Extracellular Vesicles Modulate Liver Cells Viability and Reactive Oxygen Species in Patients Following a Very Low-Calorie Ketogenic Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Extracellular Vesicles Extraction and Characterization
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Reactive Oxygen Species Detection
2.6. Statistical Analysis
3. Results
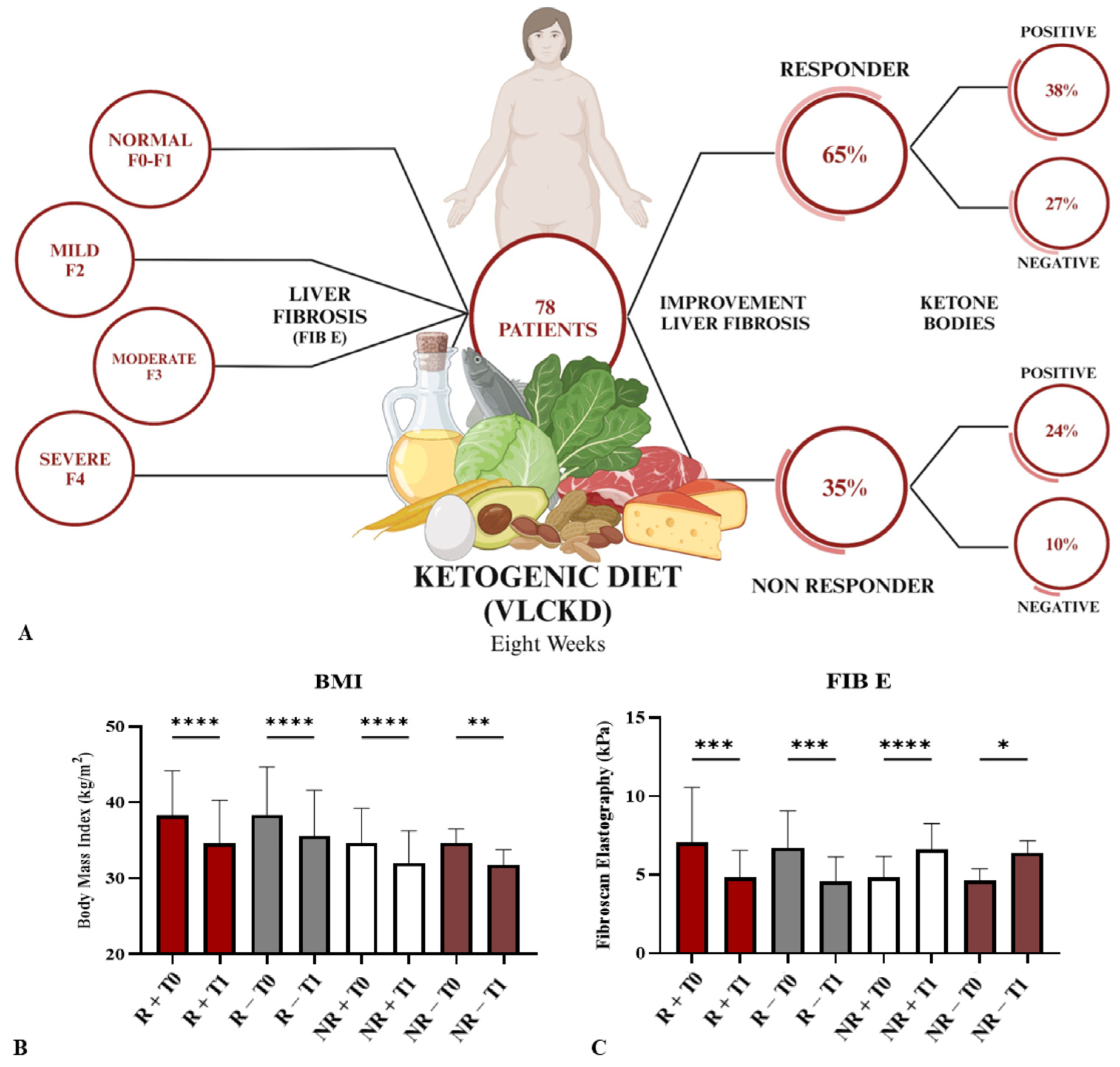
3.1. Patients Clinical Parameters after VLCKD
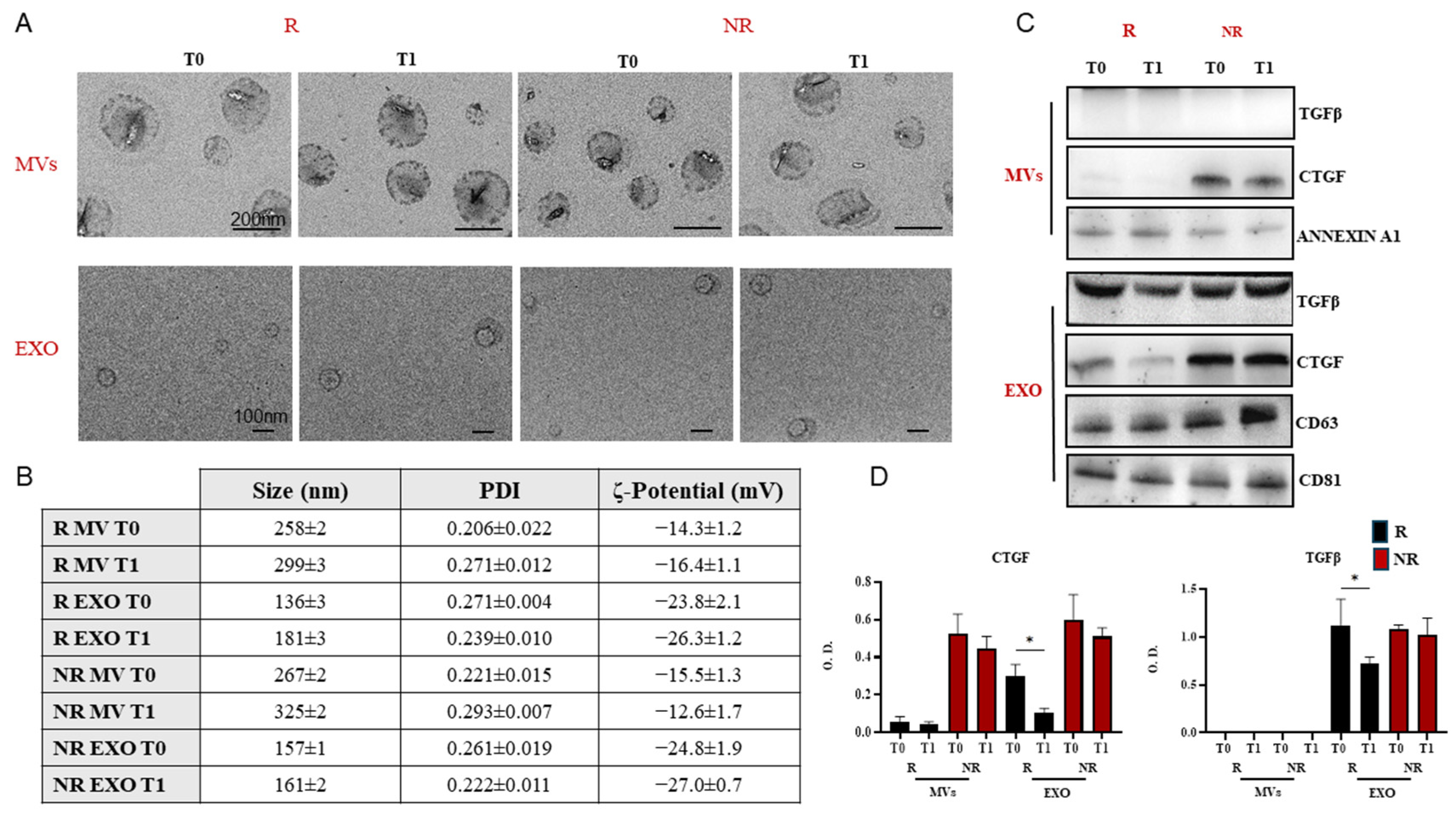
3.2. Extracellular Vesicles Extraction and Characterization
3.3. Effect of Exosomes on Liver Cell Viability
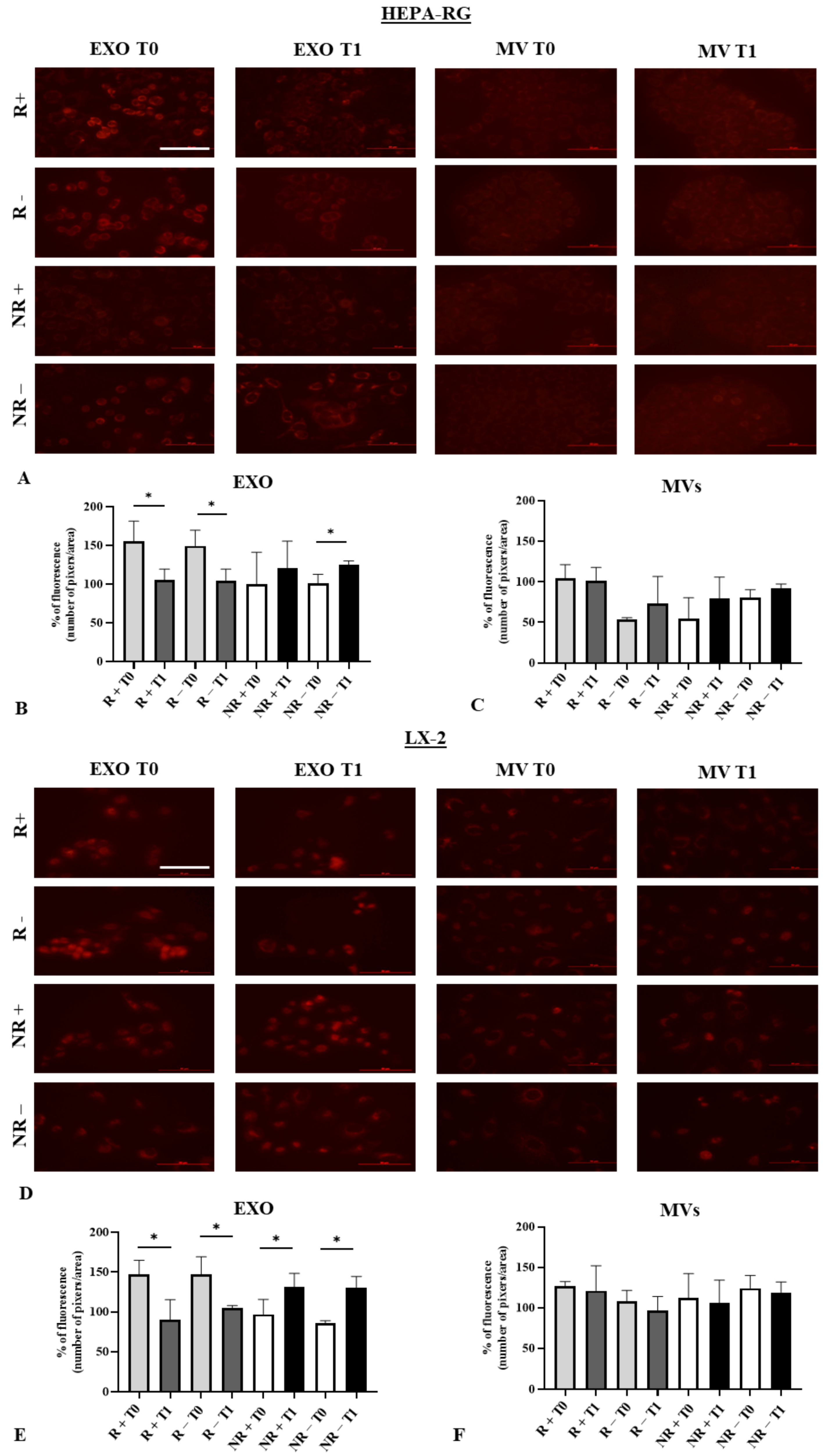
3.4. Modulation of Reactive Oxygen Species (ROS) in HEPA-RG and LX2 Treated with EXO and MVs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellis, R.W.B. Some Effects of a Ketogenic Diet. Arch. Dis. Child. 1931, 6, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Swink, T.D.; Vining, E.P.; Freeman, J.M. The Ketogenic Diet: 1997. Adv. Pediatr. 1997, 44, 297–329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public Health 2022, 19, 10429. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.; Raggi, P. The Ketogenic Diet: Pros and Cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef]
- Barrea, L.; Caprio, M.; Camajani, E.; Verde, L.; Elce, A.; Frias-Toral, E.; Ceriani, F.; Cucalón, G.; Garcia-Velasquez, E.; El Ghoch, M.; et al. Clinical and Nutritional Management of Very-Low-Calorie Ketogenic Diet (VLCKD) in Patients with Psoriasis and Obesity: A Practical Guide for the Nutritionist. Crit. Rev. Food Sci. Nutr. 2022, 63, 10775–10791. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, H.; Li, Y.; Hao, Y.; Wang, C.; Jia, P.; Chen, X.; Ma, S.; Xiao, Z. Crosstalk between Hepatic Stellate Cells and Surrounding Cells in Hepatic Fibrosis. Int. Immunopharmacol. 2021, 99, 108051. [Google Scholar] [CrossRef] [PubMed]
- Shojaie, L.; Iorga, A.; Dara, L. Cell Death in Liver Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. Tgf-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Khanam, A.; Saleeb, P.G.; Kottilil, S. Pathophysiology and Treatment Options for Hepatic Fibrosis: Can It Be Completely Cured? Cells 2021, 10, 1097. [Google Scholar] [CrossRef]
- Yang, L.; Inokuchi, S.; Roh, Y.S.; Song, J.; Loomba, R.; Park, E.J.; Seki, E. Transforming Growth Factor-β Signaling in Hepatocytes Promotes Hepatic Fibrosis and Carcinogenesis in Mice with Hepatocyte-Specific Deletion of TAK1. Gastroenterology 2013, 144, 1042–1054.e4. [Google Scholar] [CrossRef]
- Liu, R.M.; Desai, L.P. Reciprocal Regulation of TGF-β and Reactive Oxygen Species: A Perverse Cycle for Fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Kisin, E.R.; Murray, A.R.; Kommineni, C.; Castranova, V.; Fadeel, B.; Kagan, V.E. Increased Accumulation of Neutrophils and Decreased Fibrosis in the Lung of NADPH Oxidase-Deficient C57BL/6 Mice Exposed to Carbon Nanotubes. Toxicol. Appl. Pharmacol. 2008, 231, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Sasaki, K.; Ruiz, I.A.P.; King, K.E.; Weinman, S.A.; Wozniak, A.L. Inflammatory Macrophage to Hepatocyte Signals Can Be Prevented by Extracellular Vesicle Reprogramming. J. Cell Sci. 2023, 136, jcs260691. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Hu, R.; Runtsch, M.C.; Kagele, D.A.; Mosbruger, T.L.; Tolmachova, T.; Seabra, M.C.; Round, J.L.; Ward, D.M.; O’Connell, R.M. Exosome-Delivered MicroRNAs Modulate the Inflammatory Response to Endotoxin. Nat. Commun. 2015, 6, 7321. [Google Scholar] [CrossRef] [PubMed]
- Scavo, M.P.; Negro, R.; Arrè, V.; Depalo, N.; Carrieri, L.; Rizzi, F.; Mastrogiacomo, R.; Serino, G.; Notarnicola, M.; De Nunzio, V.; et al. The Oleic/Palmitic Acid Imbalance in Exosomes Isolated from NAFLD Patients Induces Necroptosis of Liver Cells via the Elongase-6/RIP-1 Pathway. Cell Death Dis. 2023, 14, 635. [Google Scholar] [CrossRef] [PubMed]
- De Nucci, S.; Bonfiglio, C.; Donvito, R.; Di Chito, M.; Cerabino, N.; Rinaldi, R.; Sila, A.; Shahini, E.; Giannuzzi, V.; Pesole, P.L.; et al. Effects of an Eight Week Very Low-Calorie Ketogenic Diet (VLCKD) on White Blood Cell and Platelet Counts in Relation to Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in Subjects with Overweight and Obesity. Nutrients 2023, 15, 4468. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; De Nucci, S.; Castellana, F.; Di Chito, M.; Giannuzzi, V.; Shahini, E.; Zupo, R.; Lampignano, L.; Piazzolla, G.; Triggiani, V.; et al. The Effects of Eight Weeks’ Very Low-Calorie Ketogenic Diet (VLCKD) on Liver Health in Subjects Affected by Overweight and Obesity. Nutrients 2023, 15, 825. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Bruyère, O.; Geerinck, A.; Hajaoui, M.; Scafoglieri, A.; Perkisas, S.; Bautmans, I.; Gielen, E.; Reginster, J.Y.; Buckinx, F. Equation Models Developed with Bioelectric Impedance Analysis Tools to Assess Muscle Mass: A Systematic Review. Clin. Nutr. ESPEN 2020, 35, 47–62. [Google Scholar] [CrossRef]
- Berzigotti, A. Non-Invasive Assessment of Non-Alcoholic Fatty Liver Disease: Ultrasound and Transient Elastography. Rev. Recent Clin. Trials 2015, 9, 170–177. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis—2021 Update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jayasankar, N.; Thamaraikani, T.; Viktor, P.; Mohany, M.; Al-Rejaie, S.S.; Alammar, H.K.; Anad, E.; Alhili, F.; Hussein, S.F.; et al. Quercetin Modulates Expression of Serum Exosomal Long Noncoding RNA NEAT1 to Regulate the MiR-129-5p/BDNF Axis and Attenuate Cognitive Impairment in Diabetic Mice. Life Sci. 2024, 340, 122449. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, R.; Eckert, S.; Wirrell, E. Ketogenic Diet. Can. J. Neurol. Sci. 2013, 40, 158–167. [Google Scholar] [CrossRef]
- Guarnotta, V.; Emanuele, F.; Amodei, R.; Giordano, C. Very Low-Calorie Ketogenic Diet: A Potential Application in the Treatment of Hypercortisolism Comorbidities. Nutrients 2022, 14, 2388. [Google Scholar] [CrossRef]
- Yao, A.; Li, Z.; Lyu, J.; Yu, L.; Wei, S.; Xue, L.; Wang, H.; Chen, G.Q. On the Nutritional and Therapeutic Effects of Ketone Body D-β-Hydroxybutyrate. Appl. Microbiol. Biotechnol. 2021, 105, 6229–6243. [Google Scholar] [CrossRef] [PubMed]
- Sarnyai, Z.; Palmer, C.M. Ketogenic Therapy in Serious Mental Illness: Emerging Evidence. Int. J. Neuropsychopharmacol. 2021, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, K.; Przeliorz, A. The Effect of the Ketogenic Diet on the Therapy of Neurodegenerative Diseases and Its Impact on Improving Cognitive Functions. Dement. Geriatr. Cogn. Dis. Extra 2022, 12, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Koutnik, A.P.; Goldberg, E.L.; Upadhyay, V.; Turnbaugh, P.J.; Verdin, E.; Newman, J.C. Investigating Ketone Bodies as Immunometabolic Countermeasures against Respiratory Viral Infections. Med 2020, 1, 43–65. [Google Scholar] [CrossRef]
- Koutnik, A.P.; Poff, A.M.; Ward, N.P.; DeBlasi, J.M.; Soliven, M.A.; Romero, M.A.; Roberson, P.A.; Fox, C.D.; Roberts, M.D.; D’Agostino, D.P. Ketone Bodies Attenuate Wasting in Models of Atrophy. J. Cachexia Sarcopenia Muscle 2020, 11, 973–996. [Google Scholar] [CrossRef]
- Goossens, C.; Weckx, R.; Derde, S.; Vander Perre, S.; Derese, I.; Van Veldhoven, P.P.; Ghesquière, B.; Van den Berghe, G.; Langouche, L. Altered Cholesterol Homeostasis in Critical Illness-Induced Muscle Weakness: Effect of Exogenous 3-Hydroxybutyrate. Crit. Care 2021, 25, 252. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic Diet for Obesity: Friend or Foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Harmon, D.M.; Kludtke, E.; Mickow, A.; Simha, V.; Kopecky, S. Dramatic Elevation of LDL Cholesterol from Ketogenic-Dieting: A Case Series. Am. J. Prev. Cardiol. 2023, 14, 100495. [Google Scholar] [CrossRef] [PubMed]
- Burén, J.; Ericsson, M.; Damasceno, N.R.T.; Sjödin, A. A Ketogenic Low-carbohydrate High-fat Diet Increases Ldl Cholesterol in Healthy, Young, Normal-weight Women: A Randomized Controlled Feeding Trial. Nutrients 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Alarim, R.A.; Alasmre, F.A.; Alotaibi, H.A.; Alshehri, M.A.; Hussain, S.A. Effects of the Ketogenic Diet on Glycemic Control in Diabetic Patients: Meta-Analysis of Clinical Trials. Cureus 2020, 12, e10796. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Verde, L.; Vetrani, C.; Marino, F.; Aprano, S.; Savastano, S.; Colao, A.; Muscogiuri, G. VLCKD: A Real Time Safety Study in Obesity. J. Transl. Med. 2022, 20, 23. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The Management of Very Low-Calorie Ketogenic Diet in Obesity Outpatient Clinic: A Practical Guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-Low-Calorie Ketogenic Diet (VLCKD) in the Management of Metabolic Diseases: Systematic Review and Consensus Statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic Diets for Drug-Resistant Epilepsy. Cochrane Database Syst. Rev. 2020, 2020, CD001903. [Google Scholar] [CrossRef]
- Lima, P.A.; Sampaio, L.P.D.B.; Damasceno, N.R.T. Ketogenic Diet in Epileptic Children: Impact on Lipoproteins and Oxidative Stress. Nutr. Neurosci. 2015, 18, 337–344. [Google Scholar] [CrossRef]
- Meyer, C.; Liu, Y.; Kaul, A.; Peipe, I.; Dooley, S. Caveolin-1 Abrogates TGF-β Mediated Hepatocyte Apoptosis. Cell Death Dis. 2013, 4, e466. [Google Scholar] [CrossRef]
- Trampuž, S.R.; van Riet, S.; Nordling, Å.; Ingelman-Sundberg, M. The Role of CTGF in Liver Fibrosis Induced in 3D Human Liver Spheroids. Cells 2023, 12, 302. [Google Scholar] [CrossRef]
- Suwanabol, P.A.; Seedial, S.M.; Zhang, F.; Shi, X.; Si, Y.; Liu, B.; Craig Kent, K. TGF-β and Smad3 Modulate PI3K/Akt Signaling Pathway in Vascular Smooth Muscle Cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2211–H2219. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.V.; Yin, Y.; Jang, S.C.; Lässer, C.; Wennmalm, S.; Hoffmann, H.J.; Li, L.; Gho, Y.S.; Nilsson, J.A.; Lötvall, J. Endosomal Signalling via Exosome Surface TGFβ-1. J. Extracell. Vesicles 2019, 8, 1650458. [Google Scholar] [CrossRef] [PubMed]
- Linsalata, M.; Russo, F.; Riezzo, G.; D’Attoma, B.; Prospero, L.; Orlando, A.; Ignazzi, A.; Di Chito, M.; Sila, A.; De Nucci, S.; et al. The Effects of a Very-Low-Calorie Ketogenic Diet on the Intestinal Barrier Integrity and Function in Patients with Obesity: A Pilot Study. Nutrients 2023, 15, 2561. [Google Scholar] [CrossRef] [PubMed]
- Verde, L.; Frias-Toral, E.; Cacciapuoti, S.; Simancas-Racines, D.; Megna, M.; Caiazzo, G.; Potestio, L.; Maisto, M.; Tenore, G.C.; Colao, A.; et al. Very Low-Calorie Ketogenic Diet (VLCKD): A Therapeutic Nutritional Tool for Acne? J. Transl. Med. 2024, 22, 322. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Caprio, M.; Watanabe, M.; Cammarata, G.; Feraco, A.; Muscogiuri, G.; Verde, L.; Colao, A.; Savastano, S. Could Very Low-Calorie Ketogenic Diets Turn off Low Grade Inflammation in Obesity? Emerging Evidence. Crit. Rev. Food Sci. Nutr. 2023, 63, 8320–8336. [Google Scholar] [CrossRef]
- Fernández-Verdejo, R.; Mey, J.T.; Ravussin, E. Effects of Ketone Bodies on Energy Expenditure, Substrate Utilization, and Energy Intake in Humans. J. Lipid Res. 2023, 64, 100442. [Google Scholar] [CrossRef]
- Wilder, R.M.; Winter, M.D. The Threshold of Ketogenesis. J. Biol. Chem. 1922, 52, 393–401. [Google Scholar] [CrossRef]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.Y.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do Ketogenic Diets Really Suppress Appetite? A Systematic Review and Meta-Analysis. Obes. Rev. 2015, 16, 64–76. [Google Scholar] [CrossRef]
- Tagliabue, A.; Armeno, M.; Berk, K.A.; Guglielmetti, M.; Ferraris, C.; Olieman, J.; van der Louw, E. Ketogenic Diet for Epilepsy and Obesity: Is It the Same? Nutr. Metab. Cardiovasc. Dis. 2024, 34, 581–589. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond Oxidative Stress: An Immunologist’s Guide to Reactive Oxygen Species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [PubMed]

| Fibroscan Elastography (kPa) | |
|---|---|
| F0–F1 | <7 |
| F2 | 7.1–10 |
| F3 | 10.1–14 |
| F4 | >14 |
| Sex | Weight (kg) | BMI (kg/m2) | FIB E (kPa) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | p | T0 | T1 | p | T0 | T1 | p | ||
| F0–F1 | ||||||||||
| Responder | M | 107.7 ± 14.0 | 98.8 ± 12.6 | <0.0001 | 35.3 ± 4.0 | 32.5 ± 3.8 | <0.0001 | 5.7 ± 1.0 | 3.9 ± 1.2 | 0.0008 |
| F | 94.5 ± 12.1 | 86.1 ± 10.1 | <0.0001 | 36.1 ± 3.8 | 33.0 ± 3.6 | <0.0001 | 5.1 ± 1.0 | 4.0 ± 1.0 | <0.0001 | |
| Avg. M–F | 98.5 ± 14.0 | 89.9 ± 12.8 | <0.0001 | 36.0 ± 3.7 | 32.9 ± 3.6 | <0.0001 | 5.2 ± 1.0 | 4.0 ± 1.0 | <0.0001 | |
| Non-responder | M | 96.6 ± 3.2 | 88.3 ± 2.9 | 0.0003 | 33.7 ± 1.7 | 31.0 ± 1.7 | <0.0001 | 5.1 ± 0.9 | 5.6 ± 0.5 | 0.1532 |
| F | 85.5 ± 5.0 | 78.8 ± 3.4 | <0.0001 | 33.5 ± 2.4 | 30.9 ± 2.5 | <0.0001 | 4.1 ± 0.8 | 5.7 ± 1.1 | <0.0001 | |
| Avg. M–F | 88.3 ± 6.7 | 81.2 ± 5.4 | <0.0001 | 31.6 ± 7.2 | 30.9 ± 2.2 | <0.0001 | 4.4 ± 0.9 | 5.7 ± 1.0 | <0.0001 | |
| F2 | ||||||||||
| Responder | M | 132.2 ± 24.1 | 120.5 ± 23.8 | 0.0002 | 42.6 ± 8.0 | 39.0 ± 8.3 | <0.0001 | 8.4 ± 0.9 | 6.1 ± 0.7 | 0.0015 |
| F | 103.0 ± 12.8 | 94.8 ± 14.6 | 0.0874 | 39.0 ± 3.3 | 35.9 ± 4.4 | 0.0845 | 8.7 ± 0.9 | 6.0 ± 1.0 | 0.0456 | |
| Avg. M–F | 124.2 ± 25.0 | 113.5 ± 24.2 | <0.0001 | 41.6 ± 7.1 | 38.1 ± 7.4 | <0.0001 | 8.4 ± 0.7 | 6.0 ± 0.7 | 0.0002 | |
| Non-responder | M | 86.8 ± 5.3 | 79.0 ± 5.0 | 0.0165 | 32.6 ± 0.2 | 29.6 ± 0.1 | 0.0212 | 6.1 ± 1.7 | 8.4 ± 1.8 | 0.0141 |
| F | 99.4 ± 17.2 | 91.3 ± 15.0 | 0.0003 | 37.5 ± 5.2 | 34.4 ± 4.9 | 0.0002 | 5.3 ± 1.3 | 7.7 ± 0.8 | 0.0002 | |
| Avg. M–F | 97.1 ± 16.3 | 89.1 ± 14.4 | <0.0001 | 36.6 ± 5.1 | 33.5 ± 4.8 | <0.0001 | 5.4 ± 1.3 | 7.8 ± 0.9 | <0.0001 | |
| F3–F4 | ||||||||||
| Responder | M | 152.2 ± 22.8 | 141.2 ± 25.3 | 0.0187 | 48.5 ± 4.1 | 44.9 ± 5.1 | 0.0325 | 18.6 ± 11.1 | 6.4 ± 2.5 | 0.1412 |
| F | 113.5 ± 19.6 | 102.0 ± 17.5 | 0.0260 | 41.8 ± 6.0 | 38.9 ± 3.6 | 0.1803 | 14.4 ± 4.0 | 6.8 ± 3.8 | 0.0495 | |
| Avg. M–F | 132.8 ± 28.4 | 121.6 ± 29.0 | 0.0004 | 45.2 ± 5.9 | 41.9 ± 5.2 | 0.0195 | 16.5 ± 7.8 | 6.6 ± 2.8 | 0.0440 | |
| Code | Sex | Age | AST (U/L) | ALT (U/L) | γ GT (U/L) | Glycemia (U/L) | Triglycerides (mg/dL) | Total Cholesterol (mg/dL) | LDL (mg/dL) | HDL (mg%) | Ketone Bodies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | |||||
| F0–F1 | Responder | |||||||||||||||||||
| 1 | F | 46 | 14 | 11 | 12 | 13 | 17 | 12 | 92 | 70 | 66 | 76 | 189 | 160 | 130 | 114 | 49 | 41 | + | |
| 2 | M | 36 | 19 | 40 | 27 | 68 | 25 | 23 | 86 | 75 | 47 | 80 | 124 | 117 | 73 | 59 | 45 | 42 | + | |
| 3 | F | 18 | 20 | 17 | 19 | 19 | 9 | 9 | 93 | 80 | 53 | 40 | 157 | 166 | 80 | 86 | 66 | 72 | − | |
| 4 | M | 62 | 16 | 17 | 22 | 16 | 13 | 10 | 104 | 96 | 112 | 56 | 177 | 121 | 124 | 79 | 35 | 34 | − | |
| 5 | F | 23 | 25 | 21 | 51 | 37 | 37 | 18 | 89 | 70 | 194 | 123 | 212 | 182 | 157 | 130 | 31 | 27 | + | |
| 6 | F | 32 | 17 | 21 | 24 | 31 | 18 | 14 | 86 | 89 | 87 | 84 | 193 | 158 | 138 | 111 | 51 | 45 | + | |
| 7 | F | 39 | 12 | 16 | 16 | 12 | 15 | 14 | 100 | 89 | 101 | 73 | 175 | 199 | 108 | 127 | 61 | 50 | − | |
| 8 | M | 29 | 53 | 35 | 110 | 94 | 40 | 33 | 83 | 78 | 130 | 111 | 256 | 201 | 182 | 141 | 48 | 38 | + | |
| 9 | F | 49 | 34 | 26 | 48 | 35 | 30 | 19 | 113 | 109 | 102 | 47 | 219 | 196 | 147 | 140 | 52 | 47 | + | |
| 10 | F | 51 | 16 | 15 | 20 | 16 | 22 | 19 | 101 | 98 | 91 | 88 | 54 | 43 | 120 | 126 | 54 | 43 | − | |
| 11 | F | 53 | 15 | 18 | 21 | 22 | 29 | 14 | 115 | 91 | 76 | 69 | 182 | 164 | 132 | 109 | 58 | 47 | + | |
| 12 | F | 51 | 18 | 20 | 12 | 9 | 30 | 19 | 89 | 84 | 89 | 72 | 204 | 146 | 127 | 90 | 64 | 51 | + | |
| 13 | F | 19 | 12 | 14 | 14 | 13 | 20 | 13 | 87 | 86 | 108 | 117 | 177 | 186 | 105 | 119 | 50 | 44 | − | |
| 14 | M | 25 | 20 | 22 | 23 | 31 | 29 | 27 | 87 | 83 | 90 | 92 | 154 | 147 | 97 | 94 | 40 | 37 | + | |
| 15 | M | 39 | 25 | 16 | 41 | 24 | 31 | 25 | 79 | 95 | 133 | 185 | 204 | 202 | 148 | 149 | 37 | 41 | − | |
| 16 | F | 43 | 18 | 14 | 21 | 17 | 51 | 37 | 95 | 93 | 128 | 77 | 212 | 209 | 160 | 158 | 36 | 39 | − | |
| 17 | F | 23 | 19 | 22 | 37 | 35 | 19 | 15 | 95 | 90 | 72 | 118 | 176 | 193 | 130 | 137 | 47 | 39 | + | |
| 18 | M | 44 | 32 | 33 | 22 | 20 | 15 | 11 | 87 | 89 | 83 | 68 | 158 | 189 | 83 | 112 | 65 | 70 | − | |
| 19 | F | 37 | 19 | 15 | 25 | 16 | 55 | 16 | 90 | 90 | 62 | 55 | 193 | 196 | 142 | 139 | 59 | 45 | + | |
| 20 | F | 21 | 16 | 15 | 16 | 20 | 13 | 12 | 94 | 91 | 115 | 84 | 150 | 120 | 69 | 68 | 76 | 42 | + | |
| 21 | F | 54 | 18 | 17 | 19 | 16 | 14 | 12 | 95 | 94 | 117 | 85 | 242 | 183 | 153 | 118 | 65 | 54 | + | |
| 22 | F | 53 | 14 | 21 | 18 | 43 | 12 | 14 | 82 | 95 | 141 | 78 | 280 | 239 | 199 | 186 | 47 | 53 | − | |
| 23 | F | 50 | 17 | 19 | 25 | 23 | 22 | 23 | 88 | 90 | 56 | 36 | 196 | 165 | 135 | 109 | 50 | 49 | − | |
| 24 | M | 37 | 22 | 20 | 38 | 21 | 38 | 10 | 105 | 95 | 67 | 108 | 167 | 170 | 85 | 108 | 59 | 44 | + | |
| 25 | F | 53 | 17 | 18 | 18 | 14 | 14 | 7 | 92 | 96 | 237 | 94 | 190 | 128 | 115 | 72 | 39 | 44 | + | |
| 26 | F | 50 | 12 | 11 | 12 | 9 | 10 | 6 | 86 | 83 | 71 | 48 | 204 | 163 | 105 | 94 | 94 | 61 | + | |
| 27 | F | 42 | 12 | 14 | 16 | 21 | 17 | 16 | 95 | 97 | 126 | 73 | 246 | 200 | 174 | 145 | 55 | 49 | − | |
| 28 | F | 32 | 18 | 19 | 19 | 19 | 14 | 9 | 78 | 76 | 90 | 52 | 211 | 122 | 153 | 36 | 48 | 36 | + | |
| 29 | M | 26 | 31 | 26 | 56 | 33 | 32 | 17 | 75 | 71 | 191 | 122 | 214 | 161 | 144 | 110 | 41 | 38 | − | |
| 30 | F | 57 | 18 | 22 | 15 | 18 | 16 | 11 | 82 | 77 | 83 | 54 | 210 | 183 | 134 | 112 | 67 | 54 | − | |
| 31 | F | 38 | 17 | 22 | 15 | 19 | 12 | 12 | 92 | 77 | 68 | 65 | 179 | 142 | 99 | 73 | 75 | 60 | + | |
| 32 | F | 61 | 14 | 18 | 18 | 14 | 17 | 17 | 89 | 80 | 142 | 92 | 193 | 141 | 125 | 94 | 42 | 37 | − | |
| 33 | M | 61 | 18 | 15 | 19 | 18 | 23 | 15 | 115 | 100 | 169 | 114 | 219 | 208 | 151 | 137 | 35 | 48 | − | |
| 34 | F | 38 | 17 | 13 | 19 | 15 | 14 | 18 | 92 | 81 | 64 | 52 | 232 | 156 | 149 | 97 | 70 | 49 | + | |
| Average | 9M + 25F | 19.6 ± 8.0 | 19.5 ± 6.4 | 26.1 ± 18.6 | 24.4 ± 16.9 | 22.7 ± 11.4 | 16.1 ± 6.9 | 92.1 ± 9.8 | 87.0 ± 9.4 | 104.7 ± 44.0 | 82.0 ± 30.4 | 192.6 ± 40.4 | 166.4 ± 37.0 | 128.6 ± 31.0 | 111.1 ± 30.9 | 53.3 ± 14.0 | 46.2 ± 9.6 | |||
| p value | 0.9734 | 0.6983 | 0.0049 | 0.327 | 0.0158 | 0.0068 | 0.0232 | 0.0174 | ||||||||||||
| Non-Responder | ||||||||||||||||||||
| 35 | F | 33 | 12 | 12 | 12 | 13 | 18 | 11 | 93 | 85 | 81 | 74 | 162 | 153 | 106 | 106 | 43 | 41 | + | |
| 36 | F | 33 | 13 | 16 | 12 | 21 | 12 | 10 | 98 | 97 | 61 | 41 | 163 | 122 | 108 | 68 | 59 | 51 | + | |
| 37 | F | 21 | 19 | 11 | 32 | 16 | 13 | 13 | 90 | 91 | 96 | 52 | 205 | 184 | 104 | 100 | 82 | 74 | + | |
| 38 | F | 47 | 21 | 16 | 27 | 17 | 18 | 13 | 87 | 86 | 76 | 106 | 166 | 158 | 103 | 107 | 49 | 47 | − | |
| 39 | F | 60 | 15 | 17 | 13 | 18 | 26 | 21 | 103 | 101 | 66 | 65 | 174 | 178 | 114 | 101 | 59 | 64 | + | |
| 40 | F | 43 | 17 | 14 | 18 | 15 | 15 | 11 | 100 | 106 | 80 | 53 | 191 | 166 | 129 | 97 | 72 | 64 | − | |
| 41 | F | 32 | 23 | 21 | 26 | 17 | 21 | 11 | 77 | 79 | 95 | 48 | 150 | 139 | 91 | 81 | 60 | 51 | + | |
| 42 | M | 25 | 56 | 20 | 106 | 42 | 63 | 32 | 86 | 83 | 120 | 97 | 180 | 175 | 126 | 125 | 35 | 35 | + | |
| 43 | M | 48 | 19 | 18 | 26 | 30 | 55 | 24 | 90 | 86 | 85 | 103 | 226 | 206 | 155 | 151 | 57 | 47 | − | |
| 44 | M | 46 | 18 | 13 | 30 | 19 | 45 | 19 | 97 | 94 | 261 | 111 | 341 | 236 | 237 | 173 | 47 | 44 | − | |
| 45 | M | 38 | 27 | 23 | 47 | 24 | 32 | 16 | 95 | 95 | 330 | 216 | 156 | 151 | 88 | 90 | 28 | 31 | + | |
| 46 | F | 55 | 35 | 21 | 49 | 18 | 61 | 22 | 96 | 92 | 96 | 63 | 234 | 178 | 151 | 117 | 69 | 63 | + | |
| 47 | F | 36 | 13 | 18 | 12 | 15 | 18 | 12 | 91 | 99 | 90 | 103 | 190 | 176 | 130 | 127 | 47 | 41 | − | |
| 48 | F | 62 | 20 | 17 | 20 | 11 | 25 | 19 | 87 | 85 | 101 | 69 | 210 | 215 | 130 | 143 | 68 | 59 | + | |
| 49 | F | 21 | 35 | 35 | 19 | 11 | 16 | 12 | 105 | 92 | 66 | 62 | 170 | 172 | 106 | 105 | 61 | 53 | + | |
| 50 | F | 54 | 20 | 24 | 24 | 21 | 15 | 11 | 93 | 82 | 81 | 77 | 250 | 229 | 160 | 135 | 78 | 81 | + | |
| Average | 4M + 12F | 22.7 ± 11.2 | 18.5 ± 5.8 | 29.6 ± 23.3 | 19.3 ± 7.7 | 28.3 ± 17.6 | 16.1 ± 6.2 | 93.0 ± 7.1 | 90.8 ± 7.6 | 111.6 ± 74.4 | 83.8 ± 41.9 | 198.0 ± 48.2 | 177.4 ± 31.4 | 127.4 ± 31.0 | 114.1 ± 27.2 | 57.1 ± 15.0 | 52.9 ± 13.8 | |||
| p value | 0.1950 | 0.1030 | 0.0137 | 0.4048 | 0.2024 | 0.1617 | 0.2526 | 0.4109 | ||||||||||||
| Responder | ||||||||||||||||||||
| F2 | 51 | M | 44 | 28 | 25 | 39 | 34 | 48 | 21 | 109 | 86 | 137 | 83 | 194 | 156 | 140 | 101 | 44 | 39 | + |
| 52 | M | 50 | 47 | 26 | 105 | 42 | 23 | 21 | 105 | 104 | 98 | 126 | 203 | 193 | 124 | 126 | 52 | 51 | − | |
| 53 | F | 34 | 22 | 18 | 32 | 20 | 22 | 13 | 85 | 75 | 86 | 71 | 181 | 123 | 134 | 69 | 45 | 42 | + | |
| 54 | M | 21 | 29 | 29 | 55 | 36 | 21 | 13 | 90 | 84 | 93 | 69 | 164 | 153 | 102 | 94 | 43 | 45 | + | |
| 55 | F | 53 | 30 | 25 | 33 | 30 | 16 | 10 | 100 | 95 | 83 | 47 | 164 | 134 | 88 | 71 | 59 | 55 | + | |
| 56 | M | 44 | 50 | 28 | 90 | 35 | 76 | 24 | 94 | 110 | 174 | 121 | 209 | 172 | 151 | 109 | 40 | 37 | − | |
| 57 | M | 32 | 34 | 35 | 59 | 79 | 58 | 53 | 95 | 94 | 191 | 132 | 226 | 191 | 159 | 152 | 32 | 30 | − | |
| 58 | F | 40 | 17 | 14 | 15 | 13 | 9 | 11 | 73 | 82 | 43 | 65 | 144 | 141 | 84 | 90 | 55 | 52 | − | |
| 59 | M | 49 | 16 | 22 | 27 | 43 | 20 | 19 | 98 | 100 | 111 | 92 | 204 | 195 | 163 | 138 | 60 | 44 | + | |
| 60 | M | 52 | 59 | 34 | 105 | 47 | 49 | 15 | 106 | 90 | 102 | 86 | 208 | 162 | 145 | 104 | 47 | 46 | + | |
| 61 | M | 33 | 54 | 70 | 97 | 159 | 40 | 35 | 115 | 90 | 271 | 176 | 198 | 186 | 158 | 115 | 40 | 35 | + | |
| Average | 8M + 3F | 35.1 ± 15.1 | 29.6 ± 14.7 | 59.7 ± 33.8 | 48.9 ± 40.2 | 34.7 ± 20.9 | 21.4 ± 12.7 | 97.3 ± 11.8 | 91.8 ± 10.2 | 126.3 ± 63.8 | 97.1 ± 37.7 | 190.5 ± 24.4 | 164.2 ± 25.3 | 131.6 ± 28.6 | 106.3 ± 25.8 | 47.0 ± 8.7 | 43.3 ± 7.7 | |||
| p value | 0.4010 | 0.5024 | 0.0851 | 0.2610 | 0.2063 | 0.0222 | 0.0409 | 0.2987 | ||||||||||||
| Non-Responder | ||||||||||||||||||||
| 62 | F | 56 | 18 | 17 | 20 | 14 | 13 | 10 | 97 | 94 | 150 | 145 | 233 | 201 | 159 | 133 | 44 | 39 | − | |
| 63 | F | 50 | 24 | 23 | 34 | 24 | 18 | 12 | 91 | 93 | 102 | 108 | 217 | 272 | 164 | 190 | 53 | 42 | − | |
| 64 | F | 57 | 15 | 14 | 20 | 18 | 18 | 15 | 113 | 95 | 101 | 63 | 231 | 196 | 148 | 115 | 63 | 67 | + | |
| 65 | M | 64 | 16 | 22 | 18 | 23 | 16 | 10 | 89 | 93 | 68 | 50 | 221 | 204 | 144 | 119 | 74 | 76 | + | |
| 66 | F | 26 | 29 | 24 | 68 | 47 | 27 | 13 | 96 | 96 | 80 | 53 | 193 | 174 | 129 | 127 | 48 | 38 | − | |
| 67 | F | 37 | 12 | 14 | 12 | 10 | 7 | 8 | 85 | 76 | 64 | 57 | 172 | 128 | 102 | 77 | 66 | 50 | + | |
| 68 | F | 20 | 33 | 11 | 35 | 12 | 23 | 9 | 100 | 91 | 101 | 101 | 134 | 129 | 80 | 78 | 58 | 44 | + | |
| 69 | F | 33 | 22 | 14 | 30 | 14 | 13 | 8 | 92 | 87 | 79 | 70 | 220 | 139 | 151 | 87 | 61 | 42 | + | |
| 70 | F | 25 | 12 | 15 | 12 | 22 | 20 | 20 | 97 | 83 | 266 | 174 | 222 | 205 | 130 | 140 | 35 | 30 | + | |
| 71 | F | 55 | 20 | 18 | 11 | 11 | 13 | 9 | 84 | 71 | 91 | 63 | 162 | 143 | 97 | 78 | 59 | 53 | + | |
| 72 | M | 42 | 19 | 14 | 24 | 18 | 18 | 14 | 106 | 98 | 60 | 85 | 192 | 175 | 140 | 125 | 47 | 43 | + | |
| Average | 2M + 9F | 20.0 ± 6.7 | 16.9 ± 4.3 | 25.8 ± 16.4 | 19.4 ± 10.4 | 16.9 ± 5.4 | 11.6 ± 3.7 | 95.5 ± 8.7 | 88.8 ± 8.7 | 105.6 ± 58.8 | 88.1 ± 40.5 | 199.7 ± 32.2 | 178.7 ± 43.3 | 131.3 ± 27.2 | 115.4 ± 34.3 | 55.3 ± 11.2 | 47.6 ± 13.4 | |||
| p value | 0.2116 | 0.2827 | 0.0150 | 0.0892 | 0.4247 | 0.2115 | 0.2427 | 0.1615 | ||||||||||||
| Responder | ||||||||||||||||||||
| F3–F4 | 73 | F | 57 | 19 | 24 | 27 | 28 | 60 | 21 | 92 | 90 | 75 | 70 | 193 | 180 | 107 | 89 | 95 | 79 | − |
| 74 | M | 44 | 29 | 29 | 34 | 53 | 22 | 19 | 102 | 98 | 257 | 176 | 196 | 174 | 128 | 102 | 42 | 42 | − | |
| 75 | M | 54 | 33 | 28 | 41 | 28 | 25 | 12 | 116 | 89 | 378 | 78 | 220 | 130 | 119 | 77 | 33 | 41 | + | |
| 76 | M | 33 | 35 | 21 | 57 | 40 | 82 | 32 | 109 | 88 | 181 | 136 | 207 | 185 | 147 | 142 | 39 | 29 | + | |
| 77 | F | 20 | 21 | 18 | 19 | 17 | 10 | 7 | 86 | 88 | 84 | 158 | 155 | 151 | 91 | 69 | 62 | 47 | + | |
| 78 | F | 33 | 26 | 34 | 51 | 59 | 53 | 38 | 99 | 82 | 113 | 78 | 167 | 124 | 117 | 85 | 34 | 30 | + | |
| Average | 3M + 3F | 27.2 ± 6.4 | 25.7 ± 5.8 | 38.2 ± 14.4 | 37.5 ± 16.2 | 42.0 ± 27.4 | 21.5 ± 11.7 | 100.7 ± 10.9 | 89.2 ± 5.2 | 181.3 ± 118.2 | 116.0 ± 46.4 | 189.7 ± 24.4 | 157.3 ± 26.3 | 118.2 ± 18.9 | 94.0 ± 26.0 | 50.8 ± 24.1 | 44.7 ± 18.3 | |||
| p value | 0.6800 | 0.9414 | 0.1232 | 0.0422 | 0.2362 | 0.0519 | 0.0958 | 0.6278 | ||||||||||||
| Average Tot | 26M + 52F | 23.0 ± 10.9 | 20.8 ± 8.6 | 32.4 ± 24.2 | 27.1 ± 21.8 | 26.2 ± 16.6 | 16.6 ± 8.3 | 94.1 ± 9.7 | 88.9 ± 8.8 | 115.2 ± 64.9 | 88.0 ± 37.0 | 194.2 ± 37.6 | 169.4 ± 37.6 | 128.4 ± 30.1 | 110.3 ± 29.5 | 53.3 ± 14.2 | 47.2 ± 11.8 | |||
| p value | 0.1628 | 0.1501 | <0.0001 | 0.0005 | 0.0016 | <0.0001 | 0.0002 | 0.0044 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestra, F.; Negro, R.; De Luca, M.; Depalo, N.; Rizzi, F.; Panzetta, G.; Arrè, V.; Mastrogiacomo, R.; Coletta, S.; Stabile, D.; et al. Extracellular Vesicles Modulate Liver Cells Viability and Reactive Oxygen Species in Patients Following a Very Low-Calorie Ketogenic Diet. Nutrients 2024, 16, 2386. https://doi.org/10.3390/nu16152386
Balestra F, Negro R, De Luca M, Depalo N, Rizzi F, Panzetta G, Arrè V, Mastrogiacomo R, Coletta S, Stabile D, et al. Extracellular Vesicles Modulate Liver Cells Viability and Reactive Oxygen Species in Patients Following a Very Low-Calorie Ketogenic Diet. Nutrients. 2024; 16(15):2386. https://doi.org/10.3390/nu16152386
Chicago/Turabian StyleBalestra, Francesco, Roberto Negro, Maria De Luca, Nicoletta Depalo, Federica Rizzi, Giorgia Panzetta, Valentina Arrè, Rita Mastrogiacomo, Sergio Coletta, Dolores Stabile, and et al. 2024. "Extracellular Vesicles Modulate Liver Cells Viability and Reactive Oxygen Species in Patients Following a Very Low-Calorie Ketogenic Diet" Nutrients 16, no. 15: 2386. https://doi.org/10.3390/nu16152386






