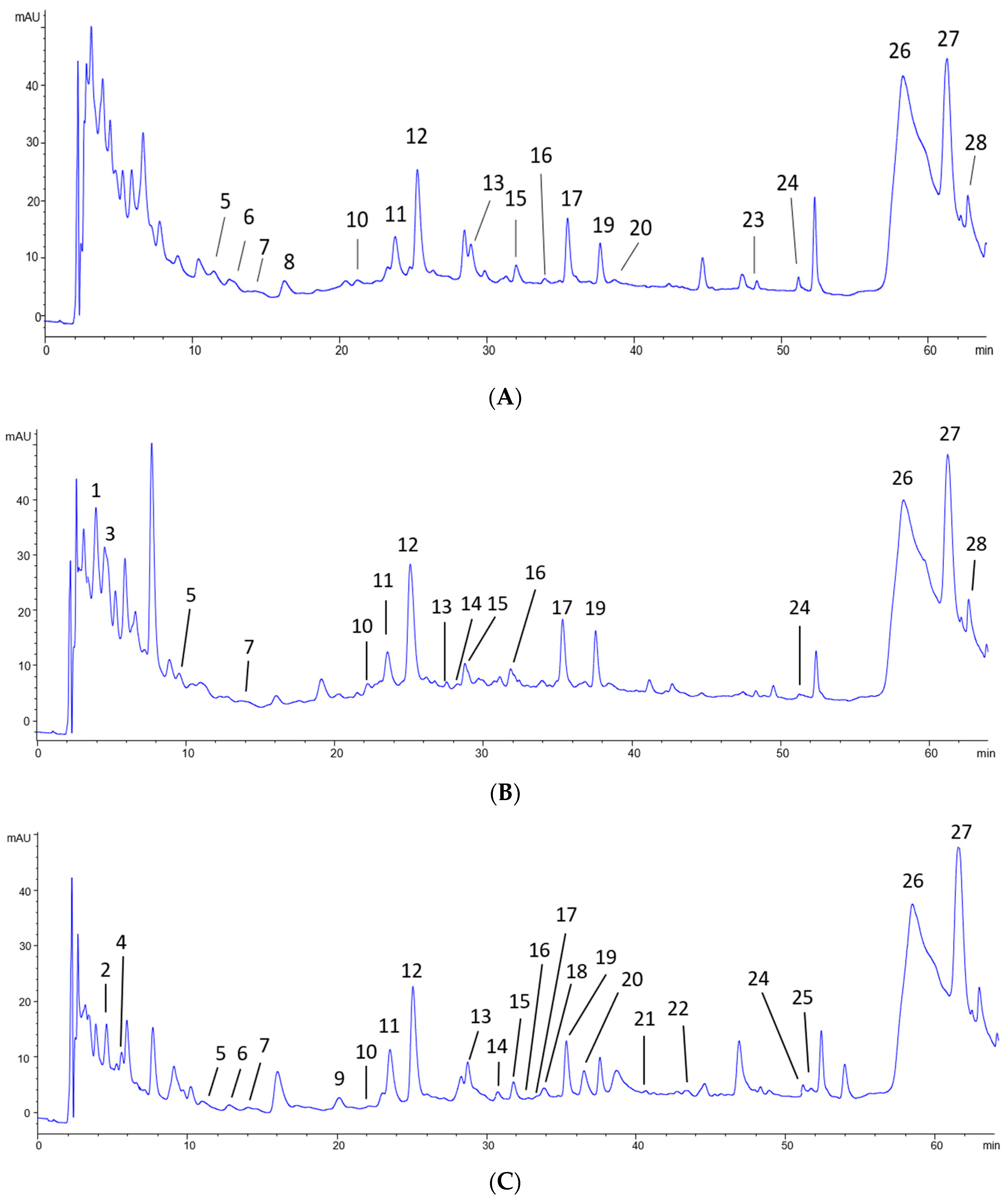
3.2. Chromatographic Analysis of Non-Anthocyanin Phenolic Compounds
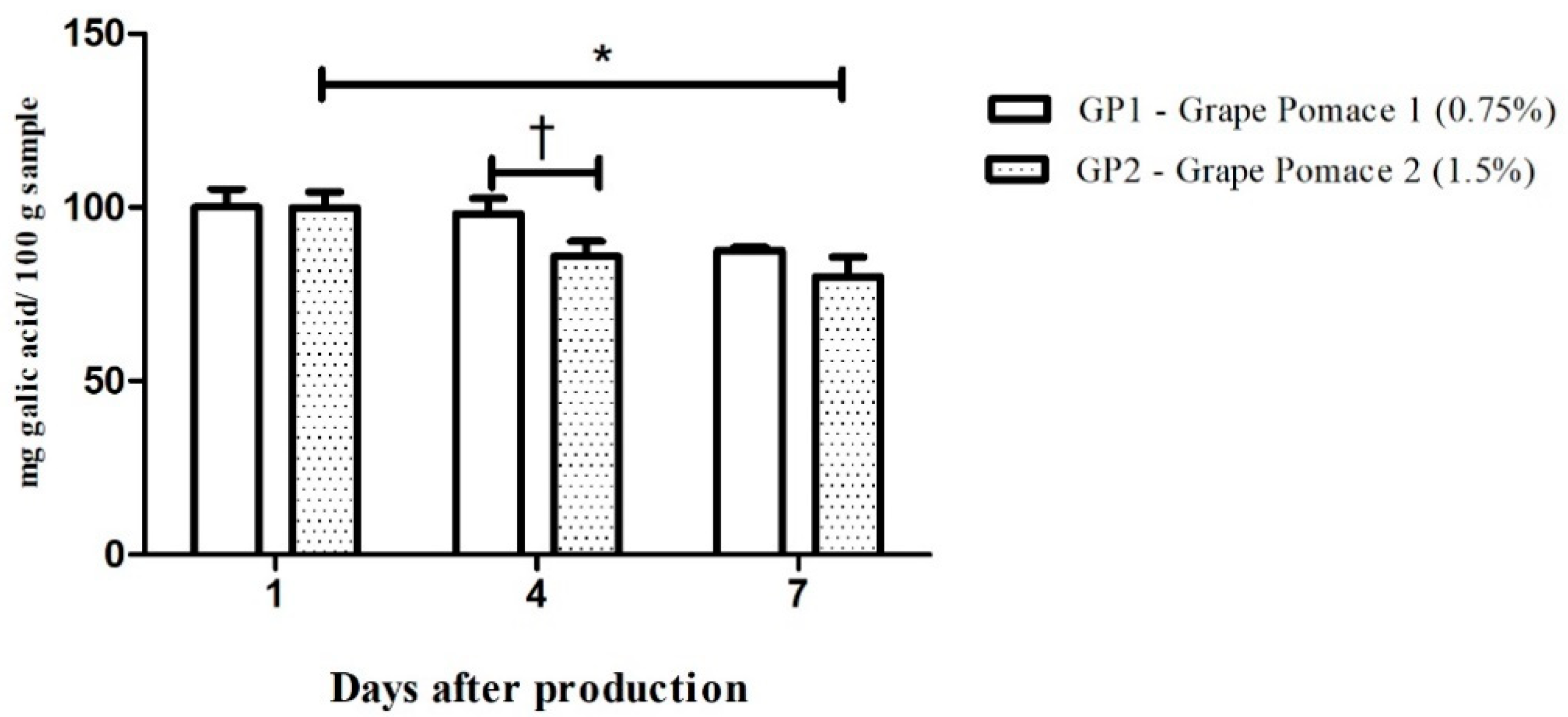
3.3. Grape Pomace-Enriched Chow and Biological Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; de Souza Schmidt Gonçalves, A.E.; Genovese, M.I.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; de Morais, S.M.; de Lima, A.; Travassos Ferreira, P.A.; Moreira Rodrigues, A.L.; Praciano Rodrigues, S.; do Nascimento Silva, J.; Lages Rodrigues, L. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef] [PubMed]
- Barcia, M.T.; Pertuzatti, P.B.; Rodrigues, D.; Bochi, V.C.; Hermosín-Gutiérrez, I.; Teixeira Godoy, H. Effect of drying methods on the phenolic content and antioxidant capacity of Brazilian winemaking byproducts and their stability over storage. Int. J. Food Sci. Nutr. 2015, 66, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro Fernandes, C.; Santana, L.F.; Dos Santos, J.R.; Fernandes, D.S.; Hiane, P.A.; Pott, A.; de Cassia Freitas, K.; Bogo, D.; Aragao do Nascimento, V.; de Oliveira Filiu, W.F.; et al. Nutraceutical Potential of Grape (Vitis vinifera L.) Seed Oil in Oxidative Stress, Inflammation, Obesity and Metabolic Alterations. Molecules 2023, 28, 7811. [Google Scholar] [CrossRef] [PubMed]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Patrasca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Schön, C.; Allegrini, P.; Engelhart-Jentzsch, K.; Riva, A.; Petrangolini, G. Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers. Nutrients 2021, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1,201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Acelajado, M.C.; Hughes, Z.H.; Oparil, S.; Calhoun, D.A. Treatment of Resistant and Refractory Hypertension. Circ. Res. 2019, 124, 1061–1070. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ruiz, Z.; Toledo, E.; Vitelli-Storelli, F.; Goni, L.; de la, O.V.; Bes-Rastrollo, M.; Martinez-Gonzalez, M.A. Effect of Dietary Phenolic Compounds on Incidence of Cardiovascular Disease in the SUN Project; 10 Years of Follow-Up. Antioxidants 2022, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and others oxidation substrates and antioxidants by means of Folin-Ciocalteau Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Giusti, M.; Wrolstad, R.E. Characterization and measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.J. Herderich, P.A. et al. Quantification of condensed tannins by precipitation with methylcellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brncic, M.; Bysted, A.; Cano, M.P.; Graca Dias, M.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Lourdes, M.C.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Galanakis, C.M. Handbook of Grape Processing By-Products-Sustainable Solutions, 1st ed.; Academic Press: London, UK, 2017; ISBN 9780128098707. [Google Scholar]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Screening of pomace polyphenols from red and white grape varieties (Vitis vinifera L.) by HPLC–DAD–MS/MS. J. Agric. Food Chem 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Schieber, A.; Reinhold, C. A new process for the recovery of polyphenols from grape pomace (Vitis vinifera L.). J. Food Sci. 2005, 70, 157–163. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Lago-Vanzela, E.S.; Barcia, M.T.; Ramos, A.M.; Stringheta, P.C.; Da Silva, R.; Castillo-Munez, N.; Gomez-Alonso, S.; Hermosin-Gutierrez, I. Phenolic composition of the berry parts of hybrid grape cultivar BRS Violeta (BRS Rubea×IAC 1398-21) using HPLC–DAD–ESI-MS/MS. Food Res. Int. 2013, 54, 354–366. [Google Scholar] [CrossRef]
- Barcia, M.T.; Pertuzatti, P.B.; Gómez-Alonso, S.; Godoy, H.T.; Hermosín-Gutiérrez, I. Phenolic composition of grape and winemaking by-products of Brazilian hybrid cultivars BRS Violeta and BRS Lorena. Food Chem. 2014, 159, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, K.; Esmaeilzadeh, F.; Hatami, M.; Forough, M.; Molaie, R. Determination of phenolic compounds content and antioxidant activity in skin, pulp, seed, cane and leaf of five native grape cultivars in West Azerbaijan province, Iran. Food Chem. 2016, 199, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Lima, N.E.; Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Impact of Pressing Conditions on the Phenolic Composition, Radical Scavenging Activity and Glutathione Content of Brazilian Vitis vinifera White Wines and Evolution During Bottle Ageing. Food Bioprocess. Technol. 2016, 9, 944–957. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Mendonça, S.C.; Alcoléa, M.; Guedes, C.T.; Amorim, F.E.; Simoes Beckmann, A.P.; Almeida Gama, L.; Americo, M.F. Bordo grape marc (Vitis labrusca): Evaluation of bioactive compounds in vitro and in vivo. LWT 2020, 129, 109625. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Fernández-González, M.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. J. Agric. Food Chem. 2009, 57, 7883–7891. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons Ltd: Chichester, UK, 2006; ISBN 13 978-0-470-01034-1. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd: Chichester, UK, 2006; ISBN 978-0-470-01037-2. [Google Scholar]
- Colombo, R.C.; Roberto, S.R.; Nixdorf, S.L.; Pérez-Navarro, J.; Gómez-Alonso, S.; Mena-Morales, A.; Garcia-Romero, E.; Simoes Azeredo Goncalves, L.; Aparecida da Cruz, M.; de Carvalho, D.U.; et al. Analysis of the phenolic composition and yield of ‘BRS Vitoria’ seedless table grape under different bunch densities using HPLC-DAD-ESI-MS/MS. Food Res. Int. 2020, 130, 108955. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Ferrandino, A.; Carra, A.; Rolle, L.; Schneider, A.; Schubert, A. Profiling of hydroxycinnamoyl tartrates and acylated anthocyanins in the skin of 34 Vitis vinifera genotypes. J. Agric. Food Chem. 2012, 60, 4931–4945. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; Cazals., G.; Enjalbal., C.; Izquierdo-Cañas, P.M.; Gómez-Alonso, S.; Saucier, C. Flavonol Glycoside Content of Grape Seeds and Skins of Vitis vinifera Varieties Grown in Castilla-La Mancha, Spain. Molecules 2019, 24, 4001. [Google Scholar] [CrossRef]
- Simões, C.M.O.; Schenkel, E.P.; Mello, J.C.P.; Mentz, L.A.; Petrovick, P.R. Pharmacognosy: From Natural Product to Medicine, 1st ed.; Artmed Editor LTDA: Porto Alegre, Brazil, 2017; ISBN 978-8582713594. [Google Scholar]
- Eskin, N.A.M.; Shahidi, F. Food Biochemistry, 3rd ed.; Academic Press: London, UK, 2012; ISBN 9780080918099. [Google Scholar]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality-A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar] [CrossRef]
- Lewis, K.V.; Barros, F.; Cure, M.B.; Davies, C.A.; Furtado, M.N.; Hill, T.C.; Hirota, M.; Martins, D.L.; Mazzochini, G.G.; Mitchard, E.T.A.; et al. Mapping native and non-native vegetation in the Brazilian Cerrado using freely available satellite products. Sci. Rep. 2022, 12, 1588. [Google Scholar] [CrossRef]
- Pellizzon, M.A.; Ricci, M.R. The common use of improper control diets in diet-induced metabolic disease research confounds data interpretation: The fiber factor. Nutr. Metab. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Del Pino-García, R.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Croft, K.D.; Muñiz, P. Antihypertensive and antioxidant effects of supplementation with red wine pomace in spontaneously hypertensive rats. Food Funct. 2017, 8, 2444–2454. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science–Principles and Applications, 5th ed.; Academic Press: London, UK, 2008; ISBN 9780128161180. [Google Scholar]
- López-Fernández-Sobrino, R.; Soliz-Rueda, J.R.; Ávila-Román, J.; Arola-Arnal, A.; Suárez, M.; Muguerza, B.; Bravo, F.I. Blood Pressure-Lowering Effect of Wine Lees Phenolic Compounds Is Mediated by Endothelial-Derived Factors: Role of Sirtuin 1. Antioxidants 2021, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; Sander, G.E.; Nossaman, B.D.; Kadowitz, P.J. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J. Clin. Hypertens. 2012, 14, 198–205. [Google Scholar] [CrossRef]
- Goulopoulou, S.; Webb, R.C. Symphony of vascular contraction: How smooth muscle cells lose harmony to signal increased vascular resistance in hypertension. Hypertension 2014, 63, e33–e39. [Google Scholar] [CrossRef] [PubMed]
- Arzola-Rodríguez, S.I.; Muñoz-Castellanos, L.N.; López-Camarillo, C.; Salas, E. Phenolipids, Amphipilic Phenolic Antioxidants with Modified Properties and Their Spectrum of Applications in Development: A Review. Biomolecules 2022, 12, 1897. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Hidalgo, J.M.; Martínez-Rodríguez, A.J.; Martín-Álvarez, P.J.; Pueyo, E. Influence of the elaboration process on the peptide fraction with angiotensin I-converting enzyme inhibitor activity in sparkling wines and red wines aged on lees. Food Chem. 2008, 111, 965–969. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Schini-Kerth, V.B.; Auger, C.; Kim, J.H.; Etienne-Selloum, N.; Chataigneau, T. Nutritional improvement of the endothelial control of vascular tone by polyphenols: Role of NO and EDHF. Pflug. Arch. 2010, 459, 853–862. [Google Scholar] [CrossRef]
- Chacar, S.; Hajal, J.; Saliba, Y.; Bois, P.; Louka, N.; Maroun, R.G.; Faivre, J.-F.; Fares, N. Long-term intake of phenolic compounds attenuates age-related cardiac remodeling. Aging Cell 2019, 18, e12894. [Google Scholar] [CrossRef]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Perdicaro, D.J.; Rodriguez Lanzi, C.; Fontana, A.R.; Antoniolli, A.; Piccoli, P.; Miatello, R.M.; Diez, E.R.; Vazquez Prieto, M. Grape pomace reduced reperfusion arrhythmias in rats with a high-fat-fructose diet. Food Funct. 2017, 8, 3501–3509. [Google Scholar] [CrossRef] [PubMed]
- Balea, Ş.S.; Pârvu, A.E.; Pop., N.; Marín, F.Z.; Pârvu, M. Polyphenolic Compounds, Antioxidant, and Cardioprotective Effects of Pomace Extracts from Fetească Neagră Cultivar. Oxid. Med. Cell. Longev. 2018, 2018, 8194721. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, L.; El-Kubbe, A.; Roney, S.K. Potential Cardio-Protective Effects of Green Grape Juice: A Review. Curr. Nutr. Food Sci. 2019, 15, 202–207. [Google Scholar] [CrossRef]
- Larson, A.J.; Symons, J.D.; Jalili, T. Therapeutic potential of quercetin to decrease blood pressure: Review of efficacy and mechanisms. Adv. Nutr. 2012, 3, 39–46. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J.; Jimenez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive effects of the flavonoid quercetin. Pharmacol. Rep. 2009, 61, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Blaszczak, P.; Fornal, E. Dietary Isorhamnetin Intake Is Associated with Lower Blood Pressure in Coronary Artery Disease Patients. Nutrients 2022, 14, 4586. [Google Scholar] [CrossRef] [PubMed]
- Lucas Filho, M.D. Phytochemical Study of Species of the Genus Erythroxylum with Potential Vasodilatory and Angiotensin-Converting Enzyme Inhibitory Activity. Master’s Thesis, Federal University of Ouro Preto, Ouro Preto, Brazil, 2009. [Google Scholar]
- Quaresma, D.M.O. Phytochemical and Pharmacological Study of Solanum subumbellatum Vell. Ph.D. Thesis, Federal University of Uberlândia, Uberlândia, Brazil, 2023. [Google Scholar]


| Analysis | Grape | Grape Pomace | Lees |
|---|---|---|---|
| Total phenolic compounds (mg GAE/100 g sample) | 350.3 ± 8.3 | 895.5 ± 15.8 * | 102.43 ± 6.1 |
| Condensed tannins (mg epicatechin/100 g sample) | 1098.3 ± 48.1 | 2211.6 ± 207.3 * | 295.60 ± 29.1 *,† |
| Total monomeric anthocyanins (mg M-3-glc/100 g sample) | 20.84 ± 0.15 | 25.68 ± 1.6 * | 13.47 ± 0.36 *,† |
| Total carotenoids (μg β-carotene/g sample) | 747.33 ± 12.7 | 782.45 ± 55 | 616.30 ± 21.3 *,† |
| Nº | Phenolics Compounds | Rt (min) | Molecular Ion; Product Ions (m/z) a | Grape (molar%) | Grape Pomace (molar%) | Lees (molar%) |
|---|---|---|---|---|---|---|
| Flavonols | ||||||
| Total (mg/kg sample) b | 60.08 ± 22.20 | 68.65 ± 12.03 | 111.16 ± 7.43 *,† | |||
| 5 | M-3-glcU | 14.1 | 493; 317 | 1.40 ± 0.44 | 1.00 ± 0.36 | 1.34 ± 0.09 |
| 6 | M-3-gal | 14.5 | 479; 317 | 5.38 ± 0.22 | ND | 0.44 ± 0.04 * |
| 7 | M-3-glc | 16.1 | 479.2; 317 | 3.01 ± 0.53 | 2.46 ± 0.99 | 14.93 ± 0.55 *,† |
| 8 | M-3-dihex | 16.2 | 479.2; 317 | 7.26 ± 0.85 | ND | ND |
| 9 | M-3-rhm | 20 | 463; 317 | ND | ND | 4.27 ± 0.76 |
| 10 | Q-3-gal | 23 | 463.2; 301 | 1.73 ± 0.50 | 1.83 ± 0.42 | 0.80 ± 0.07 * |
| 11 | Q-3-glcU | 23.7 | 477.3; 301 | 9.30 ± 0.50 | 11.24 ± 2.37 | 9.18 ± 0.21 |
| 12 | Q-3-glc | 25.3 | 463.3; 301 | 31.20 ± 1.62 | 35.99 ± 1.92 * | 22.29 ± 0.92 *,† |
| 13 | L-3-glc | 28.7 | 493; 331 | 4.04 ± 0.51 | 5.94 ± 0.65 * | 4.02 ± 0.16 b |
| 14 | L-3-gal | 29.9 | 493; 331 | ND | 2.88 ± 0.61 | ND |
| 15 | K-3-glc | 31.8 | 447; 285 | 5.29 ± 0.71 | 5.89 ± 1.68 | 3.05 ± 0.16 † |
| 16 | I-3-gal | 33.8 | 477.4; 315 | 1.28 ± 0.34 | 1.67 ± 0.52 | 1.76 ± 0.17 |
| 17 | I-3-glc | 35.4 | 477.3; 315 | 14.23 ± 1.16 | 15.68 ± 1.44 | 8.08 ± 0.26 *,† |
| 18 | I-3-hex | 36.2 | 477.2; 315 | ND | ND | 5.01 ± 0.54 |
| 19 | S-3-gal | 37.6 | 507.3; 345 | 10.07 ± 0.49 | 14.40 ± 0.88 * | 6.09 ± 0.35 *,† |
| 20 | S-3-glc | 38.6 | 623; 344 | 1.54 ± 0.12 | ND | 9.30 ± 0.93 * |
| 21 | Free Q | 39.3 | 301; 301 | ND | ND | 0.87 ± 0.15 |
| 22 | Q-3-rut | 43.4 | 609; 301 | ND | ND | 1.23 ± 0.06 |
| 23 | I-3-rut | 48.3 | 623; 315 | 1.60 ± 0.71 | ND | ND |
| 24 | S-3-rut | 51.3 | 623; 345 | 2.68 ± 0.18 | 1.02 ± 0.18 * | 1.57 ± 0.16 *,† |
| 25 | Free I | 51.7 | 315; | ND | ND | 5.78 ± 0.60 |
| Hydroxynamic acid derivatives | ||||||
| Total (mg/kg sample) c | 132.15 ± 3.23 | 150.91 ± 8.18 * | 139.00 ± 1.25 | |||
| 1 | Trans-caffeic acid | 3.9 | 341; 179; 161; 135 | ND | 5.0 ± 1.04 | ND |
| 2 | Cis-coutaric acid | 4.4 | 295; 149 | ND | ND | 2.71 ± 0.11 |
| 3 | Cis-caffeic acid | 4.5 | 341; 179; 135 | ND | 4.59 ± 1.44 | ND |
| 4 | Trans-fertaric acid | 5.9 | 325; 193 | ND | ND | 3.25 ± 0.19 |
| 26 | Ethyl caffeate | 59.6 | 207; 179 | 77.21 ± 1.91 | 68.93 ± 0.78 * | 70.97 ± 0.12 * |
| 27 | Trans-caftaric acid | 60.7 | 311; 149 | 22.44 ± 1.82 | 21.09 ± 1.60 | 23.08 ±0.21 |
| 28 | Cis-caftaric acid | 62.1 | 311; 179 | 0.34 ± 0.10 | 0.39 ± 0.03 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, K.C.M.; Oliveira, L.d.S.; Silva, J.C.; Santana, T.S.; de Freitas, R.A.; Bressan, A.F.M.; Gómez-Alonso, S.; Pérez-Navarro, J.; Pertuzatti, P.B.; Giachini, F.R. Enhancing Vascular Health and Lowering Blood Pressure in Spontaneously Hypertensive Rats through Syrah Grape (Vitis vinifera) Pomace: The Role of Phenolic Compounds. Nutrients 2024, 16, 2312. https://doi.org/10.3390/nu16142312
da Costa KCM, Oliveira LdS, Silva JC, Santana TS, de Freitas RA, Bressan AFM, Gómez-Alonso S, Pérez-Navarro J, Pertuzatti PB, Giachini FR. Enhancing Vascular Health and Lowering Blood Pressure in Spontaneously Hypertensive Rats through Syrah Grape (Vitis vinifera) Pomace: The Role of Phenolic Compounds. Nutrients. 2024; 16(14):2312. https://doi.org/10.3390/nu16142312
Chicago/Turabian Styleda Costa, Kelly C. M., Lorrayne de S. Oliveira, Júlia C. Silva, Taynara S. Santana, Raiany A. de Freitas, Alecsander F. M. Bressan, Sérgio Gómez-Alonso, José Pérez-Navarro, Paula B. Pertuzatti, and Fernanda R. Giachini. 2024. "Enhancing Vascular Health and Lowering Blood Pressure in Spontaneously Hypertensive Rats through Syrah Grape (Vitis vinifera) Pomace: The Role of Phenolic Compounds" Nutrients 16, no. 14: 2312. https://doi.org/10.3390/nu16142312





