Proteins and Peptides Studied In Silico and In Vivo for the Treatment of Diabetes Mellitus: A Systematic Review
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategies
2.2. Elegibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Extraction
2.4. Assessment of Risk of Bias and Study Quality
3. Results
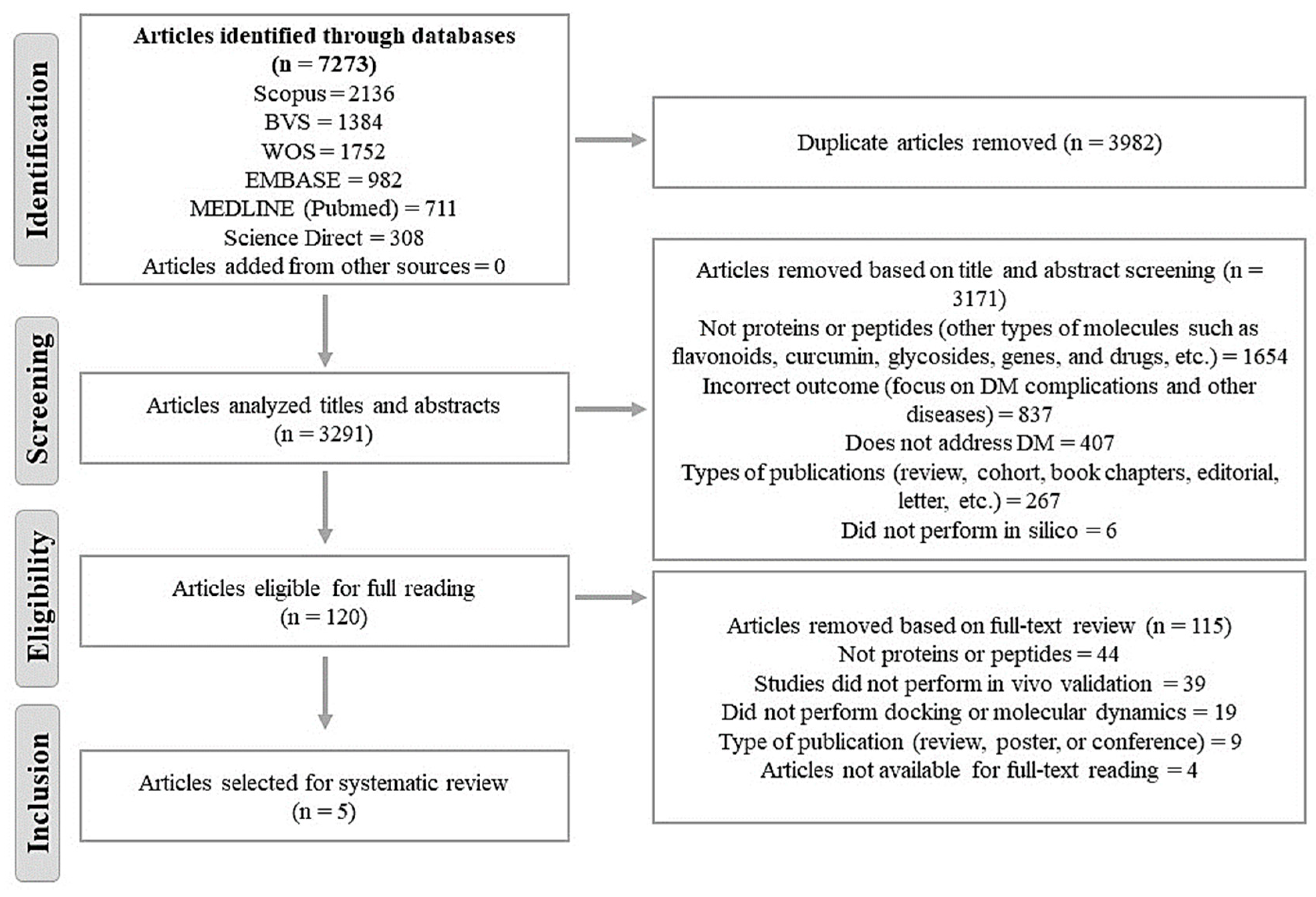
3.1. Selection and Characteristics of Studies
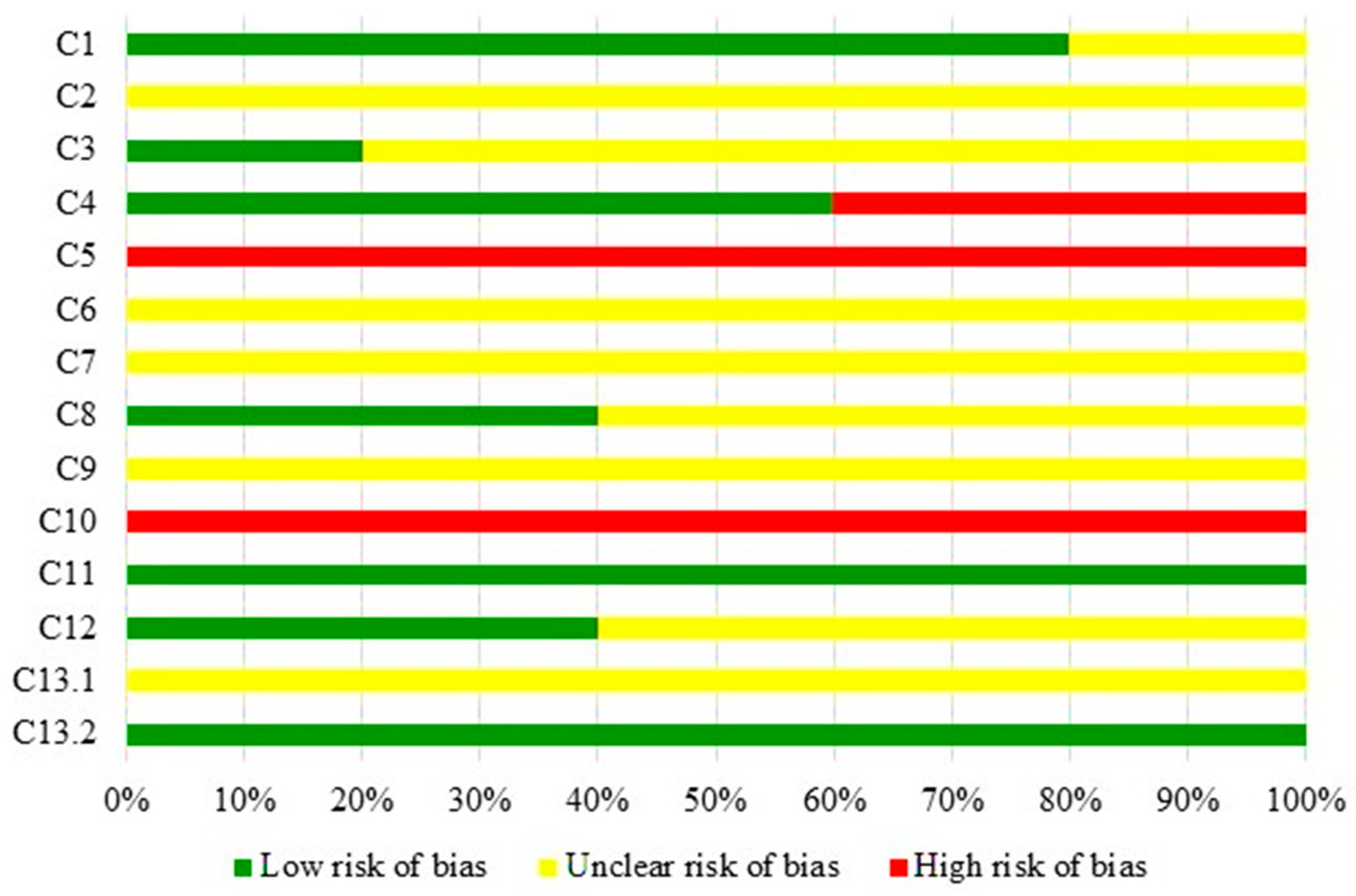
3.2. Quality of Studies
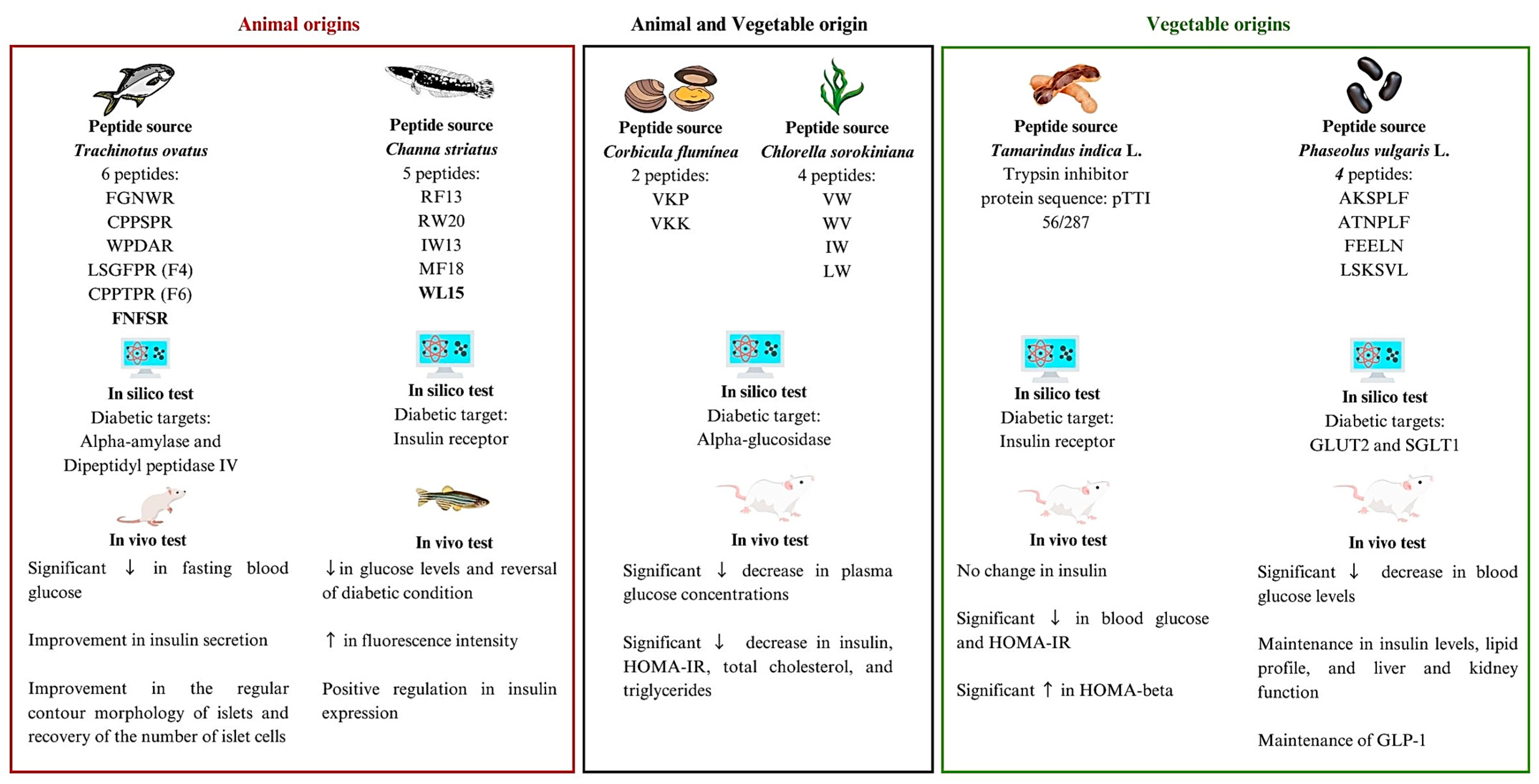
4. Bioactive Proteins and Peptides Evaluated In Silico and In Vivo for the Treatment of DM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitra, D.; Mitra, D.; Sabri Bensaad, M.; Sinha, S.; Pant, K.; Pant, M.; Priyadarshini, A.; Singh, P.; Dassamiour, S.; Hambaba, L.; et al. Evolution of Bioinformatics and Its Impact on Modern Bio-Science in the Twenty-First Century: Special Attention to Pharmacology, Plant Science and Drug Discovery. Comput. Toxicol. 2022, 24, 100248. [Google Scholar] [CrossRef]
- Genovesi, L.A.; Millar, A.; Tolson, E.; Singleton, M.; Hassall, E.; Kojic, M.; Brighi, C.; Girard, E.; Andradas, C.; Kuchibhotla, M.; et al. Systems Pharmacogenomics Identifies Novel Targets and Clinically Actionable Therapeutics for Medulloblastoma. Genome Med. 2021, 13, 103. [Google Scholar] [CrossRef]
- Vemula, D.; Jayasurya, P.; Sushmitha, V.; Kumar, Y.N.; Bhandari, V. CADD, AI and ML in Drug Discovery: A Comprehensive Review. Eur. J. Pharm. Sci. 2023, 181, 106324. [Google Scholar] [CrossRef]
- Zhao, S.; Li, X.; Peng, W.; Wang, L.; Ye, W.; Zhao, Y.; Yin, W.; Chen, W.-D.; Li, W.; Wang, Y.-D. Ligand-Based Pharmacophore Modeling, Virtual Screening and Biological Evaluation to Identify Novel TGR5 Agonists. RSC Adv. 2021, 11, 9403–9409. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, A.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care 2023, 46, e151–e199. [Google Scholar] [CrossRef]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II Diabetes Mellitus: A Review on Recent Drug Based Therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Howse, P.M.; Chibrikova, L.N.; Twells, L.K.; Barrett, B.J.; Gamble, J.-M. Safety and Efficacy of Incretin-Based Therapies in Patients With Type 2 Diabetes Mellitus and CKD: A Systematic Review and Meta-Analysis. Am. J. Kidney Dis. 2016, 68, 733–742. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Antony, P.; Vijayan, R. Bioactive Peptides as Potential Nutraceuticals for Diabetes Therapy: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 1059. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.S.; Medeiros, A.F.; Piuvezam, G.; Medeiros, G.C.; Maciel, B.L.; Morais, A.H.A.M. Insulin-Like Proteins in Plant Sources: A Systematic Review. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3421–3431. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, B.G.; Albericio, F. Peptide Therapeutics 2.0. Molecules 2020, 25, 2293. [Google Scholar] [CrossRef] [PubMed]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, I.; de Oliveira, G.S.; Aguiar, A.J.F.C.; Maia, J.K.D.S.; Piuvezam, G.; Morais, A.H. In Silico Structure-Based Design of Peptides or Proteins as Therapeutic Tools for Obesity or Diabetes Mellitus: A Protocol for Systematic Review and Meta Analysis. 2022. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022355540 (accessed on 6 May 2024).
- Medeiros, I.; Aguiar, A.J.F.C.; Fortunato, W.M.; Teixeira, A.F.G.; Oliveira E Silva, E.G.; Bezerra, I.W.L.; Maia, J.K.D.S.; Piuvezam, G.; Morais, A.H.D.A. In Silico Structure-Based Design of Peptides or Proteins as Therapeutic Tools for Obesity or Diabetes Mellitus: A Protocol for Systematic Review and Meta Analysis. Medicine 2023, 102, e33514. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Cai, B.; Chen, H.; Chen, D.; Zhao, X.; Yuan, H.; Huang, J.; Chen, X.; Luo, L.; Pan, J. Antidiabetic Effects of Protein Hydrolysates from Trachinotus Ovatus and Identification and Screening of Peptides with α-Amylase and DPP-IV Inhibitory Activities. Curr. Res. Food Sci. 2023, 6, 100446. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Sudhakaran, G.; Almutairi, M.H.; Almutairi, B.O.; Juliet, A.; Arockiaraj, J. β-Cells Regeneration by WL15 of Cysteine and Glycine-Rich Protein 2 Which Reduces Alloxan Induced β-Cell Dysfunction and Oxidative Stress through Phosphoenolpyruvate Carboxykinase and Insulin Pathway in Zebrafish in-Vivo Larval Model. Mol. Biol. Rep. 2022, 49, 11867–11879. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.; Lima, M.; Medeiros, A.; Bezerra, L.; Santos, P.; Serquiz, A.; Lima, M.; Oliveira, G.; Santos, E.; Maciel, B.; et al. An Insulin Receptor-Binding Multifunctional Protein from Tamarindus Indica L. Presents a Hypoglycemic Effect in a Diet-Induced Type 2 Diabetes—Preclinical Study. Foods 2022, 11, 2207. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, P.; Lin, Y.; Tsai, J. Hypoglycemic Peptide-enriched Hydrolysates of Corbicula fluminea and Chlorella sorokiniana Possess Synergistic Hypoglycemic Activity through Inhibiting A-glucosidase and Dipeptidyl Peptidase-4 Activity. J. Sci. Food Agric. 2022, 102, 716–723. [Google Scholar] [CrossRef]
- Mojica, L.; Gonzalez De Mejia, E.; Granados-Silvestre, M.Á.; Menjivar, M. Evaluation of the Hypoglycemic Potential of a Black Bean Hydrolyzed Protein Isolate and Its Pure Peptides Using in Silico, in Vitro and in Vivo Approaches. J. Funct. Foods 2017, 31, 274–286. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.; Nikitin, I.; Zhevlakova, A.; Selivanova, I. Insights into the Pharmacological Effects of Flavonoids: The Systematic Review of Computer Modeling. Int. J. Mol. Sci. 2022, 23, 6023. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, W.F.; Gomes, A.F.T.; Aguiar, A.J.F.C.; De Queiroz, J.L.C.; Bezerra, I.W.L.; Da Silva-Maia, J.K.; Piuvezam, G.; Morais, A.H.D.A. Anti-Obesity Therapeutic Targets Studied In Silico and In Vivo: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 4699. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular Docking as a Tool for the Discovery of Molecular Targets of Nutraceuticals in Diseases Management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef]
- Potluri, H.; Prasanth, D.S.N.B.K.; Atmakuri, L.R. In Vivo Antinociceptive Effect of Methanolic Extract of Ipomoea Marginata Desr. in Rodents as Well as In Silico Molecular Docking of Some Phytoconstituents from the Plant. Indian J. Pharm. Sci. 2021, 83, 732–741. [Google Scholar] [CrossRef]
- Ghode, P.; Jain, S.K. Structural Requirements for Some 3-Amino-N-Substituted-4-(Substituted Phenyl) Butanamides as Dipeptidyl Peptidase-IV Inhibitors Using 3D-QSAR and Molecular Docking Approaches. Indian J. Pharm. Sci. 2017, 79, 974–986. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Salmaso, V.; Moro, S. Bridging Molecular Docking to Molecular Dynamics in Exploring Ligand-Protein Recognition Process: An Overview. Front. Pharmacol. 2018, 9, 923. [Google Scholar] [CrossRef]
- Muhammed, M.T.; Aki-Yalcin, E. Homology Modeling in Drug Discovery: Overview, Current Applications, and Future Perspectives. Chem. Biol. Drug Des. 2019, 93, 12–20. [Google Scholar] [CrossRef]
- Pantsar, T.; Poso, A. Binding Affinity via Docking: Fact and Fiction. Molecules 2018, 23, 1899. [Google Scholar] [CrossRef]
- Grimes, J.M.; Hall, D.R.; Ashton, A.W.; Evans, G.; Owen, R.L.; Wagner, A.; McAuley, K.E.; Von Delft, F.; Orville, A.M.; Sorensen, T.; et al. Where Is Crystallography Going? Acta Crystallogr. Sect. Struct. Biol. 2018, 74, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA Methods to Estimate Ligand-Binding Affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, G.; Pinzi, L. Refinement and Rescoring of Virtual Screening Results. Front. Chem. 2019, 7, 498. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef]
- Sun, J.L.; Jiang, T.; Gu, Y.; Song, F.B.; Wen, X.; Luo, J. Differential Immune and Metabolic Responses Underlie Differences in the Resistance of Siganus Oramin and Trachinotus Blochii to Cryptocaryon Irritans Infection. Fish Shellfish Immunol. 2022, 120, 166–179. [Google Scholar] [CrossRef]
| Database | Search Strategies |
|---|---|
| PubMed, Scopus, Science Direct, and BVS | (Protein OR peptides) AND (“in silico” OR “computer simulation”) AND diabetes |
| WOF 1 and EMBASE | (Protein OR peptides) AND (“in silico” OR “computer simulation”) AND diabetes |
| Description | Abbreviation | Elements of the Question |
|---|---|---|
| Population | P | Proteins or peptides of natural origin |
| Exhibition | E | Diabetes mellitus |
| Context | Co | In silico studies with molecular dynamics or molecular docking |
| References | Origin of Proteins and/or Peptides | Proteins and/or Peptides | Techniques Employed | Docking Score or Potential Interaction Energy (PIE) with Target of Interest | In Vivo Assessments (Validation) | Outcomes |
|---|---|---|---|---|---|---|
| Wan et al. (2023) [17] | Carnivorous marine fish (Trachinotus ovatus) | Protein hydrolysates from carnivorous marine fish (TOH)—6 peptides: FGNWR, CPPSPR, FNFSR, WPDAR, LSGFPR (F4), and CPPTPR (F6) | Molecular docking | Not reported | Fasting blood glucose, insulin, renal histopathology, and pancreatic histopathology | Significant ↓ in fasting blood glucose 1 Significant ↑ in fasting blood glucose 2,3 - Improvement in insulin secretion, but associated with loss of pancreatic β cells - Improvement in the regular contour morphology of islets and recovery of the number of islet cells after treatment with dose-dependent TOH and metformin |
| Guru et al. (2022) [18] | Teleost fish (Channa striatus) | 5 Teleost fish peptides extracted using PeptideRanker: WL15 (WHKNCFRCAKCGKSL), RF13 (RRGKGGRRVTMSF), RW20 (RPVKRKKGWPKGVKRGPPKW), IW13 (IKHFKKQRRLIPW), and MF18 (MRKKAVKVKHVKRREKKF) | Molecular docking | IR: WL15 = −218.16 kcal/mol RF13 = −189.35 kcal/mol RW20 = −191.54 kcal/mol IW13 = −186.32 kcal/mol MF18 = −177.15 kcal/mol | 2NBDG uptake, glucose estimation in larvae, and gene expression of insulin and PEPCK | ↑ in fluorescence intensity with co-treatment of WL15 (50 µM) ↓ in glucose levels and reversal of diabetic condition with co-treatment of WL15 (50 μM) - Positive regulation in insulin expression when co-treated with WL15 (50 µM) |
| Costa et al. (2022) [19] | Tamarind seed (Tamarindus indica L.) | Trypsin inhibitor protein sequence: pTTI 56/287 | Docking and molecular dynamics | IR: TTIp 56/287: −100.6 kJ mol−1 | Fasting glycemia, insulinemia, HOMA-IR, and HOMA-beta | - No change in insulin Significant ↓ in blood glucose and HOMA-IR 1 Significant ↑ in HOMA-beta 1 |
| Huang et al. (2022) [20] | The Asian clam (Corbicula flumínea) and green microalgae (Chlorella sorokiniana) | Corbicula fluminea protein hydrolyzate (ACH)—2 peptides: VKP and VKK Chlorella sorokiniana protein hydrolyzate (PCH)—4 peptides: VW, WV, IW, and LW | Molecular docking | Not reported | Fasting blood glucose, oral glucose tolerance test, insulin, HOMA-IR, total cholesterol, and triglycerides | Significant ↓ in decrease in plasma glucose concentrations in diabetic animals treated with ACH (9.5%), PCH (4.1%), or ACH/PCH (14.6%) Significant ↓ in plasma glucose concentration at 30 min in diabetic rats fed ACH/PCH in OGTT Significant ↓ in insulin, HOMA-IR, total cholesterol, and triglycerides 1 |
| Mojica et al. (2017) [21] | Black bean (Phaseolus vulgaris L.) | Black bean protein hydrolyzate (HPI)—4 peptides: AKSPLF, ATNPLF, FEELN, and LSKSVL | Molecular docking | GLUT2: AKSPLF: −0.6 kcal/mol ATNPLF: −7.5 kcal/mol FEELN: −7.91 kcal/mol LSKSVL: −5.24 kcal/mol SGLT1: AKSPLF: −8.66 kcal/mol ATNPLF: −7.20 kcal/mol FEELN: −6.44 kcal/mol LSKSVL: −4.4 kcal/mol | Oral glucose tolerance test Determination of glucose, lipid profile, and renal function Insulin and GLP-1 | Significant ↓ in blood glucose levels (22.7 to 47.7%) 1 - Maintenance in insulin levels, lipid profile, liver, and kidney function 1 - Maintenance of GLP-1 1,2 (except for diabetic treated with HPI 150 mg/kg, where there was a significant increase) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, I.; Gomes, A.F.T.; Oliveira e Silva, E.G.; Bezerra, I.W.L.; da Silva Maia, J.K.; Piuvezam, G.; Morais, A.H.d.A. Proteins and Peptides Studied In Silico and In Vivo for the Treatment of Diabetes Mellitus: A Systematic Review. Nutrients 2024, 16, 2395. https://doi.org/10.3390/nu16152395
Medeiros I, Gomes AFT, Oliveira e Silva EG, Bezerra IWL, da Silva Maia JK, Piuvezam G, Morais AHdA. Proteins and Peptides Studied In Silico and In Vivo for the Treatment of Diabetes Mellitus: A Systematic Review. Nutrients. 2024; 16(15):2395. https://doi.org/10.3390/nu16152395
Chicago/Turabian StyleMedeiros, Isaiane, Ana Francisca Teixeira Gomes, Emilly Guedes Oliveira e Silva, Ingrid Wilza Leal Bezerra, Juliana Kelly da Silva Maia, Grasiela Piuvezam, and Ana Heloneida de Araújo Morais. 2024. "Proteins and Peptides Studied In Silico and In Vivo for the Treatment of Diabetes Mellitus: A Systematic Review" Nutrients 16, no. 15: 2395. https://doi.org/10.3390/nu16152395






