Therapeutic Potential of Natural Compounds Acting through Epigenetic Mechanisms in Cardiovascular Diseases: Current Findings and Future Directions
Abstract
:1. Introduction
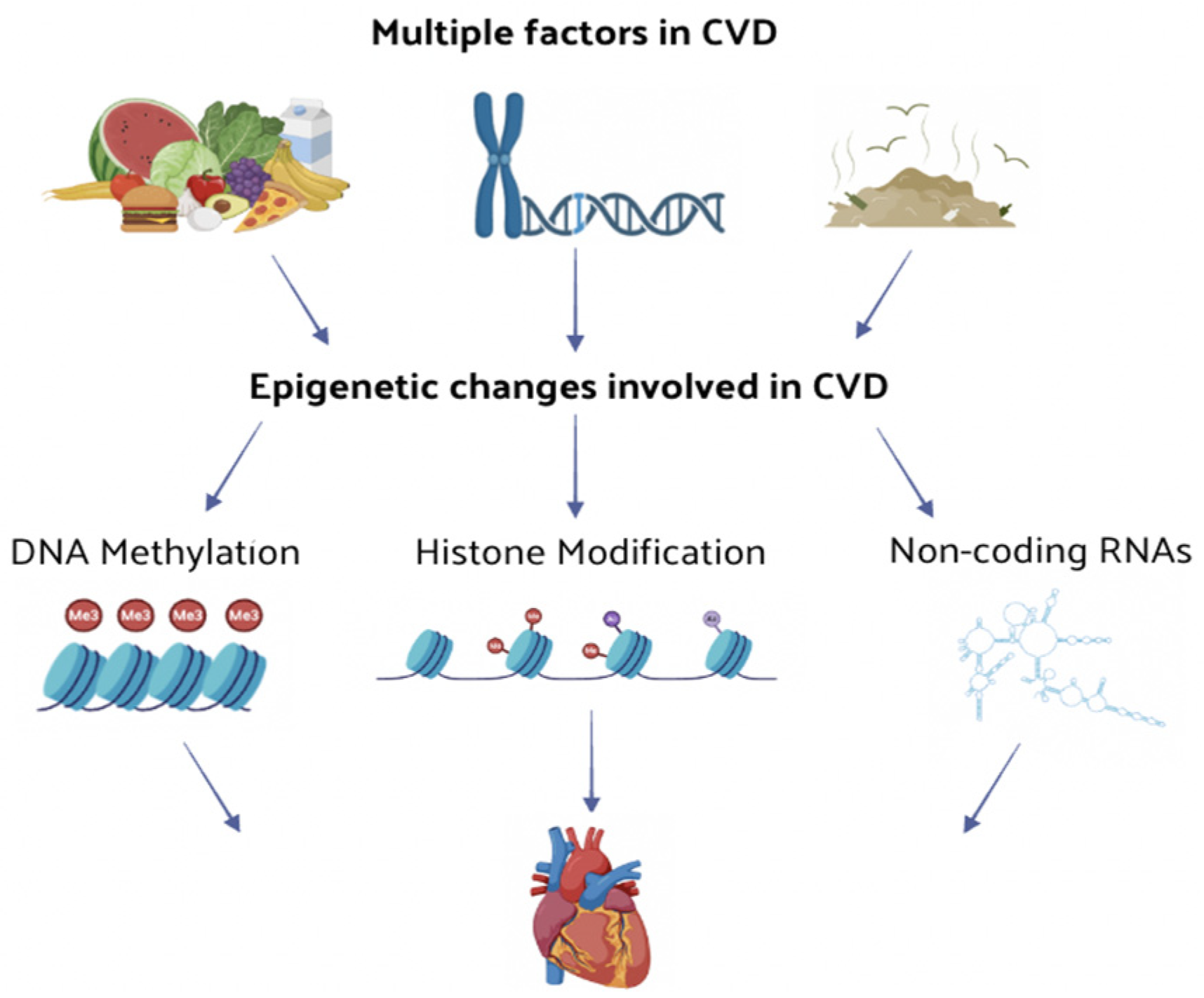
2. Epigenetic Regulatory Mechanisms
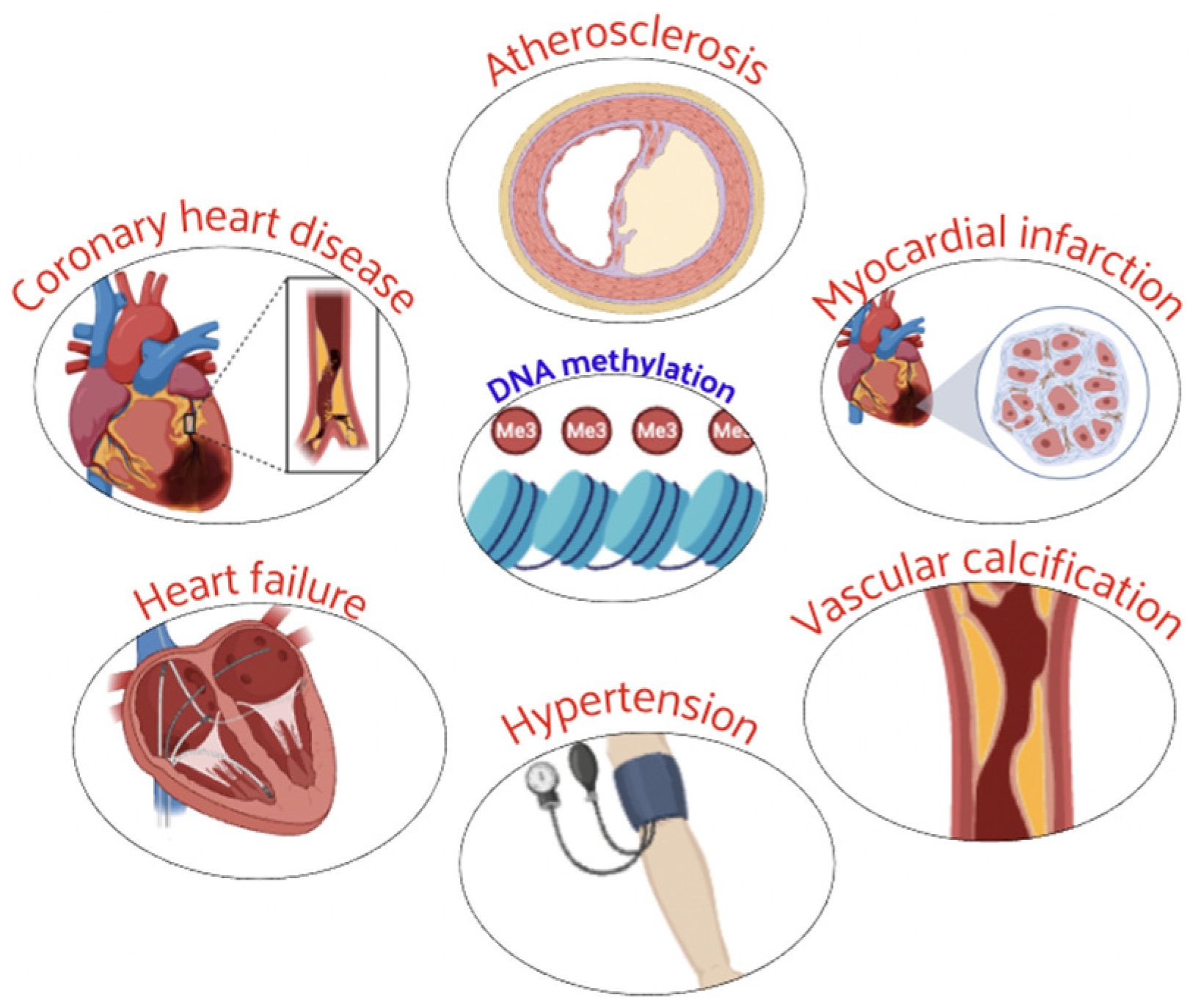
2.1. DNA Methylation Regulation
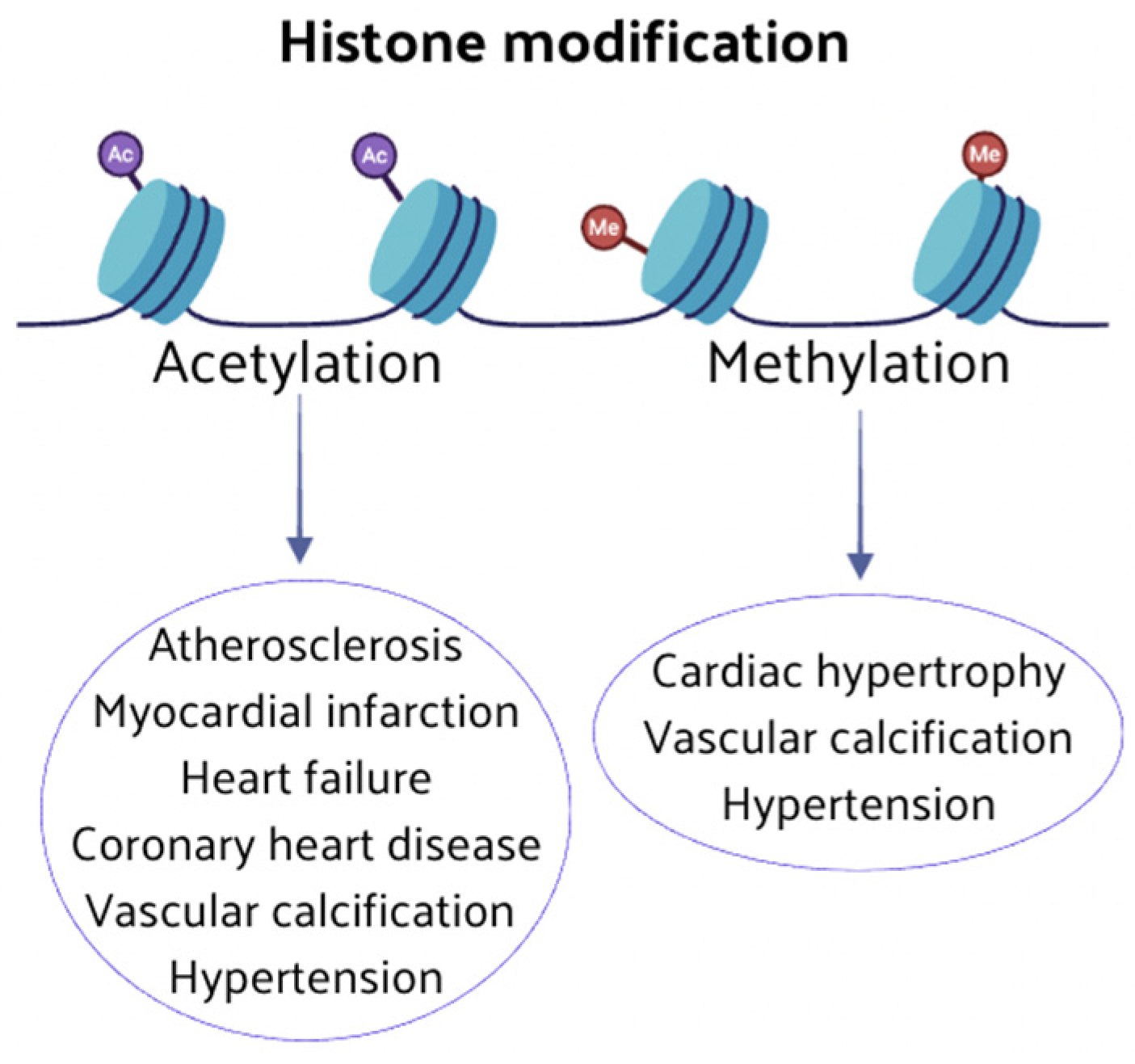
2.2. Histone Modification Regulation
2.2.1. Histone Methylation
2.2.2. Histone Acetylation
2.3. Non-Coding RNA Regulation
3. Epigenetics in Cardiovascular Diseases (CVD)
4. Epigenetic Modulation as Therapeutic Strategy for Cardiovascular Diseases (CVD)
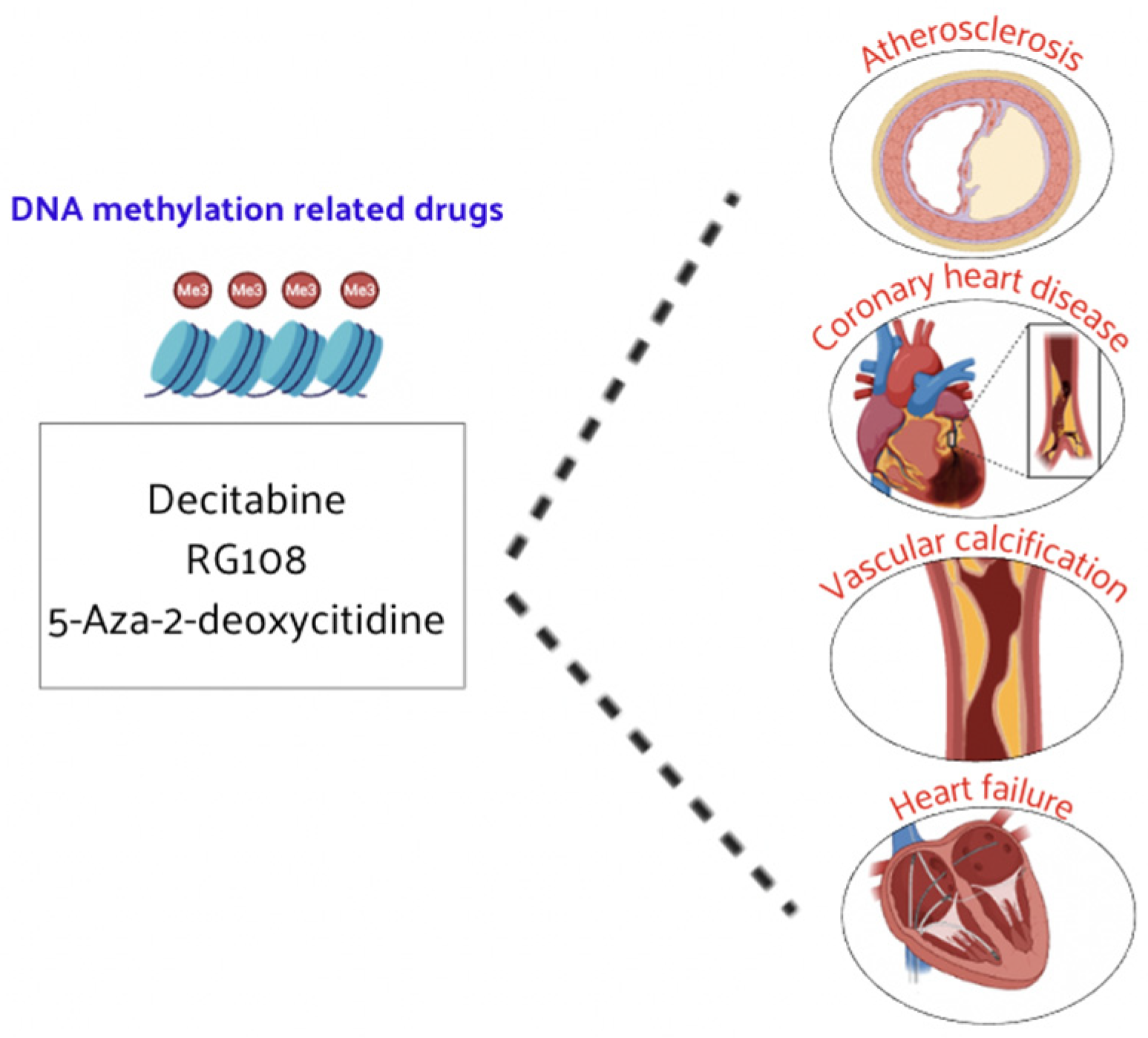
4.1. DNA Methylation-Related Drugs
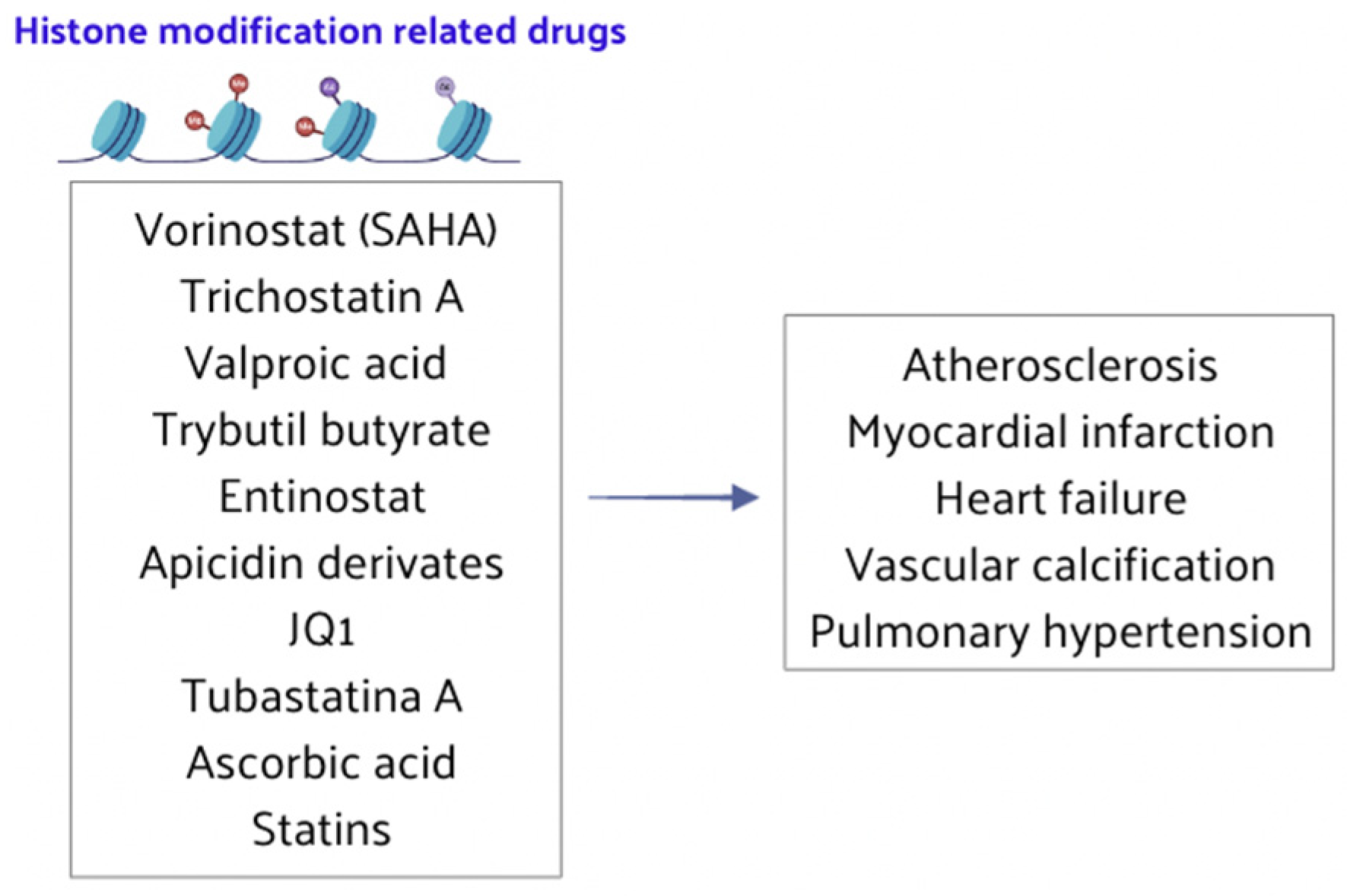
4.2. Histone Modification-Related Drugs
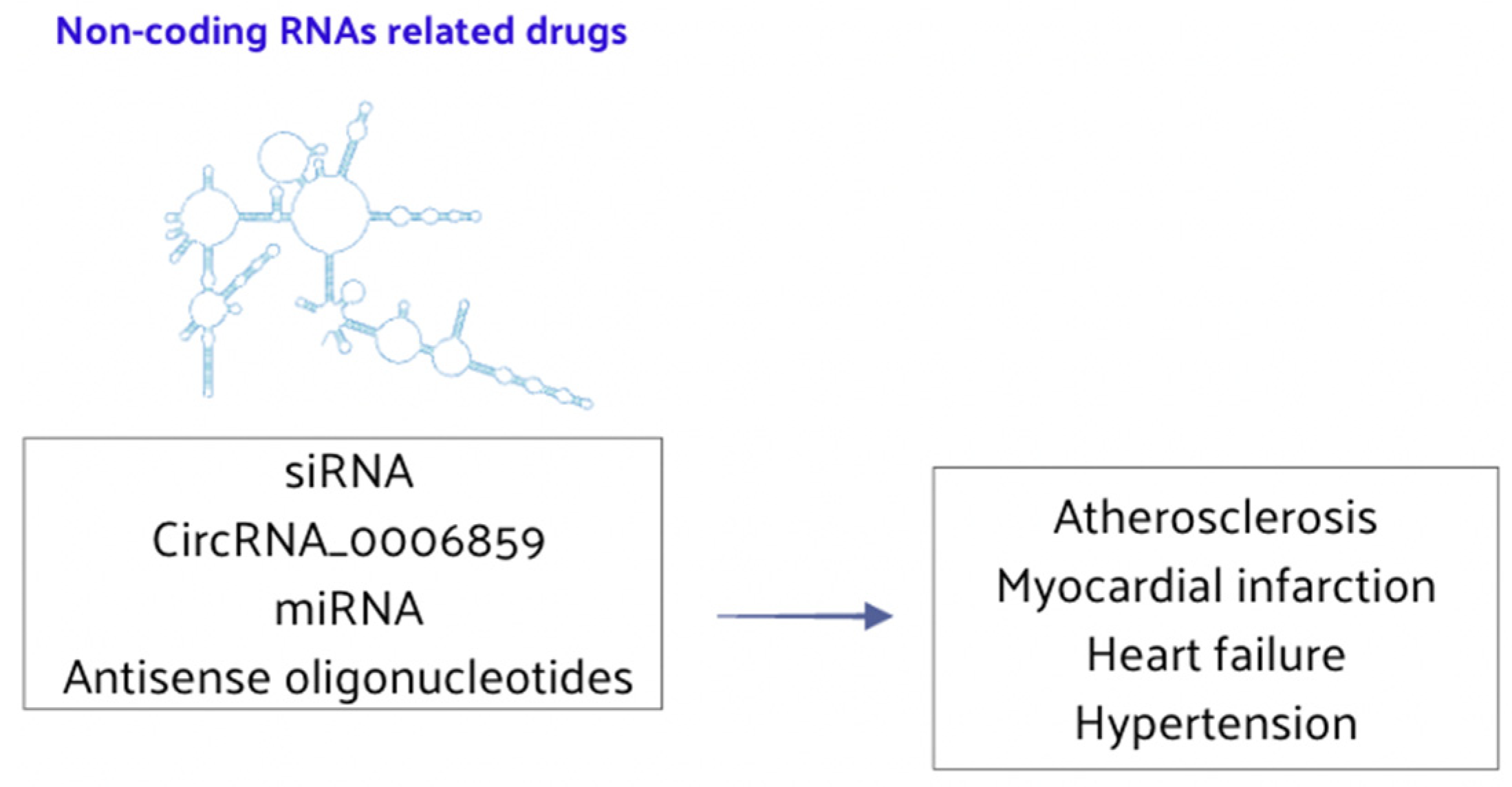
4.3. Non-Coding RNA-Related Drugs
5. Epigenetic Dietary Components in Cardiovascular Diseases (CVD)
Epigenetic Natural Compounds (ENC) in Cardiovascular Diseases (CVD)
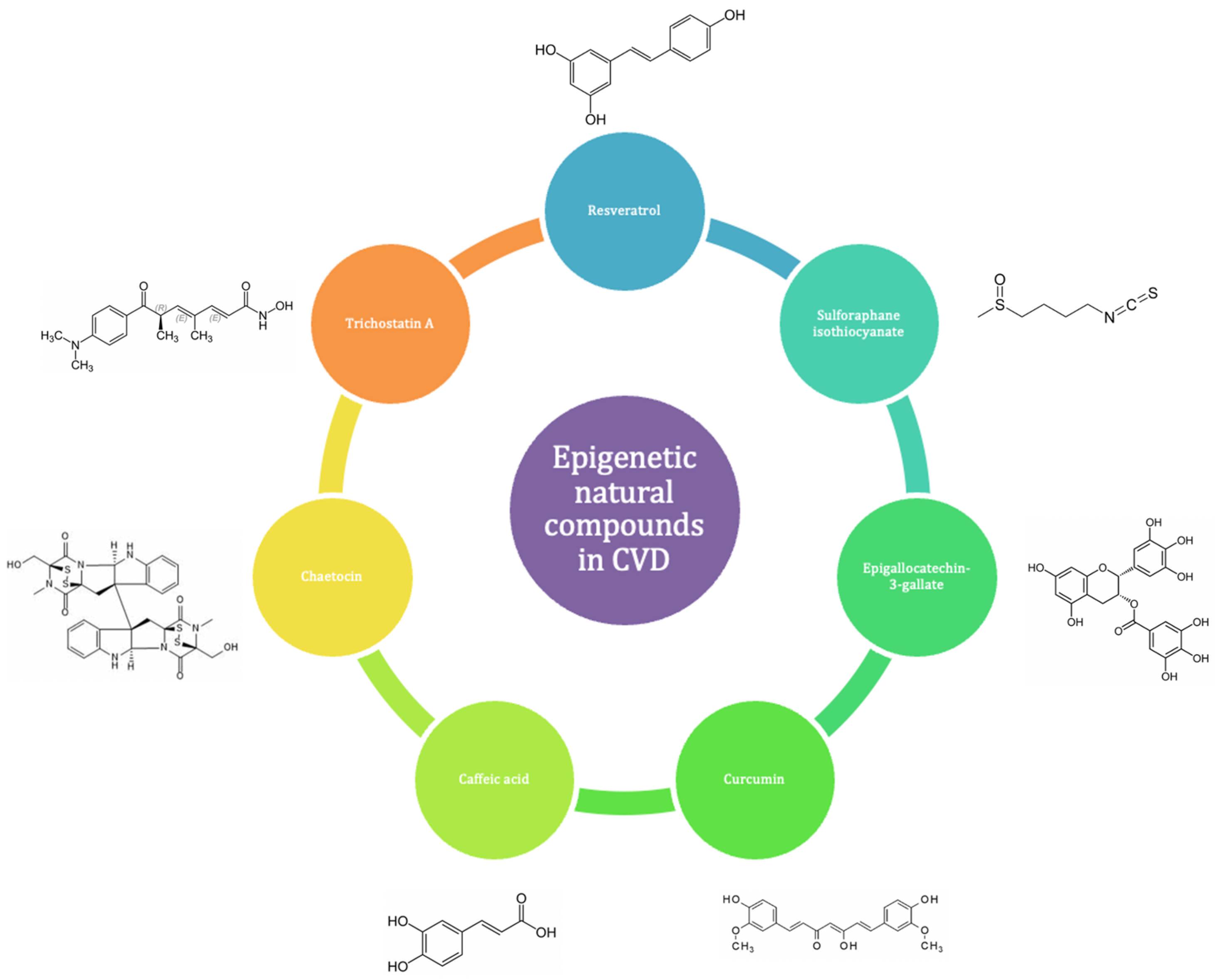
6. Epigenetic Natural Compounds (ENCs) as Potential Therapeutic Interventions in Cardiovascular Diseases (CVD)
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the gbd 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Johnson, A.D.; Benjamin, E.J.; Levy, D.; Vasan, R.S. 70-year legacy of the framingham heart study. Nat. Rev. Cardiol. 2019, 16, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P. The key role of epigenetics in human disease prevention and mitigation. N. Engl. J. Med. 2018, 378, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Shao, K.; Chen, X.; Li, Z.; Liu, Z.; Yu, Z.; Aung, L.H.H.; Wang, Y.; Li, P. The involvement of post-translational modifications in cardiovascular pathologies: Focus on sumoylation, neddylation, succinylation, and prenylation. J. Mol. Cell. Cardiol. 2020, 138, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.; Arimondo, P.B.; Rots, M.G.; Jeronimo, C.; Berdasco, M. The timeline of epigenetic drug discovery: From reality to dreams. Clin. Epigenetics 2019, 11, 174. [Google Scholar] [CrossRef]
- Aggarwal, R.; Jha, M.; Shrivastava, A.; Jha, A.K. Natural compounds: Role in reversal of epigenetic changes. Biochemistry 2015, 80, 972–989. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Neale, E.P.; Probst, Y. Dietary patterns and cardiovascular disease: Insights and challenges for considering food groups and nutrient sources. Curr. Atheroscler. Rep. 2019, 21, 9. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Drosatos, K.; Buxton, J.L. Nutriepigenetics and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 252–259. [Google Scholar] [CrossRef]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA methylation and DNA methyltransferases. Epigenetics Chromatin 2017, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Deplus, R.; Didelot, C.; Loriot, A.; Viré, E.; De Smet, C.; Gutierrez, A.; Danovi, D.; Bernard, D.; Boon, T.; et al. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 2005, 24, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, B.; Bird, A. Identification and characterization of a family of mammalian methyl-cpg binding proteins. Mol. Cell. Biol. 1998, 18, 6538–6547. [Google Scholar] [CrossRef]
- Unoki, M.; Sasaki, H. The uhrf protein family in epigenetics, development, and carcinogenesis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2022, 98, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Filion, G.J.; Zhenilo, S.; Salozhin, S.; Yamada, D.; Prokhortchouk, E.; Defossez, P.A. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol. Cell. Biol. 2006, 26, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Oh, S.; Ro, D.H.; Yoo, H.; Kwon, Y.W. The key role of DNA methylation and histone acetylation in epigenetics of atherosclerosis. J. Lipid Atheroscler. 2020, 9, 419–434. [Google Scholar] [CrossRef]
- Miller, J.L.; Grant, P.A. The role of DNA methylation and histone modifications in transcriptional regulation in humans. Subcell. Biochem. 2013, 61, 289–317. [Google Scholar] [CrossRef]
- Rea, S.; Eisenhaber, F.; O’Carroll, D.; Strahl, B.D.; Sun, Z.W.; Schmid, M.; Opravil, S.; Mechtler, K.; Ponting, C.P.; Allis, C.D.; et al. Regulation of chromatin structure by site-specific histone h3 methyltransferases. Nature 2000, 406, 593–599. [Google Scholar] [CrossRef]
- Teperino, R.; Schoonjans, K.; Auwerx, J. Histone methyl transferases and demethylases; can they link metabolism and transcription? Cell Metab. 2010, 12, 321–327. [Google Scholar] [CrossRef]
- Ogryzko, V.V.; Schiltz, R.L.; Russanova, V.; Howard, B.H.; Nakatani, Y. The transcriptional coactivators p300 and cbp are histone acetyltransferases. Cell 1996, 87, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.J.; Kwon, J.S.; Shin, S.; Ahn, Y.; Jeong, M.H.; Kook, H. Trichostatin a prevents neointimal hyperplasia via activation of krüppel like factor 4. Vascul. Pharmacol. 2011, 55, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kook, T.; Min, H.K.; Kwon, D.H.; Cho, Y.K.; Kim, M.; Shin, S.; Joung, H.; Jeong, S.H.; Lee, S.; et al. Pp2a negatively regulates the hypertrophic response by dephosphorylating hdac2 s394 in the heart. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.F.; Magliocca, K.R.; Kim, S.; Muller, S.; Chen, Z.; Owonikoko, T.K.; Sarlis, N.J.; Eggers, C.; Phelan, V.; Grist, W.J.; et al. Acetylated tubulin (at) as a prognostic marker in squamous cell carcinoma of the head and neck. Head. Neck Pathol. 2014, 8, 66–72. [Google Scholar] [CrossRef]
- McLendon, P.M.; Ferguson, B.S.; Osinska, H.; Bhuiyan, M.S.; James, J.; McKinsey, T.A.; Robbins, J. Tubulin hyperacetylation is adaptive in cardiac proteotoxicity by promoting autophagy. Proc. Natl. Acad. Sci. USA 2014, 111, E5178–E5186. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Baker, A.H.; Dimmeler, S.; Heymans, S.; Mayr, M.; Thum, T. Non-coding rnas in vascular disease—From basic science to clinical applications: Scientific update from the working group of myocardial function of the european society of cardiology. Cardiovasc. Res. 2018, 114, 1281–1286. [Google Scholar] [CrossRef]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding rnas as regulators in epigenetics (review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Poddar, S.; Kesharwani, D.; Datta, M. Interplay between the mirnome and the epigenetic machinery: Implications in health and disease. J. Cell. Physiol. 2017, 232, 2938–2945. [Google Scholar] [CrossRef]
- Reddy, S.; Hu, D.Q.; Zhao, M.; Blay, E., Jr.; Sandeep, N.; Ong, S.G.; Jung, G.; Kooiker, K.B.; Coronado, M.; Fajardo, G.; et al. Mir-21 is associated with fibrosis and right ventricular failure. JCI Insight 2017, 2, e91625. [Google Scholar] [CrossRef]
- Petrovic, N.; Ergun, S. Mirnas as potential treatment targets and treatment options in cancer. Mol. Diagn. Ther. 2018, 22, 157–168. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding rna biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Long, T.; Du, T.; Chen, Y.; Dong, Y.; Huang, Z.P. Circle the cardiac remodeling with circrnas. Front. Cardiovasc. Med. 2021, 8, 702586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, B.; Huang, S.; Zhao, L. Roles of circular rnas in immune regulation and autoimmune diseases. Cell Death Dis. 2019, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, A.S.; Aonuma, T.; Teoh, J.P.; Tang, Y.L.; Kim, I.M. Circular noncoding rnas as potential therapies and circulating biomarkers for cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, W.; Wang, X.Q.; He, Y.; Wang, S.S.; Yan, Y.X. A novel strategy of identifying circrna biomarkers in cardiovascular disease by meta-analysis. J. Cell. Physiol. 2019, 234, 21601–21612. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2022, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Wołowiec, A.; Wołowiec, Ł.; Grześk, G.; Jaśniak, A.; Osiak, J.; Husejko, J.; Kozakiewicz, M. The role of selected epigenetic pathways in cardiovascular diseases as a potential therapeutic target. Int. J. Mol. Sci. 2023, 24, 13723. [Google Scholar] [CrossRef] [PubMed]
- Westerman, K.; Sebastiani, P.; Jacques, P.; Liu, S.; DeMeo, D.; Ordovás, J.M. DNA methylation modules associate with incident cardiovascular disease and cumulative risk factor exposure. Clin. Epigenetics 2019, 11, 142. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Domingo-Relloso, A.; Subedi, P.; Riffo-Campos, A.L.; Xia, R.; Gomez, L.; Haack, K.; Goldsmith, J.; Howard, B.V.; Best, L.G.; et al. Blood DNA methylation and incident coronary heart disease: Evidence from the strong heart study. JAMA Cardiol. 2021, 6, 1237–1246. [Google Scholar] [CrossRef]
- Luo, X.; Hu, Y.; Shen, J.; Liu, X.; Wang, T.; Li, L.; Li, J. Integrative analysis of DNA methylation and gene expression reveals key molecular signatures in acute myocardial infarction. Clin. Epigenetics 2022, 14, 46. [Google Scholar] [CrossRef]
- Palou-Márquez, G.; Subirana, I.; Nonell, L.; Fernández-Sanlés, A.; Elosua, R. DNA methylation and gene expression integration in cardiovascular disease. Clin. Epigenetics 2021, 13, 75. [Google Scholar] [CrossRef]
- Teng, N.; Maghzal, G.J.; Talib, J.; Rashid, I.; Lau, A.K.; Stocker, R. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep. 2017, 22, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Chen, G.; Song, C.; Keefe, J.; Mendelson, M.; Huan, T.; Sun, B.B.; Laser, A.; Maranville, J.C.; Wu, H.; et al. Genome-wide mapping of plasma protein qtls identifies putatively causal genes and pathways for cardiovascular disease. Nat. Commun. 2018, 9, 3268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, T.W.; Su, M.C.; Chen, C.J.; Chen, K.D.; Liou, C.W.; Tang, P.; Wang, T.Y.; Chang, J.C.; Wang, C.C.; et al. Whole genome DNA methylation analysis of obstructive sleep apnea: Il1r2, npr2, ar, sp140 methylation and clinical phenotype. Sleep. 2016, 39, 743–755. [Google Scholar] [CrossRef]
- Fernández-Sanlés, A.; Sayols-Baixeras, S.; Subirana, I.; Sentí, M.; Pérez-Fernández, S.; de Castro Moura, M.; Esteller, M.; Marrugat, J.; Elosua, R. DNA methylation biomarkers of myocardial infarction and cardiovascular disease. Clin. Epigenetics 2021, 13, 86. [Google Scholar] [CrossRef]
- Slater, N.D.; Raftery, A.T. An evaluation of expanded polytetrafluoroethylene (ptfe) loop grafts in the thigh as vascular access for haemodialysis in patients with access problems. Ann. R. Coll. Surg. Engl. 1988, 70, 243–245. [Google Scholar] [PubMed]
- Laugier, L.; Frade, A.F.; Ferreira, F.M.; Baron, M.A.; Teixeira, P.C.; Cabantous, S.; Ferreira, L.R.P.; Louis, L.; Rigaud, V.O.C.; Gaiotto, F.A.; et al. Whole-genome cardiac DNA methylation fingerprint and gene expression analysis provide new insights in the pathogenesis of chronic chagas disease cardiomyopathy. Clin. Infect. Dis. 2017, 65, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cui, Y.; Huang, F.; Zeng, H.; Xia, W.; Zeng, F.; He, C.; Chen, J.; Chen, Z.; Chen, H.; et al. Long non-coding rna h19 promotes osteogenic differentiation of renal interstitial fibroblasts through wnt-β-catenin pathway. Mol. Cell. Biochem. 2020, 470, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liu, S.; Cheng, L.; Huang, T.; Guo, H.; Wang, D.; Xia, M.; Ling, W.; Xiao, Y. Epigenetic upregulation of h19 and ampk inhibition concurrently contribute to s-adenosylhomocysteine hydrolase deficiency-promoted atherosclerotic calcification. Circ. Res. 2022, 130, 1565–1582. [Google Scholar] [CrossRef]
- Montes de Oca, A.; Madueño, J.A.; Martinez-Moreno, J.M.; Guerrero, F.; Muñoz-Castañeda, J.; Rodriguez-Ortiz, M.E.; Mendoza, F.J.; Almaden, Y.; Lopez, I.; Rodriguez, M.; et al. High-phosphate-induced calcification is related to sm22α promoter methylation in vascular smooth muscle cells. J. Bone Miner. Res. 2010, 25, 1996–2005. [Google Scholar] [CrossRef]
- Lin, X.; Li, F.; Xu, F.; Cui, R.R.; Xiong, D.; Zhong, J.Y.; Zhu, T.; Shan, S.K.; Wu, F.; Xie, X.B.; et al. Aberration methylation of mir-34b was involved in regulating vascular calcification by targeting notch1. Aging 2019, 11, 3182–3197. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.L.; Kotchen, T.A.; Pan, X.; Li, Y.; Yang, C.; Liu, P.; Wang, T.; Laud, P.W.; Chelius, T.H.; Munyura, Y.; et al. Unique associations of DNA methylation regions with 24-hour blood pressure phenotypes in black participants. Hypertension 2022, 79, 761–772. [Google Scholar] [CrossRef]
- Jin, F.; Li, X.; Wang, Z.; Liu, Y.; Liu, J.; Sun, D.; Jin, Y.; Wang, S.; Wen, S.; Wei, Y. Association of mitofusin 2 methylation and essential hypertension: A case-control study in a chinese population. Hypertens. Res. 2018, 41, 605–613. [Google Scholar] [CrossRef]
- Bao, X.J.; Mao, S.Q.; Gu, T.L.; Zheng, S.Y.; Zhao, J.S.; Zhang, L.N. Hypomethylation of the interferon γ gene as a potential risk factor for essential hypertension: A case-control study. Tohoku J. Exp. Med. 2018, 244, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, G.; Maron, B.A.; Affinito, O.; D’Alto, M.; Franzese, M.; Argiento, P.; Schiano, C.; Romeo, E.; Bontempo, P.; Golino, P.; et al. Association between circulating cd4(+) t cell methylation signatures of network-oriented socs3 gene and hemodynamics in patients suffering pulmonary arterial hypertension. J. Cardiovasc. Transl. Res. 2023, 16, 17–30. [Google Scholar] [CrossRef]
- Papait, R.; Serio, S.; Pagiatakis, C.; Rusconi, F.; Carullo, P.; Mazzola, M.; Salvarani, N.; Miragoli, M.; Condorelli, G. Histone methyltransferase g9a is required for cardiomyocyte homeostasis and hypertrophy. Circulation 2017, 136, 1233–1246. [Google Scholar] [CrossRef]
- Kurozumi, A.; Nakano, K.; Yamagata, K.; Okada, Y.; Nakayamada, S.; Tanaka, Y. Il-6 and sil-6r induces stat3-dependent differentiation of human vsmcs into osteoblast-like cells through jmjd2b-mediated histone demethylation of runx2. Bone 2019, 124, 53–61. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. Sirt1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.B.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Mattagajasingh, I.; Hoffman, T.A.; Cole, M.P.; Kumar, A.; Dericco, J.S.; Jeon, B.H.; et al. Histone deacetylase 3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ. Res. 2010, 107, 877–887. [Google Scholar] [CrossRef]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef]
- Shinozaki, S.; Chang, K.; Sakai, M.; Shimizu, N.; Yamada, M.; Tanaka, T.; Nakazawa, H.; Ichinose, F.; Yamada, Y.; Ishigami, A.; et al. Inflammatory stimuli induce inhibitory s-nitrosylation of the deacetylase sirt1 to increase acetylation and activation of p53 and p65. Sci. Signal. 2014, 7, ra106. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Lei, J.; Han, H.; Li, W.; Qu, Y.; Fu, E.; Fu, F.; Wang, X. Sirt1 protects against myocardial ischemia-reperfusion injury via activating enos in diabetic rats. Cardiovasc. Diabetol. 2015, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Feng, Y.; Liu, D.; Zhang, Z.; Gao, K.; Zhang, W.; Tang, H. Thymoquinone attenuates myocardial ischemia/reperfusion injury through activation of sirt1 signaling. Cell. Physiol. Biochem. 2018, 47, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mu, Y.; Zhou, X.; Ji, H.; Gao, X.; Cai, W.W.; Guan, Q.; Xu, T. Sirt2-mediated foxo3a deacetylation drives its nuclear translocation triggering fasl-induced cell apoptosis during renal ischemia reperfusion. Apoptosis 2017, 22, 519–530. [Google Scholar] [CrossRef]
- Leng, Y.; Wu, Y.; Lei, S.; Zhou, B.; Qiu, Z.; Wang, K.; Xia, Z. Inhibition of hdac6 activity alleviates myocardial ischemia/reperfusion injury in diabetic rats: Potential role of peroxiredoxin 1 acetylation and redox regulation. Oxid. Med. Cell. Longev. 2018, 2018, 9494052. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, X.F.; Wang, N.Y.; Wang, X.M.; Liang, S.T.; Zheng, W.; Lu, Y.B.; Zhao, X.; Hao, D.L.; Zhang, Z.Q.; et al. Sirt2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation 2017, 136, 2051–2067. [Google Scholar] [CrossRef]
- Luo, Y.X.; Tang, X.; An, X.Z.; Xie, X.M.; Chen, X.F.; Zhao, X.; Hao, D.L.; Chen, H.Z.; Liu, D.P. Sirt4 accelerates ang ii-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur. Heart J. 2017, 38, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Yang, J.J.; Shi, K.H.; Li, J. Epigenetic factors mecp2 and hdac6 control α-tubulin acetylation in cardiac fibroblast proliferation and fibrosis. Inflamm. Res. 2016, 65, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Collesi, C.; Felician, G.; Secco, I.; Gutierrez, M.I.; Martelletti, E.; Ali, H.; Zentilin, L.; Myers, M.P.; Giacca, M. Reversible notch1 acetylation tunes proliferative signalling in cardiomyocytes. Cardiovasc. Res. 2018, 114, 103–122. [Google Scholar] [CrossRef]
- Vakhrusheva, O.; Smolka, C.; Gajawada, P.; Kostin, S.; Boettger, T.; Kubin, T.; Braun, T.; Bober, E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 2008, 102, 703–710. [Google Scholar] [CrossRef]
- Li, W.; Feng, W.; Su, X.; Luo, D.; Li, Z.; Zhou, Y.; Zhu, Y.; Zhang, M.; Chen, J.; Liu, B.; et al. Sirt6 protects vascular smooth muscle cells from osteogenic transdifferentiation via runx2 in chronic kidney disease. J. Clin. Investig 2022, 132, e150051. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.F.; Climent, M.; Anselmi, C.V.; Papa, L.; Tragante, V.; Lambroia, L.; Farina, F.M.; Kleber, M.E.; März, W.; Biguori, C.; et al. Rs41291957 controls mir-143 and mir-145 expression and impacts coronary artery disease risk. EMBO Mol. Med. 2021, 13, e14060. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Li, Q.; Liu, W.; Song, Q.; Jiang, H. Circrna acap2 induces myocardial apoptosis after myocardial infarction by sponging mir-29. Minerva Med. 2022, 113, 128–134. [Google Scholar] [CrossRef]
- Xing, X.; Li, Z.; Yang, X.; Li, M.; Liu, C.; Pang, Y.; Zhang, L.; Li, X.; Liu, G.; Xiao, Y. Adipose-derived mesenchymal stem cells-derived exosome-mediated microrna-342-5p protects endothelial cells against atherosclerosis. Aging 2020, 12, 3880–3898. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, J.L.; Peng, Z.Y.; Xu, W.F.; Yu, G.L. Exosomal mir-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and ezh2. Cell Death Dis. 2020, 11, 317. [Google Scholar] [CrossRef]
- Ling, H.; Guo, Z.; Shi, Y.; Zhang, L.; Song, C. Serum exosomal microrna-21, microrna-126, and pten are novel biomarkers for diagnosis of acute coronary syndrome. Front. Physiol. 2020, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Mano, M.; Ferro, M.D.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional screening identifies mirnas inducing cardiac regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Danielson, K.M.; Shah, R.; Yeri, A.; Liu, X.; Garcia, F.C.; Silverman, M.; Tanriverdi, K.; Das, A.; Xiao, C.; Jerosch-Herold, M.; et al. Plasma circulating extracellular rnas in left ventricular remodeling post-myocardial infarction. EBioMedicine 2018, 32, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xiang, J.; Wang, Q.; Wang, A.; Li, C.; Tian, G.; Zhang, H.; Chen, S. Revealing the interactions between diabetes, diabetes-related diseases, and cancers based on the network connectivity of their related genes. Front. Genet. 2020, 11, 617136. [Google Scholar] [CrossRef]
- Leimena, C.; Qiu, H. Non-coding rna in the pathogenesis, progression and treatment of hypertension. Int. J. Mol. Sci. 2018, 19, 927. [Google Scholar] [CrossRef]
- Ritter, N.; Ali, T.; Kopitchinski, N.; Schuster, P.; Beisaw, A.; Hendrix, D.A.; Schulz, M.H.; Müller-McNicoll, M.; Dimmeler, S.; Grote, P. The lncrna locus handsdown regulates cardiac gene programs and is essential for early mouse development. Dev. Cell 2019, 50, 644–657.e8. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Shen, D.; Ge, D.; Chen, J.; Pei, J.; Li, Y.; Yue, Z.; Feng, J.; Chu, M.; et al. A long noncoding rna nr_045363 controls cardiomyocyte proliferation and cardiac repair. J. Mol. Cell. Cardiol. 2019, 127, 105–114. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, Y.; Li, B.; Bu, K.; Wu, L.; Lu, Y.; Lu, Y.; Qiu, Y. Downregulation of lncrna-sra participates in the development of cardiovascular disease in type ii diabetic patients. Exp. Ther. Med. 2019, 17, 3367–3372. [Google Scholar] [CrossRef]
- Sallam, T.; Sandhu, J.; Tontonoz, P. Long noncoding rna discovery in cardiovascular disease: Decoding form to function. Circ. Res. 2018, 122, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Beutner, F.; Scholz, M.; Gielen, S.; Gäbel, G.; Bergert, H.; Schuler, G.; Thiery, J.; Teupser, D. Anril expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 620–627. [Google Scholar] [CrossRef]
- Wu, G.; Cai, J.; Han, Y.; Chen, J.; Huang, Z.P.; Chen, C.; Cai, Y.; Huang, H.; Yang, Y.; Liu, Y.; et al. Lincrna-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 2014, 130, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, M.D.; Pinel, K.; Dakin, R.; Vesey, A.T.; Diver, L.; Mackenzie, R.; Garcia, R.; Welsh, P.; Sattar, N.; Hamilton, G.; et al. Smooth muscle enriched long noncoding rna (smilr) regulates cell proliferation. Circulation 2016, 133, 2050–2065. [Google Scholar] [CrossRef]
- Sallam, T.; Jones, M.; Thomas, B.J.; Wu, X.; Gilliland, T.; Qian, K.; Eskin, A.; Casero, D.; Zhang, Z.; Sandhu, J.; et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding rna. Nat. Med. 2018, 24, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, Q.; Mao, J.; Zhang, J.; Li, L. The roles of lncrna in myocardial infarction: Molecular mechanisms, diagnosis biomarkers, and therapeutic perspectives. Front. Cell Dev. Biol. 2021, 9, 680713. [Google Scholar] [CrossRef]
- Huang, L.; Guo, B.; Liu, S.; Miao, C.; Li, Y. Inhibition of the lncrna gpr19 attenuates ischemia-reperfusion injury after acute myocardial infarction by inhibiting apoptosis and oxidative stress via the mir-324-5p/mtfr1 axis. IUBMB Life 2020, 72, 373–383. [Google Scholar] [CrossRef]
- Yuan, T.; Chen, Y.; Zhou, X.; Lin, X.; Zhang, Q. Effectiveness and safety of danshen injection on heart failure: Protocol for a systematic review and meta-analysis. Medicine 2019, 98, e15636. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Wang, Y.Y.; Jia, P.; Xiong, Q.; Hu, Y.; Chang, Y.; Lai, S.; Xu, Y.; Zhao, Z.; Song, J. Multi-level transcriptome sequencing identifies col1a1 as a candidate marker in human heart failure progression. BMC Med. 2020, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.Y.; Cui, X.J.; Zhan, J.K.; Wang, Y.J.; Li, S.; Lin, X.; Xiang, Q.Y.; Ni, Y.Q.; Liu, L.; Liu, Y.S. Lncrna-es3 inhibition by bhlhe40 is involved in high glucose-induced calcification/senescence of vascular smooth muscle cells. Ann. N. Y. Acad. Sci. 2020, 1474, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qi, F.; Wei, S.; Lin, L.; Liu, X. The transcription factor c/ebpβ promotes hfl-1 cell migration, proliferation, and inflammation by activating lncrna has2-as1 in hypoxia. Front. Cell Dev. Biol. 2021, 9, 651913. [Google Scholar] [CrossRef]
- Mao, Y.Y.; Wang, J.Q.; Guo, X.X.; Bi, Y.; Wang, C.X. Circ-satb2 upregulates stim1 expression and regulates vascular smooth muscle cell proliferation and differentiation through mir-939. Biochem. Biophys. Res. Commun. 2018, 505, 119–125. [Google Scholar] [CrossRef]
- Shen, L.; Hu, Y.; Lou, J.; Yin, S.; Wang, W.; Wang, Y.; Xia, Y.; Wu, W. Circrna-0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting mir-107. Mol. Med. Rep. 2019, 19, 3923–3932. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.H.; Li, R.; Su, Y.M.; Xiao, J.; Pan, M.; Cai, X.X.; Ji, X.P. The circular rna cdr1as promotes myocardial infarction by mediating the regulation of mir-7a on its target genes expression. PLoS ONE 2016, 11, e0151753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pan, W.; Yang, T.; Meng, X.; Jiang, Z.; Tao, L.; Wang, L. Upregulation of circular rna circnfib attenuates cardiac fibrosis by sponging mir-433. Front. Genet. 2019, 10, 564. [Google Scholar] [CrossRef]
- Li, M.; Ding, W.; Tariq, M.A.; Chang, W.; Zhang, X.; Xu, W.; Hou, L.; Wang, Y.; Wang, J. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting mir-133a-3p. Theranostics 2018, 8, 5855–5869. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Zhai, M.; Huang, Y.; Xu, S.; An, T.; Wang, Y.H.; Zhang, R.C.; Liu, C.Y.; Dong, Y.H.; Wang, M.; et al. The circular rna acr attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the pink1/fam65b pathway. Cell Death Differ. 2019, 26, 1299–1315. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, X.; Zhan, X.; Kang, S.; Liu, H.; Luo, Y.; Lin, L. Advance in circular rna modulation effects of heart failure. Gene 2020, 763, 100036. [Google Scholar] [CrossRef]
- Wu, N.; Li, C.; Xu, B.; Xiang, Y.; Jia, X.; Yuan, Z.; Wu, L.; Zhong, L.; Li, Y. Circular rna mmu_circ_0005019 inhibits fibrosis of cardiac fibroblasts and reverses electrical remodeling of cardiomyocytes. BMC Cardiovasc. Disord. 2021, 21, 308. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Y.; Jiang, Y.; Tan, M.; Liu, C. Circular rna rbms1 inhibited the development of myocardial ischemia reperfusion injury by regulating mir-92a/bcl2l11 signaling pathway. Bioengineered 2022, 13, 3082–3092. [Google Scholar] [CrossRef]
- Garikipati VN, S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular rna circfndc3b modulates cardiac repair after myocardial infarction via fus/vegf-a axis. Nat. Commun. 2019, 10, 4317. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Perkins, J.T.; Hennig, B. Egcg prevents pcb-126-induced endothelial cell inflammation via epigenetic modifications of nf-κb target genes in human endothelial cells. J. Nutr. Biochem. 2016, 28, 164–170. [Google Scholar] [CrossRef]
- Zhang, D.; Ni, N.; Wang, Y.; Tang, Z.; Gao, H.; Ju, Y.; Sun, N.; He, X.; Gu, P.; Fan, X. Circrna-vgll3 promotes osteogenic differentiation of adipose-derived mesenchymal stem cells via modulating mirna-dependent integrin α5 expression. Cell Death Differ. 2021, 28, 283–302. [Google Scholar] [CrossRef]
- Zhuang, J.; Luan, P.; Li, H.; Wang, K.; Zhang, P.; Xu, Y.; Peng, W. The yin-yang dynamics of DNA methylation is the key regulator for smooth muscle cell phenotype switch and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 84–97. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, X.; Jia, L.; Mondal, A.K.; Diallo, A.; Hawkins, G.A.; Das, S.K.; Parks, J.S.; Yu, L.; Shi, H.; et al. Inhibiting DNA methylation by 5-aza-2’-deoxycytidine ameliorates atherosclerosis through suppressing macrophage inflammation. Endocrinology 2014, 155, 4925–4938. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, U.; Kajioka, S.; Finoti, L.S.; Palioto, D.B.; Kinane, D.F.; Benakanakere, M.R. Decitabine inhibits bone resorption in periodontitis by upregulating anti-inflammatory cytokines and suppressing osteoclastogenesis. Biomedicines 2021, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Connelly, J.J.; Cherepanova, O.A.; Doss, J.F.; Karaoli, T.; Lillard, T.S.; Markunas, C.A.; Nelson, S.; Wang, T.; Ellis, P.D.; Langford, C.F.; et al. Epigenetic regulation of col15a1 in smooth muscle cell replicative aging and atherosclerosis. Hum. Mol. Genet. 2013, 22, 5107–5120. [Google Scholar] [CrossRef]
- Xiao, D.; Dasgupta, C.; Chen, M.; Zhang, K.; Buchholz, J.; Xu, Z.; Zhang, L. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc. Res. 2014, 101, 373–382. [Google Scholar] [CrossRef]
- Watson, C.J.; Horgan, S.; Neary, R.; Glezeva, N.; Tea, I.; Corrigan, N.; McDonald, K.; Ledwidge, M.; Baugh, J. Epigenetic therapy for the treatment of hypertension-induced cardiac hypertrophy and fibrosis. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Guay, S.P.; Légaré, C.; Houde, A.A.; Mathieu, P.; Bossé, Y.; Bouchard, L. Acetylsalicylic acid, aging and coronary artery disease are associated with abca1 DNA methylation in men. Clin. Epigenetics 2014, 6, 14. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, H.; Hao, L.; Guo, X.; Ma, X.; Qian, Y.; Chen, H.; Ma, J.; Zhang, J.; Sheng, W.; et al. The roles of smyd4 in epigenetic regulation of cardiac development in zebrafish. PLoS Genet. 2018, 14, e1007578. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, J.; Bai, J.; Pu, P.; Liu, J.; Wang, F.; Ruan, B. Suv39h1 protects from myocardial ischemia-reperfusion injury in diabetic rats. Cell. Physiol. Biochem. 2014, 33, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Zhang, Y.; Li, Z.; Yu, L.; Xu, F.; Fang, M.; Hou, L.; Ge, J.; Xu, Y. Class ii transactivator (ciita) mediates ifn-γ induced enos repression by enlisting suv39h1. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Dje N’Guessan, P.; Riediger, F.; Vardarova, K.; Scharf, S.; Eitel, J.; Opitz, B.; Slevogt, H.; Weichert, W.; Hocke, A.C.; Schmeck, B.; et al. Statins control oxidized ldl-mediated histone modifications and gene expression in cultured human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Palomo, M.; Vera, M.; Martin, S.; Torramadé-Moix, S.; Martinez-Sanchez, J.; Moreno, A.B.; Carreras, E.; Escolar, G.; Cases, A.; Díaz-Ricart, M. Up-regulation of hdacs, a harbinger of uraemic endothelial dysfunction, is prevented by defibrotide. J. Cell. Mol. Med. 2020, 24, 1713–1723. [Google Scholar] [CrossRef]
- Aune, S.E.; Herr, D.J.; Mani, S.K.; Menick, D.R. Selective inhibition of class i but not class iib histone deacetylases exerts cardiac protection from ischemia reperfusion. J. Mol. Cell. Cardiol. 2014, 72, 138–145. [Google Scholar] [CrossRef]
- Herr, D.J.; Baarine, M.; Aune, S.E.; Li, X.; Ball, L.E.; Lemasters, J.J.; Beeson, C.C.; Chou, J.C.; Menick, D.R. Hdac1 localizes to the mitochondria of cardiac myocytes and contributes to early cardiac reperfusion injury. J. Mol. Cell. Cardiol. 2018, 114, 309–319. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, X.; Zhao, Y.; Fast, L.; Zhuang, S.; Liu, P.; Cheng, G.; Zhao, T.C. Inhibition of histone deacetylases preserves myocardial performance and prevents cardiac remodeling through stimulation of endogenous angiomyogenesis. J. Pharmacol. Exp. Ther. 2012, 341, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hong, S.; He, H.; Zeng, Y.; Chen, Y.; Mo, X.; Li, J.; Li, L.; Steinmetz, R.; Liu, Q. Nfκb promotes oxidative stress-induced necrosis and ischemia/reperfusion injury by inhibiting nrf2-are pathway. Free Radic. Biol. Med. 2020, 159, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, P.; Wang, L.; Zhao, J.; Zhong, Z.; Wang, Y.; Xu, J. Inhibition of histone deacetylases prevents cardiac remodeling after myocardial infarction by restoring autophagosome processing in cardiac fibroblasts. Cell. Physiol. Biochem. 2018, 49, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tang, Q.; Yang, J.; Ding, J.; Ye, M.; Dong, W. Atorvastatin ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Int. J. Clin. Exp. Med. 2014, 7, 4915–4923. [Google Scholar] [PubMed]
- Xie, M.; Kong, Y.; Tan, W.; May, H.; Battiprolu, P.K.; Pedrozo, Z.; Wang, Z.V.; Morales, C.; Luo, X.; Cho, G.; et al. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation 2014, 129, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Lin, M.S.; Chang, N.C. Inhibition of histone deacetylase on ventricular remodeling in infarcted rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H968–H977. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Marunouchi, T.; Tanonaka, K. Histone deacetylase inhibitor saha treatment prevents the development of heart failure after myocardial infarction via an induction of heat-shock proteins in rats. Biol. Pharm. Bull. 2019, 42, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, D.; Wang, S.H.; Wright, L.H.; Mani, S.K.; Kasiganesan, H.; LaRue, A.C.; Cheng, Q.; Nadig, S.N.; Atkinson, C.; Menick, D.R. Hdac inhibition helps post-mi healing by modulating macrophage polarization. J. Mol. Cell. Cardiol. 2018, 119, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Gillette, T.G.; Hill, J.A. Readers, writers, and erasers: Chromatin as the whiteboard of heart disease. Circ. Res. 2015, 116, 1245–1253. [Google Scholar] [CrossRef]
- Shalwala, M.; Zhu, S.G.; Das, A.; Salloum, F.N.; Xi, L.; Kukreja, R.C. Sirtuin 1 (sirt1) activation mediates sildenafil induced delayed cardioprotection against ischemia-reperfusion injury in mice. PLoS ONE 2014, 9, e86977. [Google Scholar] [CrossRef]
- Potenza, M.A.; Sgarra, L.; Nacci, C.; Leo, V.; De Salvia, M.A.; Montagnani, M. Activation of ampk/sirt1 axis is required for adiponectin-mediated preconditioning on myocardial ischemia-reperfusion (i/r) injury in rats. PLoS ONE 2019, 14, e0210654. [Google Scholar] [CrossRef]
- Koka, S.; Aluri, H.S.; Xi, L.; Lesnefsky, E.J.; Kukreja, R.C. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: Potential role of no/sirt1/pgc-1α signaling. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1558–H1568. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, C.; Turdi, S.; Richmond, K.L.; Zhang, Y.; Ren, J. Aldh2 protects against high fat diet-induced obesity cardiomyopathy and defective autophagy: Role of cam kinase ii, histone h3k9 methyltransferase suv39h, sirt1, and pgc-1α deacetylation. Int. J. Obes. 2018, 42, 1073–1087. [Google Scholar] [CrossRef]
- Morales, C.R.; Li, D.L.; Pedrozo, Z.; May, H.I.; Jiang, N.; Kyrychenko, V.; Cho, G.W.; Kim, S.Y.; Wang, Z.V.; Rotter, D.; et al. Inhibition of class i histone deacetylases blunts cardiac hypertrophy through tsc2-dependent mtor repression. Sci. Signal. 2016, 9, ra34. [Google Scholar] [CrossRef]
- Antos, C.L.; McKinsey, T.A.; Dreitz, M.; Hollingsworth, L.M.; Zhang, C.L.; Schreiber, K.; Rindt, H.; Gorczynski, R.J.; Olson, E.N. Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. J. Biol. Chem. 2003, 278, 28930–28937. [Google Scholar] [CrossRef]
- Gallo, P.; Latronico, M.V.; Gallo, P.; Grimaldi, S.; Borgia, F.; Todaro, M.; Jones, P.; Gallinari, P.; De Francesco, R.; Ciliberto, G.; et al. Inhibition of class i histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc. Res. 2008, 80, 416–424. [Google Scholar] [CrossRef]
- Chen, Y.; Du, J.; Zhao, Y.T.; Zhang, L.; Lv, G.; Zhuang, S.; Qin, G.; Zhao, T.C. Histone deacetylase (hdac) inhibition improves myocardial function and prevents cardiac remodeling in diabetic mice. Cardiovasc. Diabetol. 2015, 14, 99. [Google Scholar] [CrossRef]
- Kashyap, S.; Rabbani, M.; de Lima, I.; Kondrachuk, O.; Patel, R.; Shafiei, M.S.; Mukker, A.; Rajakumar, A.; Gupta, M.K. Hopx plays a critical role in antiretroviral drugs induced epigenetic modification and cardiac hypertrophy. Cells 2021, 10, 3458. [Google Scholar] [CrossRef] [PubMed]
- Nural-Guvener, H.F.; Zakharova, L.; Nimlos, J.; Popovic, S.; Mastroeni, D.; Gaballa, M.A. Hdac class i inhibitor, mocetinostat, reverses cardiac fibrosis in heart failure and diminishes cd90+ cardiac myofibroblast activation. Fibrogenesis Tissue Repair. 2014, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Nural-Guvener, H.; Zakharova, L.; Feehery, L.; Sljukic, S.; Gaballa, M. Anti-fibrotic effects of class i hdac inhibitor, mocetinostat is associated with il-6/stat3 signaling in ischemic heart failure. Int. J. Mol. Sci. 2015, 16, 11482–11499. [Google Scholar] [CrossRef]
- Nebbioso, A.; Manzo, F.; Miceli, M.; Conte, M.; Manente, L.; Baldi, A.; De Luca, A.; Rotili, D.; Valente, S.; Mai, A.; et al. Selective class ii hdac inhibitors impair myogenesis by modulating the stability and activity of hdac-mef2 complexes. EMBO Rep. 2009, 10, 776–782. [Google Scholar] [CrossRef]
- Stratton, M.S.; Bagchi, R.A.; Felisbino, M.B.; Hirsch, R.A.; Smith, H.E.; Riching, A.S.; Enyart, B.Y.; Koch, K.A.; Cavasin, M.A.; Alexanian, M.; et al. Dynamic chromatin targeting of brd4 stimulates cardiac fibroblast activation. Circ. Res. 2019, 125, 662–677. [Google Scholar] [CrossRef]
- Gu, J.; Lu, Y.; Deng, M.; Qiu, M.; Tian, Y.; Ji, Y.; Zong, P.; Shao, Y.; Zheng, R.; Zhou, B.; et al. Inhibition of acetylation of histones 3 and 4 attenuates aortic valve calcification. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Li, S.J.; Kao, Y.H.; Chung, C.C.; Chen, W.Y.; Cheng, W.L.; Chen, Y.J. Activated p300 acetyltransferase activity modulates aortic valvular calcification with osteogenic transdifferentiation and downregulation of klotho. Int. J. Cardiol. 2017, 232, 271–279. [Google Scholar] [CrossRef]
- Fu, Z.; Li, F.; Jia, L.; Su, S.; Wang, Y.; Cai, Z.; Xiang, M. Histone deacetylase 6 reduction promotes aortic valve calcification via an endoplasmic reticulum stress-mediated osteogenic pathway. J. Thorac. Cardiovasc. Surg. 2019, 158, 408–417.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.M.; Ding, Q.H.; Chen, W.P.; Luo, R.B. Vorinostat, a hdac inhibitor, showed anti-osteoarthritic activities through inhibition of inos and mmp expression, p38 and erk phosphorylation and blocking nf-κb nuclear translocation. Int. Immunopharmacol. 2013, 17, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Chabot, S.; Paulin, R.; Trinh, I.; Bourgeois, A.; Potus, F.; Lampron, M.C.; Lambert, C.; Breuils-Bonnet, S.; Nadeau, V.; et al. Hdac6: A novel histone deacetylase implicated in pulmonary arterial hypertension. Sci. Rep. 2017, 7, 4546. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Wu, J.; Liu, M.; Li, M.; Sun, Y.; Huang, W.; Li, Y.; Zhang, Y.; Tang, W.; et al. Endothelial sirt6 is vital to prevent hypertension and associated cardiorenal injury through targeting nkx3.2-gata5 signaling. Circ. Res. 2019, 124, 1448–1461. [Google Scholar] [CrossRef]
- Chi, Z.; Byeon, H.E.; Seo, E.; Nguyen, Q.T.; Lee, W.; Jeong, Y.; Choi, J.; Pandey, D.; Berkowitz, D.E.; Kim, J.H.; et al. Histone deacetylase 6 inhibitor tubastatin a attenuates angiotensin ii-induced hypertension by preventing cystathionine γ-lyase protein degradation. Pharmacol. Res. 2019, 146, 104281. [Google Scholar] [CrossRef]
- Wang, J.; Yin, N.; Deng, Y.; Wei, Y.; Huang, Y.; Pu, X.; Li, L.; Zheng, Y.; Guo, J.; Yu, J.; et al. Ascorbic acid protects against hypertension through downregulation of ace1 gene expression mediated by histone deacetylation in prenatal inflammation-induced offspring. Sci. Rep. 2016, 6, 39469. [Google Scholar] [CrossRef]
- Tang, Y.; Li, H.; Chen, C. Non-coding rna-associated therapeutic strategies in atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 889743. [Google Scholar] [CrossRef]
- Haemmig, S.; Yang, D.; Sun, X.; Das, D.; Ghaffari, S.; Molinaro, R.; Chen, L.; Deng, Y.; Freeman, D.; Moullan, N.; et al. Long noncoding rna snhg12 integrates a DNA-pk-mediated DNA damage response and vascular senescence. Sci. Transl. Med. 2020, 12, eaaw1868. [Google Scholar] [CrossRef] [PubMed]
- Parthymos, I.; Kostapanos, M.S.; Liamis, G.; Florentin, M. Early investigational and experimental therapeutics for the treatment of hypertriglyceridemia. J. Cardiovasc. Dev. Dis. 2022, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K.; Kafert-Kasting, S.; Thum, T. Preclinical and clinical development of noncoding rna therapeutics for cardiovascular disease. Circ. Res. 2020, 126, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Long, B.; Liu, F.; Wang, J.X.; Liu, C.Y.; Zhao, B.; Zhou, L.Y.; Sun, T.; Wang, M.; Yu, T.; et al. A circular rna protects the heart from pathological hypertrophy and heart failure by targeting mir-223. Eur. Heart J. 2016, 37, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Meng, Q.; Li, D.; Hu, F.Z.; Zhu, Y.Q.; Huang, Y.Y.; Liu, Y.N.; Sun, L.; Liang, Q.H. The protective effects of long non-coding rna-ancr on arterial calcification. J. Bone Miner. Metab. 2020, 38, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Ding, Y.; Wang, R.; Yang, Y.; Luo, K.; Hua, F. Exosomal hsa_circ_0006859 is a potential biomarker for postmenopausal osteoporosis and enhances adipogenic versus osteogenic differentiation in human bone marrow mesenchymal stem cells by sponging mir-431-5p. Stem Cell Res. Ther. 2021, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Bontempo, P.; Palmieri, V.; Coscioni, E.; Maiello, C.; Donatelli, F.; Benincasa, G. Epigenetic therapies for heart failure: Current insights and future potential. Vasc. Health Risk Manag. 2021, 17, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Goeman, J.J.; Monajemi, R.; Gu, H.; Putter, H.; Zhang, Y.; Slieker, R.C.; Stok, A.P.; Thijssen, P.E.; Müller, F.; et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat. Commun. 2014, 5, 5592. [Google Scholar] [CrossRef] [PubMed]
- Painter, R.C.; de Rooij, S.R.; Bossuyt, P.M.; Simmers, T.A.; Osmond, C.; Barker, D.J.; Bleker, O.P.; Roseboom, T.J. Early onset of coronary artery disease after prenatal exposure to the dutch famine. Am. J. Clin. Nutr. 2006, 84, 322–327, quiz 466–467. [Google Scholar] [CrossRef]
- Moore, S.E. Early life nutritional programming of health and disease in the gambia. J. Dev. Orig. Health Dis. 2016, 7, 123–131. [Google Scholar] [CrossRef]
- Costello, K.R.; Schones, D.E. Chromatin modifications in metabolic disease: Potential mediators of long-term disease risk. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1416. [Google Scholar] [CrossRef] [PubMed]
- Zwamborn, R.A.; Slieker, R.C.; Mulder, P.C.; Zoetemelk, I.; Verschuren, L.; Suchiman, H.E.; Toet, K.H.; Droog, S.; Slagboom, P.E.; Kooistra, T.; et al. Prolonged high-fat diet induces gradual and fat depot-specific DNA methylation changes in adult mice. Sci. Rep. 2017, 7, 43261. [Google Scholar] [CrossRef] [PubMed]
- Perfilyev, A.; Dahlman, I.; Gillberg, L.; Rosqvist, F.; Iggman, D.; Volkov, P.; Nilsson, E.; Risérus, U.; Ling, C. Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Block, T.; El-Osta, A. Epigenetic programming, early life nutrition and the risk of metabolic disease. Atherosclerosis 2017, 266, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Irvin, M.R.; Zhi, D.; Aslibekyan, S.; Claas, S.A.; Absher, D.M.; Ordovas, J.M.; Tiwari, H.K.; Watkins, S.; Arnett, D.K. Genomics of post-prandial lipidomic phenotypes in the genetics of lipid lowering drugs and diet network (goldn) study. PLoS ONE 2014, 9, e99509. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Simone, E.; Grimaldi, M.; Gagliardi, M.; Zullo, L.; Matarazzo, M.R.; Mancini, F.P. Effect of nutrient deprivation on the expression and the epigenetic signature of sirtuin genes. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.P.; Albert, S.G.; Reeds, D.N.; Kress, K.S.; McDaniel, J.L.; Klein, S.; Villareal, D.T. Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: A randomized intervention trial. Am. J. Clin. Nutr. 2016, 104, 576–586. [Google Scholar] [CrossRef]
- Nowacka-Woszuk, J.; Madeja, Z.E.; Chmurzynska, A. Prenatal caloric restriction alters lipid metabolism but not hepatic fasn gene expression and methylation profiles in rats. BMC Genet. 2017, 18, 78. [Google Scholar] [CrossRef]
- Franke, K.; Gaser, C.; Roseboom, T.J.; Schwab, M.; de Rooij, S.R. Premature brain aging in humans exposed to maternal nutrient restriction during early gestation. Neuroimage 2018, 173, 460–471. [Google Scholar] [CrossRef]
- van Dijk, S.J.; Zhou, J.; Peters, T.J.; Buckley, M.; Sutcliffe, B.; Oytam, Y.; Gibson, R.A.; McPhee, A.; Yelland, L.N.; Makrides, M.; et al. Effect of prenatal dha supplementation on the infant epigenome: Results from a randomized controlled trial. Clin. Epigenetics 2016, 8, 114. [Google Scholar] [CrossRef]
- Zeisel, S. Choline, other methyl-donors and epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; Devlieger, R.; Godderis, L. Maternal intake of methyl-group donors affects DNA methylation of metabolic genes in infants. Clin. Epigenetics 2017, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Min, K.B.; Min, J.Y. Association between leukocyte telomere length and serum carotenoid in us adults. Eur. J. Nutr. 2017, 56, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P.; Banach, M. Mineral and vitamin consumption and telomere length among adults in the united states. Pol. Arch. Intern. Med. 2017, 127, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Baragetti, A.; Palmen, J.; Garlaschelli, K.; Grigore, L.; Pellegatta, F.; Tragni, E.; Catapano, A.L.; Humphries, S.E.; Norata, G.D.; Talmud, P.J. Telomere shortening over 6 years is associated with increased subclinical carotid vascular damage and worse cardiovascular prognosis in the general population. J. Intern. Med. 2015, 277, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, I.; Herrmann, M.; Kirsch, S.H.; Werner, C.; Hübner, U.; Bodis, M.; Laufs, U.; Wagenpfeil, S.; Geisel, J.; Herrmann, W. Prospective study of telomere length and line-1 methylation in peripheral blood cells: The role of b vitamins supplementation. Eur. J. Nutr. 2016, 55, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Shin, C.; Baik, I. Longitudinal associations between micronutrient consumption and leukocyte telomere length. J. Hum. Nutr. Diet. 2017, 30, 236–243. [Google Scholar] [CrossRef]
- Pisano, S.; Gilson, E.; Giraud-Panis, M.J. Dynamics under the telomeric bridge. Mol. Cell 2017, 68, 643–644. [Google Scholar] [CrossRef]
- Miceli, M.; Bontempo, P.; Nebbioso, A.; Altucci, L. Natural compounds in epigenetics: A current view. Food Chem. Toxicol. 2014, 73, 71–83. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Scafuri, B.; Bontempo, P.; Altucci, L.; De Masi, L.; Facchiano, A. Molecular docking simulations on histone deacetylases (hdac)-1 and -2 to investigate the flavone binding. Biomedicines 2020, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wendorff, T.J.; Berger, J.M. Resveratrol: A novel type of topoisomerase ii inhibitor. J. Biol. Chem. 2017, 292, 21011–21022. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.J.; Wang, C.J.; He, Y.; Zhou, Y.L.; Peng, X.D.; Liu, S.K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through pgc-1α deacetylation. Acta Pharmacol. Sin. 2018, 39, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Togliatto, G.; Gambino, R.; Ponzo, V.; Lombardo, G.; Rosato, R.; Cassader, M.; Brizzi, M.F. Impact of sirtuin-1 expression on h3k56 acetylation and oxidative stress: A double-blind randomized controlled trial with resveratrol supplementation. Acta Diabetol. 2018, 55, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Si, H.; Babu, P.V.; Pan, D.; Fu, Y.; Brooke, E.A.; Shah, H.; Zhen, W.; Zhu, H.; Liu, D.; et al. Sulforaphane reduces vascular inflammation in mice and prevents tnf-α-induced monocyte adhesion to primary endothelial cells through interfering with the nf-κb pathway. J. Nutr. Biochem. 2014, 25, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Kaufman-Szymczyk, A.; Majewski, G.; Lubecka-Pietruszewska, K.; Fabianowska-Majewska, K. The role of sulforaphane in epigenetic mechanisms, including interdependence between histone modification and DNA methylation. Int. J. Mol. Sci. 2015, 16, 29732–29743. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shen, T.; Xie, J.; Wang, S.; He, Y.; Zhu, F. Curcumin modulates covalent histone modification and timp1 gene activation to protect against vascular injury in a hypertension rat model. Exp. Ther. Med. 2017, 14, 5896–5902. [Google Scholar] [CrossRef] [PubMed]
- Vahid, F.; Zand, H.; Nosrat-Mirshekarlou, E.; Najafi, R.; Hekmatdoost, A. The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: A review. Gene 2015, 562, 8–15. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, Y.M.; Lau, A.T.Y. The epigenetic effects of coffee. Molecules 2023, 28, 1770. [Google Scholar] [CrossRef]
- Crescenti, A.; Solà, R.; Valls, R.M.; Caimari, A.; Del Bas, J.M.; Anguera, A.; Anglés, N.; Arola, L. Cocoa consumption alters the global DNA methylation of peripheral leukocytes in humans with cardiovascular disease risk factors: A randomized controlled trial. PLoS ONE 2013, 8, e65744. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; Costantino, S.; Mügge, A.; Lebeche, D.; Tschöpe, C.; Thum, T.; Paneni, F. Leveraging clinical epigenetics in heart failure with preserved ejection fraction: A call for individualized therapies. Eur. Heart J. 2021, 42, 1940–1958. [Google Scholar] [CrossRef] [PubMed]
- Gorica, E.; Mohammed, S.A.; Ambrosini, S.; Calderone, V.; Costantino, S.; Paneni, F. Epi-drugs in heart failure. Front. Cardiovasc. Med. 2022, 9, 923014. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Kamimura, N.; Matsuhashi, T.; Nagai, T.; Nishiyama, T.; Endo, J.; Hishiki, T.; Nakanishi, T.; Shimizu, N.; Tanaka, H.; et al. The histone 3 lysine 9 methyltransferase inhibitor chaetocin improves prognosis in a rat model of high salt diet-induced heart failure. Sci. Rep. 2017, 7, 39752. [Google Scholar] [CrossRef]
- Guo, Y.; Su, Z.Y.; Kong, A.N. Current perspectives on epigenetic modifications by dietary chemopreventive and herbal phytochemicals. Curr. Pharmacol. Rep. 2015, 1, 245–257. [Google Scholar] [CrossRef]
- Han, S.; Uludag, M.O.; Usanmaz, S.E.; Ayaloglu-Butun, F.; Akcali, K.C.; Demirel-Yilmaz, E. Resveratrol affects histone 3 lysine 27 methylation of vessels and blood biomarkers in doca salt-induced hypertension. Mol. Biol. Rep. 2015, 42, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, J.T.; Guan, H.S. Resveratrol exerts pharmacological preconditioning by activating pgc-1alpha. Med. Hypotheses 2008, 71, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Sin, T.K.; Yu, A.P.; Yung, B.Y.; Yip, S.P.; Chan, L.W.; Wong, C.S.; Ying, M.; Rudd, J.A.; Siu, P.M. Modulating effect of sirt1 activation induced by resveratrol on foxo1-associated apoptotic signalling in senescent heart. J. Physiol. 2014, 592, 2535–2548. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Wu, J.C.; Ho, C.T. Epigenetic and disease targets by polyphenols. Curr. Pharm. Des. 2013, 19, 6156–6185. [Google Scholar] [CrossRef]
- Masi, S.; Ambrosini, S.; Mohammed, S.A.; Sciarretta, S.; Lüscher, T.F.; Paneni, F.; Costantino, S. Epigenetic remodeling in obesity-related vascular disease. Antioxid. Redox Signal 2021, 34, 1165–1199. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; Jurado-López, R.; Valero-Muñoz, M.; Bartolomé, M.V.; Ballesteros, S.; Luaces, M.; Briones, A.M.; López-Andrés, N.; Miana, M.; Cachofeiro, V. Leptin induces cardiac fibrosis through galectin-3, mtor and oxidative stress: Potential role in obesity. J. Hypertens. 2014, 32, 1104–1114; discussion 14. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Xie, Y.; Suzuki, S.; Tagami, M. Epigallocatechin-3-gallate inhibits vcam-1 expression and apoptosis induction associated with lc3 expressions in tnfα-stimulated human endothelial cells. Phytomedicine 2015, 22, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane protects against cardiovascular disease via nrf2 activation. Oxid. Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.N.; Huang, H.P.; Wang, C.J.; Liu, K.L.; Lii, C.K. Sulforaphane inhibits tnf-α-induced adhesion molecule expression through the rho a/rock/nf-κb signaling pathway. J. Med. Food 2014, 17, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.; Falcão-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase gα oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ku, H.C.; Kuo, Y.H.; Yang, K.C.; Tu, P.C.; Chiu, H.L.; Su, M.J. Caffeic acid ethanolamide prevents cardiac dysfunction through sirtuin dependent cardiac bioenergetics preservation. J. Biomed. Sci. 2015, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, N.; Liao, H.; Chen, S.; Xu, L.; Li, J.; Yang, Z.; Deng, W.; Tang, Q. Caffeic acid phenethyl ester attenuates pathological cardiac hypertrophy by regulation of mek/erk signaling pathway in vivo and vitro. Life Sci. 2017, 181, 53–61. [Google Scholar] [CrossRef] [PubMed]

| Dietary Factors | Epigenetic Changes | Biological Effects | Cardiovascular Risk | References |
|---|---|---|---|---|
| Excess in carbohydrates | Increase in the acetylation of DNA binding protein | Increase in acetyl-CoA, suppression of autophagy and acceleration of age-associated pathologies | ↑ | [159,161,162,165,166] |
| Excess in lipids | Increase/decrease in DNA methylation in some genes that regulate lipogenesis and lipidic metabolism i.e., APOA5, CPT1AVariation of methylation level of proinflammatory signals i.e., FTO, IL6 | Altered inflammatory response | ↑ | [159,161,162,163,164] |
| Fasting conditions | Increase in DNA methylation and chromatin accessibility i.e., SIRT genes | Benefic effect on levels of total cholesterol, HDL and TG on weight and adiposity | ↓ | [167,168,169] |
| Methyl group donors (folic acid, B group vitamins) | Influence DNA and histone methylases, increase in DNA methylation of growth-related genes, LTL, LINE-1 | Beneficial effect on metabolic function and appetite control with positive impact on cardiovascular health | ↓ | [170,171,172,173,174,175,176,177,178,179] |
| Natural Compounds and Total Extracts | Epigenetic Action | Potential Application for HF Prevention or Treatment | References |
|---|---|---|---|
| Resveratrol | Activates SIRT deacetylases, reduces H3K56ac levels | Reduces cardiac oxidative stress induced by high glucose, mitochondrial dysfunction, myocardial fibrosis, and vascular aging. | [183,184,185] |
| Sulforaphane | Suppresses NF-κB signaling, downregulates histone deacetylase activity, indirectly influences methylation | Reduces monocyte adhesion, circulating adhesion molecules, and chemokines. | [186,187] |
| Curcumin | Suppresses HDAC1 expression, decreases inflammatory markers (MMP-2, TGFβ), increases histone H3 acetylation | Reduces extracellular matrix degradation and inflammation in coronary arteries. | [188] |
| Epigallocatechin-3-gallate (EGCG) | Inhibits HAT, induces H3 hypoacetylation, suppresses HDAC1 expression | Blocks the response of inflammatory mediators in endothelial cells. | [189] |
| Coffee extract and components | Alter DNA methylation, histone modifications, ncRNA expression | Impact on gene expression and health outcomes. | [190] |
| Cocoa extract | Downregulates key genes involved in epigenetic processes (DNMT, MTHFR, MTRR) | Potential cardiovascular health benefits through regulation of epigenetic processes in peripheral blood mononuclear cells. | [191] |
| Danshen extract | Inhibits JMJD2A methyltransferase, reduces H3K9 trimethylation | Effects on heart failure. | [91,192] |
| Compound | Epigenetic Action | Epigenetic Target | Disease | References |
|---|---|---|---|---|
| Resveratrol | Histone modification | H3K27me3, Class I, II and IV HDAC, SIRT1, IL6, FOXO 1 | Hypertension, CAD, HF, atherosclerosis | [183,185,196,197,198] |
| Sulforaphane | Histone modification | Class IIa HDAC, HDAC2 | Vascular remodeling, fibrosis, HF | [186,187,203,204] |
| Curcumin | Histone modification | HAT | Ventricular hypertrophy, CAD, HF | [188,199] |
| Epigallocatechin-3-gallate | DNA methylation, histone modification, non-coding RNA | DNMT, HAT, miRNA | HF | [105,189,202] |
| Chaetocin | Histone methylation | H3K9 methyltransferase | HF | [194] |
| Caffeic acid derivative | Sirtuins modulation | Class I and II HDAC | Cardiac dysfunction and hypertrophy | [207] |
| Trichostatin A | Histone modification | HDAC | Atherosclerosis, myocardial infarction, CAD, HF | [118,135,136,137,138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bontempo, P.; Capasso, L.; De Masi, L.; Nebbioso, A.; Rigano, D. Therapeutic Potential of Natural Compounds Acting through Epigenetic Mechanisms in Cardiovascular Diseases: Current Findings and Future Directions. Nutrients 2024, 16, 2399. https://doi.org/10.3390/nu16152399
Bontempo P, Capasso L, De Masi L, Nebbioso A, Rigano D. Therapeutic Potential of Natural Compounds Acting through Epigenetic Mechanisms in Cardiovascular Diseases: Current Findings and Future Directions. Nutrients. 2024; 16(15):2399. https://doi.org/10.3390/nu16152399
Chicago/Turabian StyleBontempo, Paola, Lucia Capasso, Luigi De Masi, Angela Nebbioso, and Daniela Rigano. 2024. "Therapeutic Potential of Natural Compounds Acting through Epigenetic Mechanisms in Cardiovascular Diseases: Current Findings and Future Directions" Nutrients 16, no. 15: 2399. https://doi.org/10.3390/nu16152399






