Harnessing the Gut Microbiome: To What Extent Can Pre-/Probiotics Alleviate Immune Activation in Autism Spectrum Disorder?
Abstract
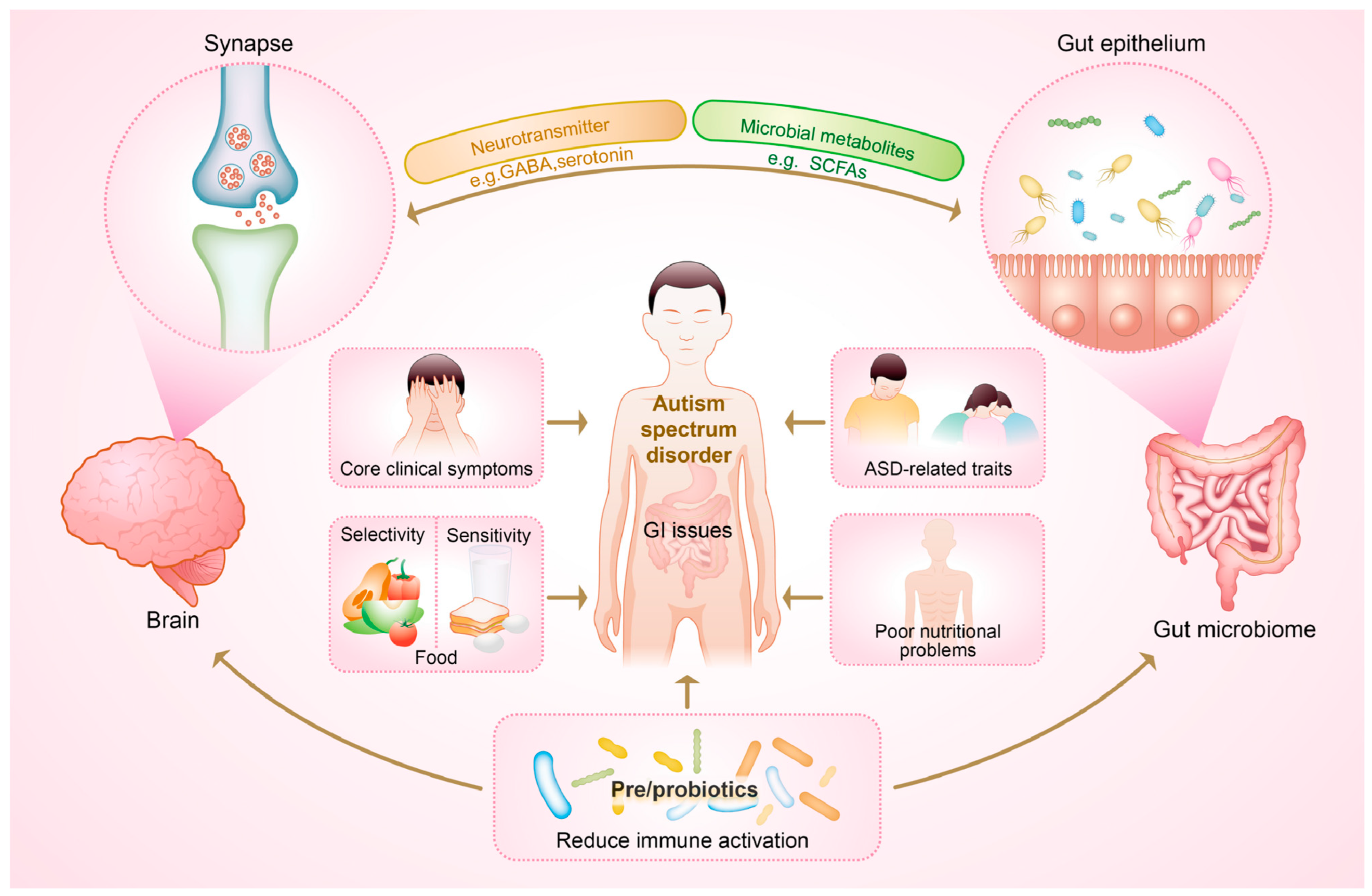
:1. Introduction
2. Gut Microbiome-Mediated Immune Dysregulation Co-Occurs with Poor Nutritional Status
2.1. Gut Dysbiosis
2.2. Gastrointestinal Symptoms
2.3. Food Allergy and Digestive Discomfort
2.4. Neuroinflammation
3. Gut Microbiome in Connection with ASD
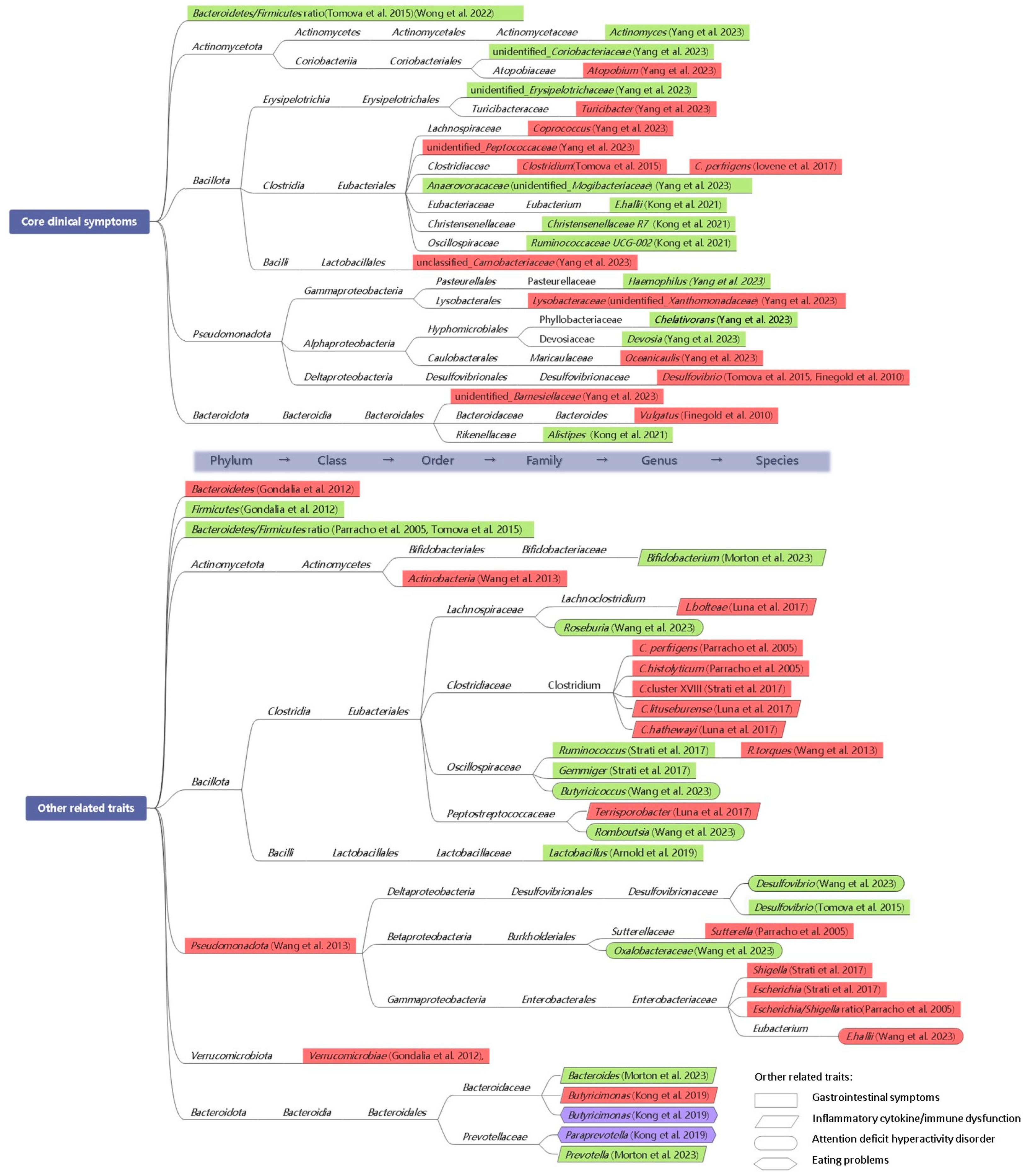
3.1. Microbiota Profile with ASD Clinical Symptoms
3.1.1. Core Clinical Symptoms
3.1.2. Other Related Traits
3.2. Microbiota Profile with Atypical Eating Behaviors and Nutritional Status
4. Beyond the “Cure”: Potential of Pre-/Probiotics
4.1. Clinical Evidence on Pre-/Probiotics
4.1.1. Probiotics
4.1.2. Prebiotics
4.1.3. Symbiotic and Combined Treatments
4.2. Potential Fecal Microbiota Transplantation (FMT) in ASD
| Methods | Pre-/Probiotics | Characteristics | Measures | Gut Microbial Changes | GI Issues | Biomarkers | Core Symptoms | Sleep | Related Traits | Parents’ Rating | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Probiotics | |||||||||||
| Crossover-controlled | Lactobacillus plantarum WCFS1 | 17 ASD children; 6-week period; Britain | Stool consistency, social behavioral score, fecal sequencing | Increases in Lactobacilli and Enterococci and decrease in Clostridium cluster XIVa. | √ | - | √ | - | - | - | [72] |
| Crossover-controlled | VISBIOME: 1 strain of Streptococcus thermophiles, 3 strains of Bifidobacteria, and 4 strains of Lactobacilli | 10 ASD children; 8-week period and 3-week washout; USA | GI module of PedsQL, PRAS-ASD, Target Symptom Rating, ABC, SRS, fecal sequencing | No alteration in microbiota composition. | √ | - | × | - | × | × | [59] |
| RCT | Lactobacillus plantarum PS128 | 39 ASD children: probiotic, 41 ASD children: placebo; 4-week period; Taiwan (China) | CGI-I, ABC-T, SRS, SNAP-IV | - | - | - | √ | - | √ | - | [74] |
| RCT | Lactobacillus Plantarum PS128 | 41 ASD children: early treat with PS128 (for 4 weeks), 41 ASD children: late treat with PS128 (start from the middle course until week 4); Taiwan (China) | ADHDT, ASEBA | - | - | - | × | - | √ | - | [75] |
| RCT | Vivomixx®: Streptococcus thermophilus, Bifidobacterium (B. breve, B. longum, B. infantis), and Lactobacillus (L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. Bulgaricus) | 42 ASD children (14 GI and 28 NGI): probiotic, 43 ASD children (16 GI and 27 NGI: placebo; 6-month period; Italy | GSI, ADOS-CSS, VABS-II, SCQ, SP, RBS-R, CBCL, PSI, plasma Leptin, TNF-α, IL-6, PAI-1, EEG | - | √ | × | √ | - | √ | × | [76,77] |
| Crossover-controlled | Limosilactobacillus fermentum LF10, Ligilactobacillus salivarius LS03, Lactiplantibacillus plantarum, and 5 trains of Bifidobacterium longum | 61 ASD children; 3-month period and 2-month washout; Italy | GSI, PSI, VABS, ASRS, fecal sequencing | Beta diversity altered, and S. thermophilus, B. longum, Lfermentum, and L. salivarius showed high abundances in the treatment group. | √ | - | × | - | √ | √ | [73] |
| RCT | Lactiplantibacillus Reuteri | 21 ASD children: probiotics, 22 ASD children: placebo; 6-month period; Italy | ADOS, GSI, RBS-R, ABAS-II, PSI, SRS, CBCL, blood CD40L and immune response, fecal sequencing | No widespread changes in the diversity and composition of gut microbiome. | × | √ | √ | - | √ | × | [78] |
| Crossover-controlled | VISBIOME: 1 strain of Streptococcus thermophiles, 3 strains of Bifidobacteria, and 4 strains of Lactobacilli | 13 ASD children; 8-week period and 3-week washout; USA | GI module of PedsQL, Target Symptom Rating, ABC, SRS, CSHQ, PSI | - | √ | - | × | × | × | × | NCT02903030 |
| Crossover-controlled | Vivomixx®: Streptococcus thermophilus, Bifidobacterium (B. breve, B. longum, B. infantis), and Lactobacillus (L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. Bulgaricus) | 69 ASD children; 12-week period and 4-week washout; Bratish | ATEC, GIH, ABC, APSI | - | × | - | × | - | × | × | NCT03369431 |
| Prebiotics | |||||||||||
| RCT | Bimuno® 1 | 30 ASD children, in which URD+B-GOS (n = 7), URD+placebo (n = 7), RD+B-GOS (n = 6), RD+placebo (n = 6); 6-week period; Britain | GI symptoms, stool consistency, ATEC, AQ, SCAS-P, sleep diary, fecal sequencing | An increase in Lachnospiraceae family. | × | - | √ | × | √ | - | [79] |
| RCT | β-1,3/1,6-glucan | 12 ASD children: conventional treatment combined with the glucan, 6 ASD children: conventional treatment; 90-day period; India | CARS, CSHQ, plasma alpha-synuclein, serum melatonin, fecal sequencing | A shift towards a healthier microbial composition with decreased Escherichia coli, Akkermansia muciniphila, Blautia, Coprobacillus, and Clostridium bolteae. | √ | √ | √ | √ | √ | - | [80,81,82] |
| Symbiotic and combined treatments | |||||||||||
| RCT | 6 bacteria (the strain not shown) | 37 ASD children: a combination of ABA training and probiotic, 28 ASD children: ABA training only; 4-week period; China | ATEC, GI score | - | √ | - | √ | √ | √ | - | [83] |
| RCT | Bifidobacterium infantis Bi-26, Lactobacillus rhamnosus HN001, Bifidobacterium lactis BL-04, and Lactobacillus paracasei LPC-37 | 16 ASD children: a combination of fructo-oligosaccharide and probiotic, 10 ASD children: placebo; 108-day period; China | ATEC, GSI, fecal sequencing | A shift towards a healthier microbial composition with increased Bifidobacteriales and B. longum, and reduced Clostridium. | √ | √ | √ | - | √ | - | [85] |
| RCT | Bifidobacterium longiformis, Lactobacillus acidophilus, Enterococcus faecalis | 21 ASD children: a combination of ABA training and probiotic, 20 ASD children: ABA training only; 3-month period; China | GSI, ATEC, fecal sequencing | A shift towards a healthier microbial composition with increased Bifidobacterium, Lactobacillus, Coprobacillus, Ruminococcus, Prevotella, and Blautia, and decreased Shigella and Clostridium. | - | - | √ | - | √ | - | [84] |
| RCT | Lactobacillus plantarum PS128 | 18 ASD children: probiotic, 17 ASD children: placebo; 28-week period and oxytocin treatment for all groups starting on 16 weeks; USA | SRS, ABC, plasma OXT, IL-1β, CGI, GSI, fecal sequencing | A trend towards the normalization of ASD-related bacteria genera and the predicted functional profile. | × | × | × | - | √ | - | [56] |
| Crossover-controlled | A combination of L. reuteri, dextran, and maltose | 15 ASD children; 28-day period and 14-day washout; USA | ADOS, SCR, WASI-II, ABC, CGI, RBANS, VABS, attentional performance, neurophysiology measures, plasma OXT, and serum inflammatory factors | - | - | × | × | - | √ | - | [86] |
| Efficacy evaluations | |||||||||||
| Cases with significant improvements | Probiotics | 5/7 | 1/2 | 4/9 | 0/1 | 5/8 | 1/6 | ||||
| Percentages (%) | 71% | 50% | 44% | 0 | 63% | 17% | |||||
| Cases with significant improvements | Prebiotics | 1/2 | 1/1 | 2/2 | 1/2 | 2/2 | - | ||||
| Percentages (%) | 50% | 100% | 100% | 50% | 100% | - | |||||
| Cases with significant improvements | Symbiotic and combined treatments | 2/3 | 1/3 | 3/5 | 1/1 | 5/5 | - | ||||
| Percentages (%) | 67% | 33% | 60% | 100% | 100% | - | |||||
| Cases with significant improvements | Overall | 8/12 | 3/6 | 9/16 | 2/4 | 12/15 | 1/6 | ||||
| Percentages (%) | 67% | 50% | 56% | 50% | 80% | 17% | |||||
5. Limitations and Future Strategies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lord, C.; Charman, T.; Havdahl, A.; Carbone, P.; Anagnostou, E.; Boyd, B.; Carr, T.; de Vries, P.J.; Dissanayake, C.; Divan, G.; et al. The Lancet Commission on the future of care and clinical research in autism. Lancet 2022, 399, 271–334. [Google Scholar] [CrossRef] [PubMed]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.K.; Davies, P.S.W. Pre- and probiotics in the management of children with autism and gut issues: A review of the current evidence. Eur. J. Clin. Nutr. 2022, 76, 913–921. [Google Scholar] [CrossRef]
- Dargenio, V.N.; Dargenio, C.; Castellaneta, S.; de Giacomo, A.; Laguardia, M.; Schettini, F.; Francavilla, R.; Cristofori, F. Intestinal Barrier Dysfunction and Microbiota-Gut-Brain Axis: Possible implications in the pathogenesis and treatment of Autism Spectrum Disorder. Nutrients 2023, 15, 1620. [Google Scholar] [CrossRef] [PubMed]
- Srikantha, P.; Mohajeri, M.H. The possible role of the microbiota-gut-brain-axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; de Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lin, H.-Y.; Chien, Y.; Tung, Y.-H.; Ni, Y.-H.; Gau, S.S.-F. Altered gut microbiota correlates with behavioral problems but not gastrointestinal symptoms in individuals with autism. Brain Behav. Immun. 2022, 106, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S.; Elbeltagi, R.; Alhawamdeh, R. Role of gastrointestinal health in managing children with autism spectrum disorder. World J. Clin. Pediatr. 2023, 12, 171–196. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Huang, S.; Wang, X.; Cao, M.; Gu, T.; Ou, X.; Pan, S.; Lin, Z.; Wang, X.; et al. Multi-omics analyses demonstrate the modulating role of gut microbiota on the associations of unbalanced dietary intake with gastrointestinal symptoms in children with autism spectrum disorder. Gut Microbes 2023, 15, 2281350. [Google Scholar] [CrossRef]
- Nogay, N.H.; Nahikian-Nelms, M. Can we reduce autism-related gastrointestinal and behavior problems by gut microbiota based dietary modulation? A review. Nutr. Neurosci. 2021, 24, 327–338. [Google Scholar] [CrossRef]
- Suprunowicz, M.; Tomaszek, N.; Urbaniak, A.; Zackiewicz, K.; Modzelewski, S.; Waszkiewicz, N. Between dysbiosis, maternal immune activation and autism: Is there a common pathway? Nutrients 2024, 16, 549. [Google Scholar] [CrossRef] [PubMed]
- Önal, S.; Sachadyn-Król, M.; Kostecka, M. A review of the nutritional approach and the role of dietary components in children with Autism Spectrum Disorders in light of the latest scientific research. Nutrients 2023, 15, 4852. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Xiong, X.; Yang, T.; Hou, N.; Liang, X.; Chen, J.; Cheng, Q.; Li, T. Correlation between nutrition and symptoms: Nutritional survey of children with Autism Spectrum Disorder in Chongqing, China. Nutrients 2016, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, L.; Zhang, Q.; Chen, L.; Dai, Y.; Liu, L.; Feng, J.; Cai, X.; Cheng, Q.; Chen, J.; et al. Vitamin and mineral status of children with autism spectrum disorder in Hainan Province of China: Associations with symptoms. Nutr. Neurosci. 2020, 23, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhu, J.; Yang, T.; Lai, X.; Lei, Y.; Chen, J.; Li, T. Vitamin A and vitamin D deficiencies exacerbate symptoms in children with autism spectrum disorders. Nutr. Neurosci. 2019, 22, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.T.; Jin, D.-M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K.; et al. Multi-level analysis of the gut-brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat. Neurosci. 2023, 26, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.X.; Henders, A.K.; Alvares, G.A.; Wood, D.L.A.; Krause, L.; Tyson, G.W.; Restuadi, R.; Wallace, L.; McLaren, T.; Hansell, N.K.; et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 2021, 184, 5916–5931.e17. [Google Scholar] [CrossRef] [PubMed]
- Sanlier, N.; Kocabas, Ş. The effect of probiotic, prebiotic and gut microbiota on ASD: A review and future perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 2319–2330. [Google Scholar] [CrossRef]
- Hughes, H.K.; Moreno, R.J.; Ashwood, P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef]
- Ibi, D.; Yamada, K. Therapeutic targets for neurodevelopmental disorders emerging from animal models with perinatal immune activation. Int. J. Mol. Sci. 2015, 16, 28218–28229. [Google Scholar] [CrossRef]
- Butler, M.J.; Perrini, A.A.; Eckel, L.A. The role of the gut microbiome, immunity, and neuroinflammation in the pathophysiology of eating disorders. Nutrients 2021, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- de Wouters d’Oplinter, A.; Huwart, S.J.P.; Cani, P.D.; Everard, A. Gut microbes and food reward: From the gut to the brain. Front. Neurosci. 2022, 16, 947240. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Shankland, E.; Richards, T.; Corrigan, N.; Shusterman, D.; Edden, R.; Estes, A.; St John, T.; Dager, S.; Kleinhans, N.M. Relationships between GABA, glutamate, and GABA/glutamate and social and olfactory processing in children with autism spectrum disorder. Psychiatry Res. Neuroimaging 2023, 336, 111745. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, M.M.H.; van Buul, V.J.; Brouns, F.J.P.H. Autism and nutrition: The role of the gut–brain axis. Nutr. Res. Rev. 2014, 27, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Bandini, L.G.; Anderson, S.E.; Curtin, C.; Cermak, S.; Evans, E.W.; Scampini, R.; Maslin, M.; Must, A. Food selectivity in children with autism spectrum disorders and typically developing children. J. Pediatr. 2010, 157, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Berland, C.; Castel, J.; Terrasi, R.; Montalban, E.; Foppen, E.; Martin, C.; Muccioli, G.G.; Luquet, S.; Gangarossa, G. Identification of an endocannabinoid gut-brain vagal mechanism controlling food reward and energy homeostasis. Mol. Psychiatry 2022, 27, 2340–2354. [Google Scholar] [CrossRef] [PubMed]
- de Pol, M.; Kolla, N.J. Endocannabinoid markers in autism spectrum disorder: A scoping review of human studies. Psychiatry Res. 2021, 306, 114256. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.L.; Anacker, A.M.J.; Veenstra-VanderWeele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef]
- Banskota, S.; Ghia, J.-E.; Khan, W.I. Serotonin in the gut: Blessing or a curse. Biochimie 2019, 161, 56–64. [Google Scholar] [CrossRef]
- Kaye, W.H.; Fudge, J.L.; Paulus, M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 2009, 10, 573–584. [Google Scholar] [CrossRef]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal dysbiosis and yeast isolation in stool of subjects with Autism Spectrum Disorders. Mycopathologia 2017, 182, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U. Prenatal Poly(I:C) Exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Gondalia, S.V.; Palombo, E.A.; Knowles, S.R.; Cox, S.B.; Meyer, D.; Austin, D.W. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res. 2012, 5, 419–427. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism–Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.; Watters, A.; Mehler, P.S. The intersect of gastrointestinal symptoms and malnutrition associated with anorexia nervosa and avoidant/restrictive food intake disorder: Functional or pathophysiologic?—A systematic review. Int. J. Eat. Disord. 2021, 54, 1019–1054. [Google Scholar] [CrossRef] [PubMed]
- Riehl, M.E.; Scarlata, K. Understanding disordered eating risks in patients with gastrointestinal conditions. J. Acad. Nutr. Diet. 2022, 122, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Bourke, C.D.; Robertson, R.C.; Shivakumar, N.; Edwards, C.A.; Preston, T.; Holmes, E.; Kelly, P.; Frost, G.; Morrison, D.J. Understanding the role of the gut in undernutrition: What can technology tell us? Gut 2021, 70, 1580–1594. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.; Burton Murray, H.; Staller, K. Assessment and management of disorders of gut-brain interaction in patients with eating disorders. J. Eat. Disord. 2023, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Wronski, M.-L.; Kuja-Halkola, R.; Hedlund, E.; Martini, M.I.; Lichtenstein, P.; Lundström, S.; Larsson, H.; Taylor, M.J.; Micali, N.; Bulik, C.M.; et al. Co-existing mental and somatic conditions in Swedish children with the avoidant restrictive food intake disorder phenotype. medRxiv 2024. preprint. [Google Scholar] [CrossRef]
- Sanchez-Cerezo, J.; Nagularaj, L.; Gledhill, J.; Nicholls, D. What do we know about the epidemiology of avoidant/restrictive food intake disorder in children and adolescents? A systematic review of the literature. Eur. Eat. Disord. Rev. 2023, 31, 226–246. [Google Scholar] [CrossRef]
- de Theije, C.G.M.; Bavelaar, B.M.; Da Lopes Silva, S.; Korte, S.M.; Olivier, B.; Garssen, J.; Kraneveld, A.D. Food allergy and food-based therapies in neurodevelopmental disorders. Pediatr. Allergy Immunol. 2014, 25, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Gargano, D.; Appanna, R.; Santonicola, A.; de Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food allergy and intolerance: A narrative review on nutritional concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psychopathology. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Saad, K.; Eltayeb, A.A.; Mohamad, I.L.; Al-Atram, A.A.; Elserogy, Y.; Bjørklund, G.; El-Houfey, A.A.; Nicholson, B. A randomized, placebo-controlled trial of digestive enzymes in children with Autism Spectrum Disorders. Clin. Psychopharmacol. Neurosci. 2015, 13, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Pandey, R.; Chauhan, N.S. Hypoxia inducible factor-1α: The curator of gut homeostasis. Front. Cell. Infect. Microbiol. 2020, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Taniya, M.A.; Chung, H.-J.; Al Mamun, A.; Alam, S.; Aziz, M.A.; Emon, N.U.; Islam, M.M.; Hong, S.-T.S.; Podder, B.R.; Ara Mimi, A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and its therapeutic regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef]
- Madore, C.; Leyrolle, Q.; Lacabanne, C.; Benmamar-Badel, A.; Joffre, C.; Nadjar, A.; Layé, S. Neuroinflammation in autism: Plausible role of maternal inflammation, dietary omega 3, and microbiota. Neural Plast. 2016, 2016, 3597209. [Google Scholar] [CrossRef]
- Layé, S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2010, 82, 295–303. [Google Scholar] [CrossRef]
- Bolte, E.R. Autism and clostridium tetani. Med. Hypotheses 1998, 51, 133–144. [Google Scholar] [CrossRef]
- Shultz, S.R.; MacFabe, D.F.; Martin, S.; Jackson, J.; Taylor, R.; Boon, F.; Ossenkopp, K.-P.; Cain, D.P. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long–Evans rat: Further development of a rodent model of autism. Behav. Brain Res. 2009, 200, 33–41. [Google Scholar] [CrossRef]
- Coley-O’Rourke, E.J.; Hsiao, E.Y. Microbiome alterations in autism spectrum disorder. Nat. Microbiol. 2023, 8, 1615–1616. [Google Scholar] [CrossRef] [PubMed]
- Waizbard-Bartov, E.; Fein, D.; Lord, C.; Amaral, D.G. Autism severity and its relationship to disability. Autism Res. 2023, 16, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wong, O.W.H.; Lam, A.M.W.; Or, B.P.N.; Mo, F.Y.M.; Shea, C.K.S.; Lai, K.Y.C.; Ma, S.L.; Hung, S.F.; Chan, S.; Kwong, T.N.Y.; et al. Disentangling the relationship of gut microbiota, functional gastrointestinal disorders and autism: A case-control study on prepubertal Chinese boys. Sci. Rep. 2022, 12, 10659. [Google Scholar] [CrossRef]
- Yang, J.; He, L.; Dai, S.; Zheng, H.; Cui, X.; Ou, J.; Zhang, X. Therapeutic efficacy of sulforaphane in autism spectrum disorders and its association with gut microbiota: Animal model and human longitudinal studies. Front. Nutr. 2023, 10, 1294057. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-J.; Liu, J.; Liu, K.; Koh, M.; Sherman, H.; Liu, S.; Tian, R.; Sukijthamapan, P.; Wang, J.; Fong, M.; et al. Probiotic and oxytocin combination therapy in patients with Autism Spectrum Disorder: A randomized, double-blinded, placebo-controlled pilot trial. Nutrients 2021, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.E.; Luna, R.A.; Williams, K.; Chan, J.; Parker, R.A.; Wu, Q.; Hollway, J.A.; Jeffs, A.; Lu, F.; Coury, D.L.; et al. Probiotics for gastrointestinal symptoms and quality of life in autism: A placebo-controlled pilot trial. J. Child Adolesc. Psychopharmacol. 2019, 29, 659–669. [Google Scholar] [CrossRef]
- Luna, R.A.; Oezguen, N.; Balderas, M.; Venkatachalam, A.; Runge, J.K.; Versalovic, J.; Veenstra-VanderWeele, J.; Anderson, G.M.; Savidge, T.; Williams, K.C. Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with Autism Spectrum Disorder. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 218–230. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Z.; Li, G.; Li, G.; Liang, J. Two-sample Mendelian randomization analysis investigates causal associations between gut microbiota and attention deficit hyperactivity disorder. Front. Microbiol. 2023, 14, 1144851. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.R.; Yang, H.; Serena, G.; Sturgeon, C.; Ma, B.; Careaga, M.; Hughes, H.K.; Angkustsiri, K.; Rose, M.; Hertz-Picciotto, I.; et al. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav. Immun. 2018, 70, 354–368. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Cetinbas, M.; Sadreyev, R.; Koh, M.; Huang, H.; Adeseye, A.; He, P.; Zhu, J.; Russell, H.; et al. New and preliminary evidence on altered oral and gut microbiota in individuals with Autism Spectrum Disorder (ASD): Implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients 2019, 11, 2128. [Google Scholar] [CrossRef] [PubMed]
- Kushak, R.I.; Winter, H.S.; Buie, T.M.; Cox, S.B.; Phillips, C.D.; Ward, N.L. Analysis of the duodenal microbiome in autistic individuals: Association with carbohydrate digestion. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e110–e116. [Google Scholar] [CrossRef] [PubMed]
- de Filippis, F.; Pasolli, E.; Tett, A.; Tarallo, S.; Naccarati, A.; de Angelis, M.; Neviani, E.; Cocolin, L.; Gobbetti, M.; Segata, N.; et al. Distinct genetic and functional traits of human intestinal prevotella copri strains are associated with different habitual diets. Cell Host Microbe 2019, 25, 444–453.e3. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Donovan, S.M. Dietary patterns impact temporal dynamics of fecal microbiota composition in children with Autism Spectrum Disorder. Front. Nutr. 2019, 6, 193. [Google Scholar] [CrossRef]
- Tomova, A.; Soltys, K.; Kemenyova, P.; Karhanek, M.; Babinska, K. The Influence of food intake specificity in children with autism on gut microbiota. Int. J. Mol. Sci. 2020, 21, 2797. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zheng, H. Dysbiosis of gut fungal microbiota in children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2021, 51, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.; Gibson, G.R.; Knott, F.J.; McCartney, L. A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int. J. Probiotics Prebiotics 2010, 5, 69–74. [Google Scholar]
- Guidetti, C.; Salvini, E.; Viri, M.; Deidda, F.; Amoruso, A.; Visciglia, A.; Drago, L.; Calgaro, M.; Vitulo, N.; Pane, M.; et al. Randomized double-blind crossover study for evaluating a probiotic mixture on gastrointestinal and behavioral symptoms of autistic children. J. Clin. Med. 2022, 11, 5263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Liong, M.T.; Chung, Y.-C.E.; Huang, H.-Y.; Peng, W.-S.; Cheng, Y.-F.; Lin, Y.-S.; Wu, Y.-Y.; Tsai, Y.-C. Effects of lactobacillus plantarum ps128 on children with Autism Spectrum Disorder in Taiwan: A randomized, double-blind, placebo-controlled trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Wang, J.-E.; Sun, F.-J.; Huang, Y.-H.; Chen, H.-J. Probiotic intervention in young children with autism spectrum disorder in Taiwan: A randomized, double-blinded, placebo-controlled trial. Res. Autism Spectr. Disord. 2023, 109, 102256. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in autism Spectrum Disorders: A Randomized controlled trial. Front. Psychiatry 2020, 11, 550593. [Google Scholar] [CrossRef]
- Billeci, L.; Callara, A.L.; Guiducci, L.; Prosperi, M.; Morales, M.A.; Calderoni, S.; Muratori, F.; Santocchi, E. A randomized controlled trial into the effects of probiotics on electroencephalography in preschoolers with autism. Autism 2023, 27, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, L.; Dooling, S.W.; Volpe, E.; Uljarević, M.; Waters, J.L.; Sabatini, A.; Arturi, L.; Abate, R.; Riccioni, A.; Siracusano, M.; et al. Precision microbial intervention improves social behavior but not autism severity: A pilot double-blind randomized placebo-controlled trial. Cell Host Microbe 2024, 32, 106–116.e6. [Google Scholar] [CrossRef]
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Leigh Gibson, E.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome 2018, 6, 133. [Google Scholar] [CrossRef]
- Raghavan, K.; Dedeepiya, V.D.; Ikewaki, N.; Sonoda, T.; Iwasaki, M.; Preethy, S.; Abraham, S.J. Improvement of behavioural pattern and alpha-synuclein levels in autism spectrum disorder after consumption of a beta-glucan food supplement in a randomised, parallel-group pilot clinical study. BMJ Neurol. Open 2022, 4, e000203. [Google Scholar] [CrossRef]
- Raghavan, K.; Dedeepiya, V.D.; Kandaswamy, R.S.; Balamurugan, M.; Ikewaki, N.; Sonoda, T.; Kurosawa, G.; Iwasaki, M.; Preethy, S.; Abraham, S.J. Improvement of sleep and melatonin in children with autism spectrum disorder after β-1,3/1,6-glucan consumption: An open-label prospective pilot clinical study. Brain Behav. 2022, 12, e2750. [Google Scholar] [CrossRef]
- Raghavan, K.; Dedeepiya, V.D.; Yamamoto, N.; Ikewaki, N.; Sonoda, T.; Iwasaki, M.; Kandaswamy, R.S.; Senthilkumar, R.; Preethy, S.; Abraham, S.J.K. Benefits of gut microbiota reconstitution by beta 1,3-1,6 glucans in subjects with Autism Spectrum Disorder and other neurodegenerative diseases. J. Alzheimer’s. Dis. 2023, 94, S241–S252. [Google Scholar] [CrossRef]
- Niu, M.; Li, Q.; Zhang, J.; Wen, F.; Dang, W.; Duan, G.; Li, H.; Ruan, W.; Yang, P.; Guan, C.; et al. Characterization of intestinal microbiota and probiotics treatment in children with Autism Spectrum Disorders in China. Front. Neurol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Sun, Y.-H.; Liang, Y.-P.; Zhou, F.; Yang, J.; Jin, S.-L. Effect of probiotics combined with applied behavior analysis in the treatment of children with autism spectrum disorder: A prospective randomized controlled trial. Zhongguo Dang Dai Er Ke Za Zhi 2021, 23, 1103–1110. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.-J.; Zhao, D.-M.; Chen, B.; Zhang, G.-Q.; Chen, S.; Cao, R.-F.; Yu, H.; Zhao, C.-Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- Schmitt, L.M.; Smith, E.G.; Pedapati, E.V.; Horn, P.S.; Will, M.; Lamy, M.; Barber, L.; Trebley, J.; Meyer, K.; Heiman, M.; et al. Results of a phase Ib study of SB-121, an investigational probiotic formulation, a randomized controlled trial in participants with autism spectrum disorder. Sci. Rep. 2023, 13, 5192. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, C.; Fan, Z.; Yang, C.; Cai, W.; Huang, Y.; Xiang, Z.; Wu, J.; Zhang, J.; Yang, J. Effect of probiotics on children with autism spectrum disorders: A meta-analysis. Ital. J. Pediatr. 2024, 50, 120. [Google Scholar] [CrossRef]
- Pan, Z.-Y.; Zhong, H.-J.; Huang, D.-N.; Wu, L.-H.; He, X.-X. Beneficial effects of repeated washed microbiota transplantation in children with autism. Front. Pediatr. 2022, 10, 928785. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Pan, Z.; He, X. Effects of washed fecal bacteria transplantation in sleep quality, stool features and autism symptomatology: A Chinese preliminary observational study. Neuropsychiatr. Dis. Treat. 2022, 18, 1165–1173. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Li, N.; Chen, H.; Cheng, Y.; Xu, F.; Ruan, G.; Ying, S.; Tang, W.; Chen, L.; Chen, M.; Lv, L.; et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front. Cell. Infect. Microbiol. 2021, 11, 759435. [Google Scholar]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Zamora, A.F.; Ramírez-Valenzuela, D.G.; Ramos-Jiménez, A. Food selectivity and its implications associated with gastrointestinal disorders in children with Autism Spectrum Disorders. Nutrients 2022, 14, 2660. [Google Scholar] [CrossRef] [PubMed]
- Díaz Vargas, D.; Leonario Rodríguez, M. Efectividad de las intervenciones nutricionales en la sintomatología conductual del trastorno del espectro autista: Revisión sistemática. Nutr. Hosp. 2022, 39, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Blaine, R.E.; Blaine, K.P.; Cheng, K.; Banuelos, C.; Leal, A. Priorities, barriers, and facilitators for nutrition-related care for autistic children: A qualitative study comparing interdisciplinary health professional and parent perspectives. Front. Pediatr. 2023, 11, 1198177. [Google Scholar] [CrossRef]
- Frye, R.E. A personalized multidisciplinary approach to evaluating and treating Autism Spectrum Disorder. JPM 2022, 12, 464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Su, Q. Harnessing the Gut Microbiome: To What Extent Can Pre-/Probiotics Alleviate Immune Activation in Autism Spectrum Disorder? Nutrients 2024, 16, 2382. https://doi.org/10.3390/nu16152382
Wu Y, Su Q. Harnessing the Gut Microbiome: To What Extent Can Pre-/Probiotics Alleviate Immune Activation in Autism Spectrum Disorder? Nutrients. 2024; 16(15):2382. https://doi.org/10.3390/nu16152382
Chicago/Turabian StyleWu, Yuqi, and Qi Su. 2024. "Harnessing the Gut Microbiome: To What Extent Can Pre-/Probiotics Alleviate Immune Activation in Autism Spectrum Disorder?" Nutrients 16, no. 15: 2382. https://doi.org/10.3390/nu16152382







