Ocoxin Oral Solution Triggers DNA Damage and Cell Death in Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture
2.3. Protein Extraction and Western Blotting
2.4. Cell Proliferation, Cell Cycle, and Apoptosis Analyses
2.5. Immunofluorescence Microscopy
2.6. In Vivo Experiments
2.7. Histological and Immunohistochemical (IHC) Analyses
2.8. Statistical Analyses
3. Results
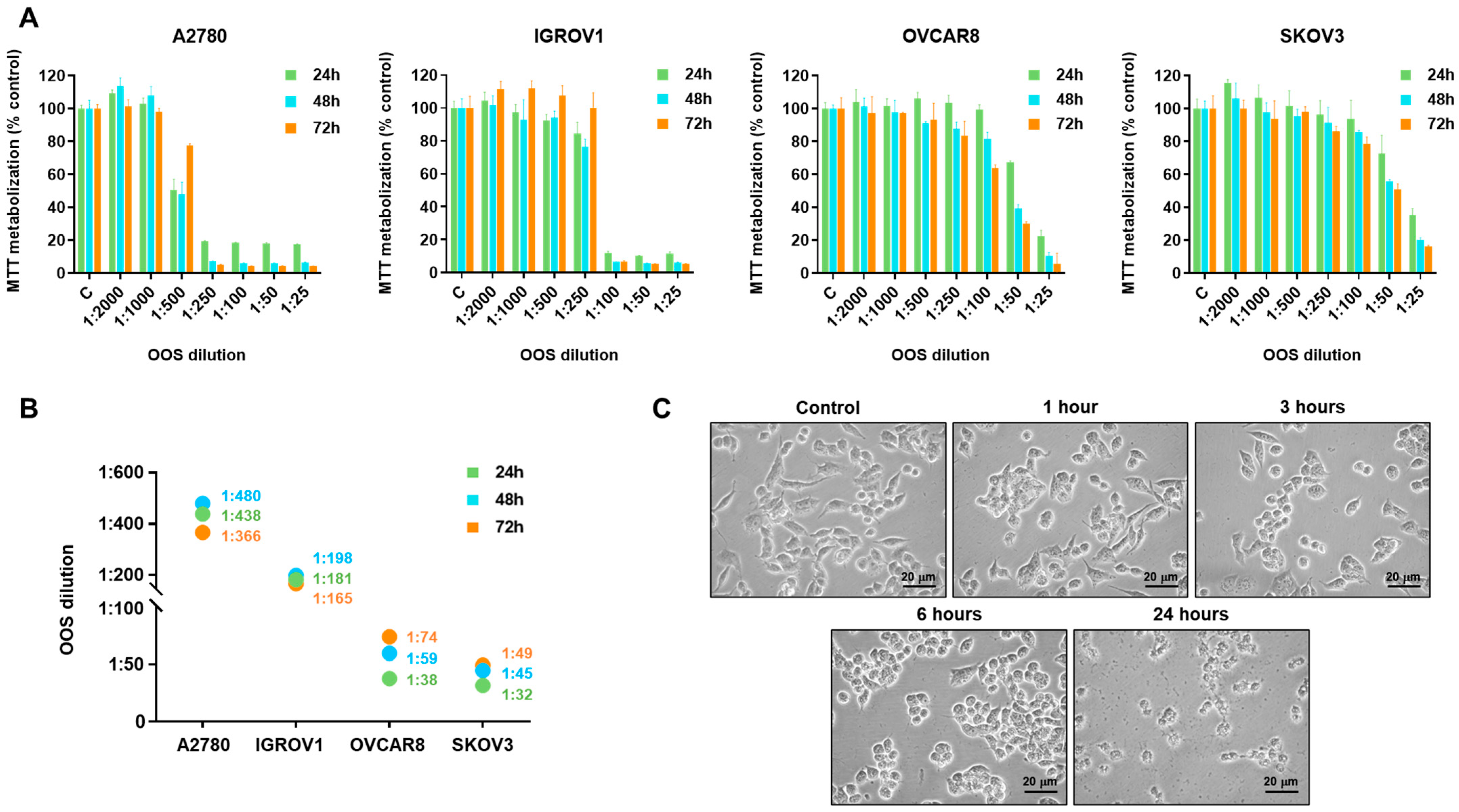
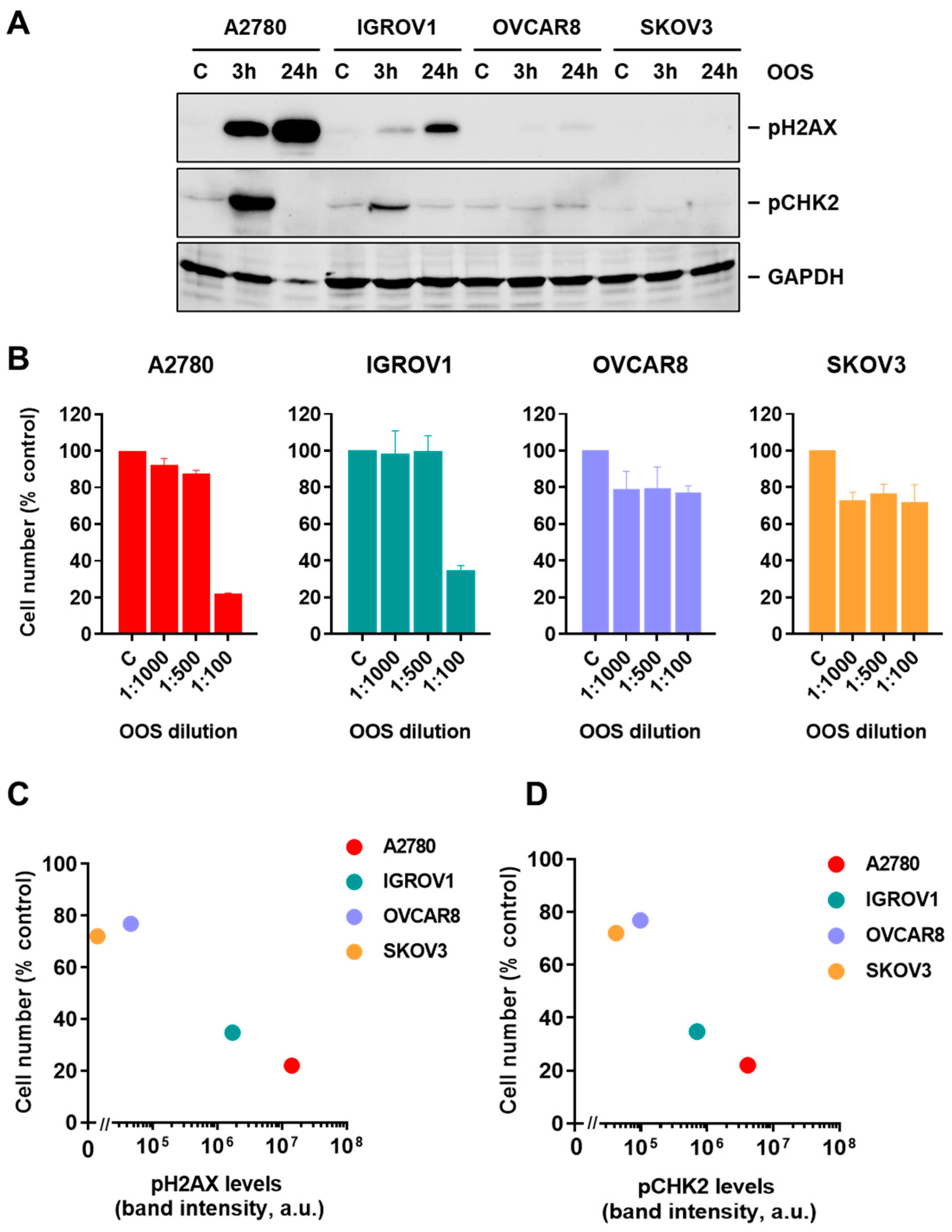
3.1. Action of OOS on Ovarian Cancer Cells
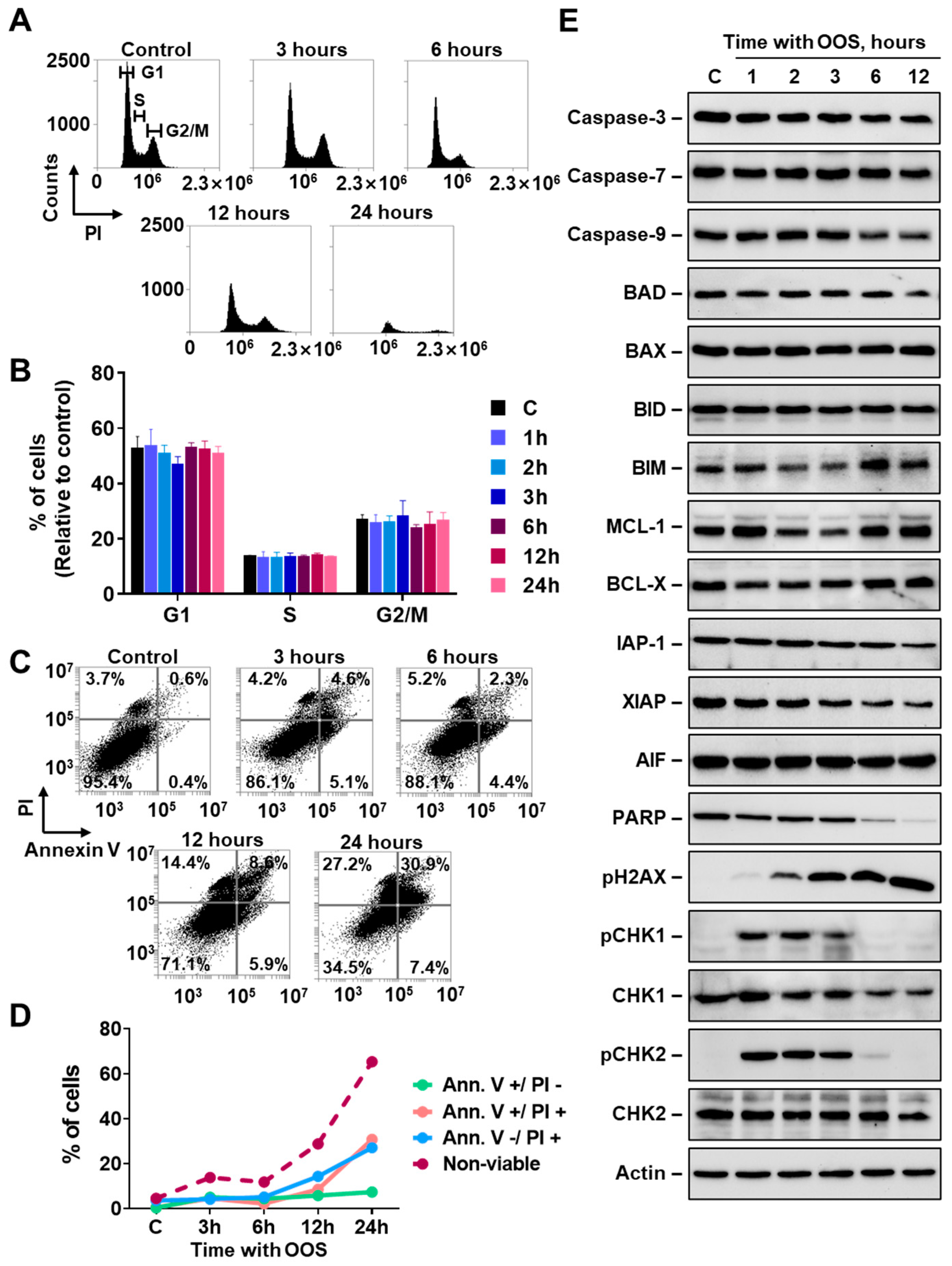
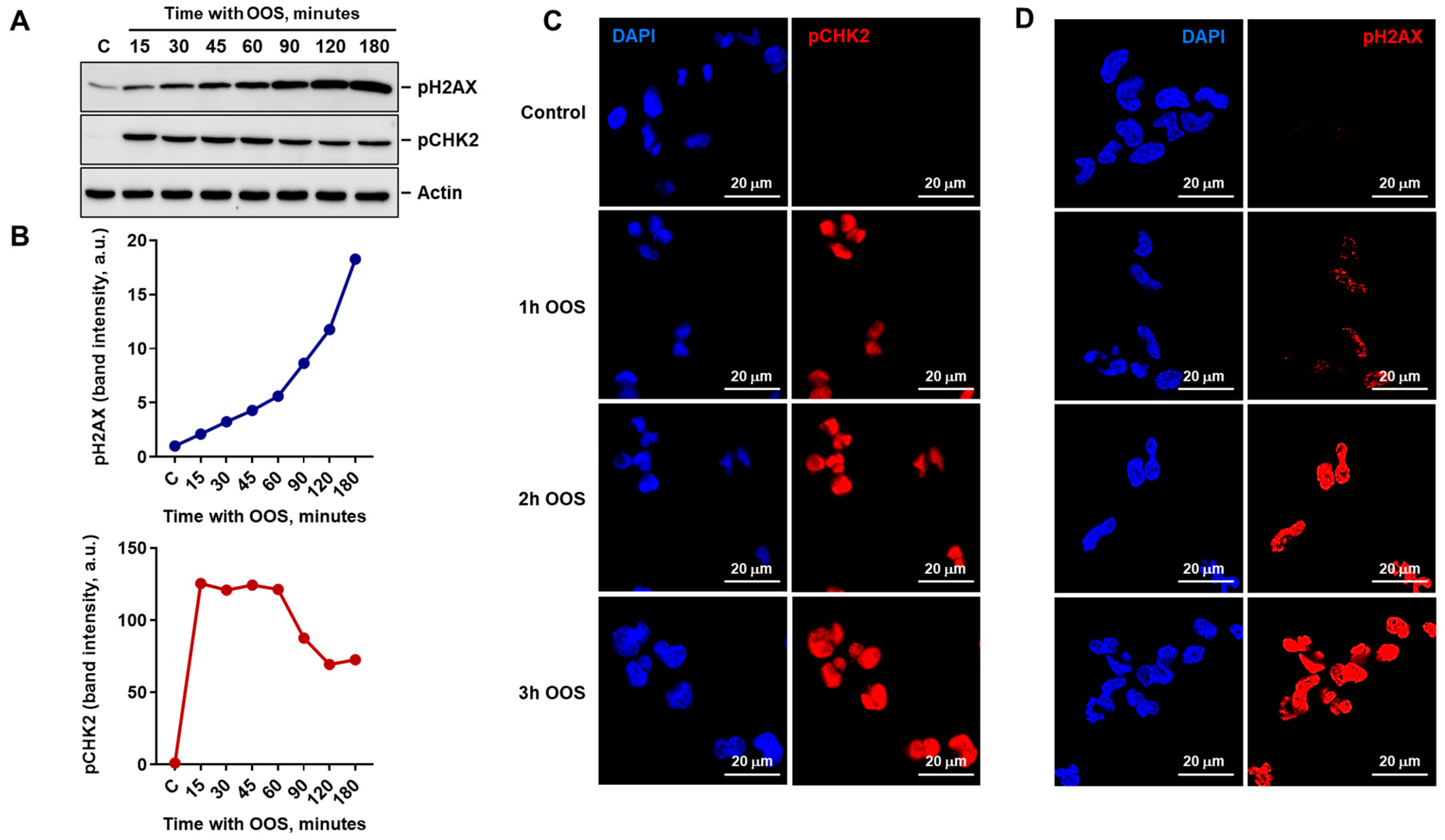
3.2. OOS Activates the DNA Damage Response in Ovarian Cancer Cells
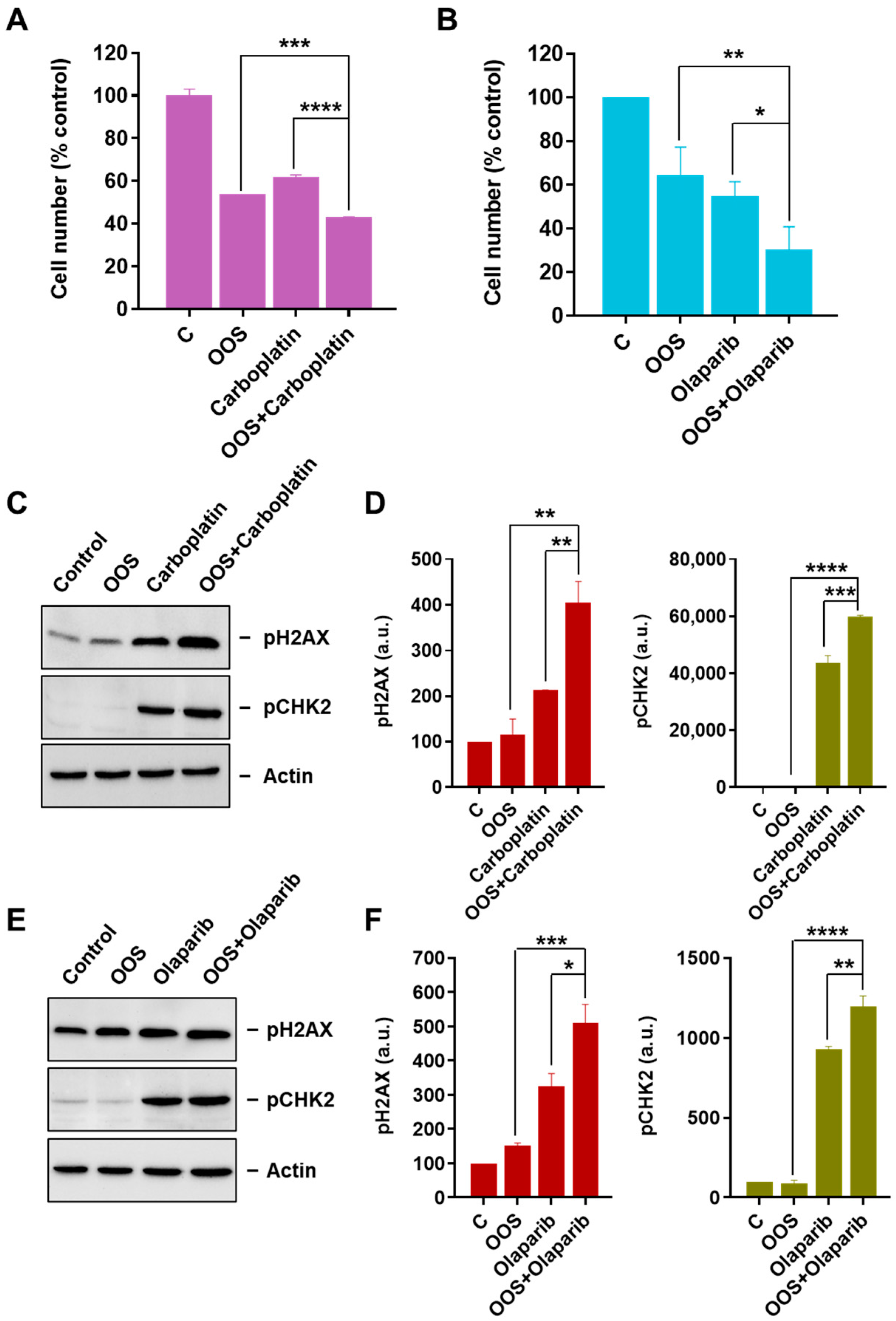
3.3. OOS Augments the Antiproliferative Action of Carboplatin and Olaparib
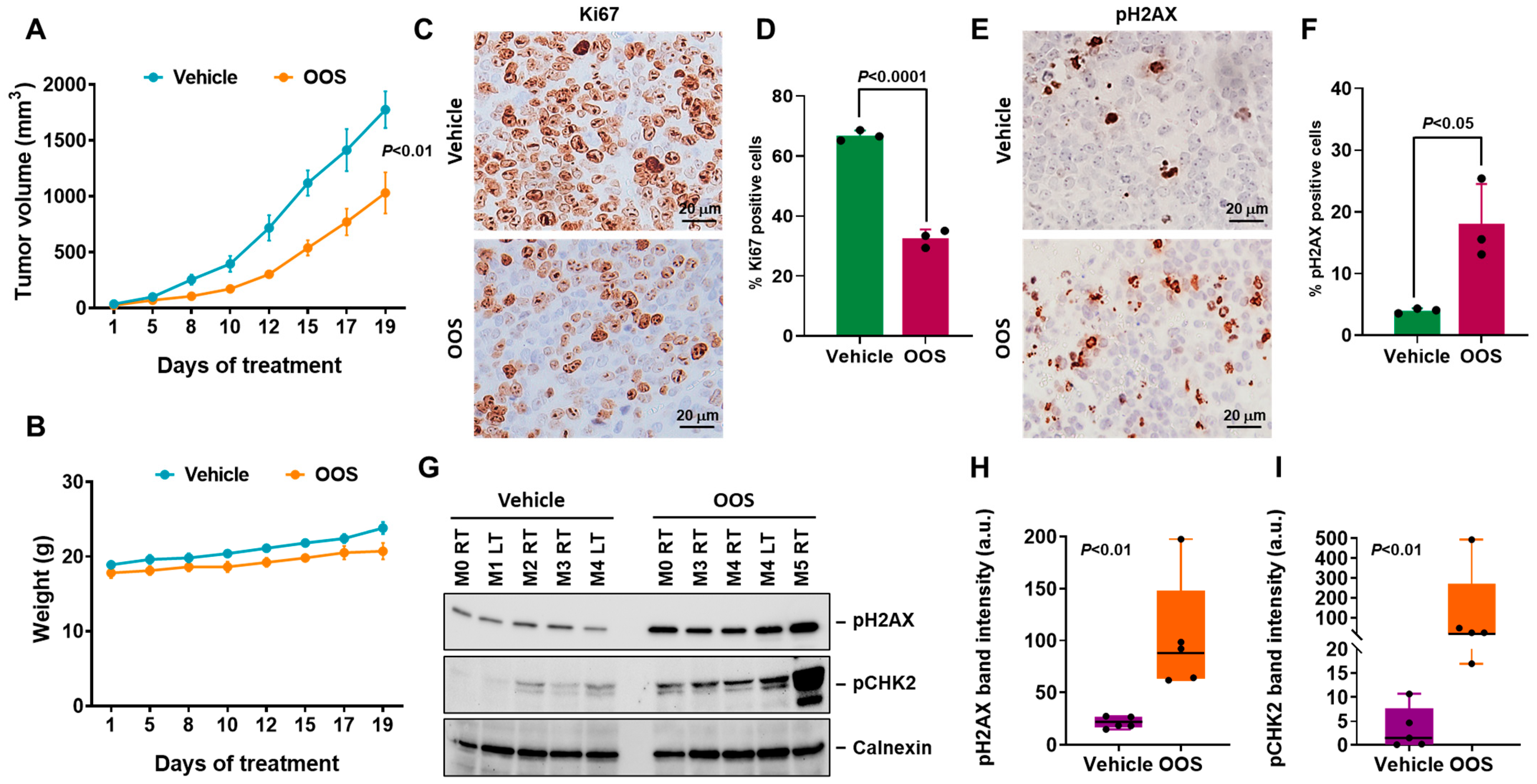
3.4. In Vivo Antitumoral Efficacy of OOS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Straubhar, A.; Chi, D.S.; Long Roche, K. Update on the role of surgery in the management of advanced epithelial ovarian cancer. Clin. Adv. Hematol. Oncol. 2020, 18, 723–731. [Google Scholar] [PubMed]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Santaballa, A.; Barretina, P.; Casado, A.; García, Y.; González-Martín, A.; Guerra, E.; Laínez, N.; Martinez, J.; Redondo, A.; Romero, I. SEOM Clinical Guideline in ovarian cancer. Clin. Transl. Oncol. 2016, 18, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Goldlust, I.S.; Guidice, E.; Lee, J.M. PARP inhibitors in ovarian cancer. Semin. Oncol. 2024, 51, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karihtala, P.; Moschetta, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Combined Strategies with Poly (ADP-Ribose) Polymerase (PARP) Inhibitors for the Treatment of Ovarian Cancer: A Literature Review. Diagnostics 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O’Malley, D.M.; et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Mirvetuximab Soravtansine: First Approval. Drugs 2023, 83, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Ray-Coquard, I.; Concin, N.; Ngoi, N.Y.L.; Morice, P.; Caruso, G.; Enomoto, T.; Takehara, K.; Denys, H.; Lorusso, D.; et al. Endometrial carcinosarcoma. Int. J. Gynecol. Cancer 2023, 33, 147–174. [Google Scholar] [CrossRef]
- Zamarin, D.; Burger, R.A.; Sill, M.W.; Powell, D.J.; Lankes, H.A.; Feldman, M.D.; Zivanovic, O.; Gunderson, C.; Ko, E.; Mathews, C.; et al. Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J. Clin. Oncol. 2020, 38, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian Cancer Immunotherapy and Personalized Medicine. Int. J. Mol. Sci. 2021, 22, 6532. [Google Scholar] [CrossRef] [PubMed]
- Moufarrij, S.; O’Cearbhaill, R.E. Novel Therapeutics in Ovarian Cancer: Expanding the Toolbox. Curr. Oncol. 2023, 31, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Pharmacological Activities of Epigallocatechin-3-gallate in Signaling Pathways: An Update on Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Tran, H.M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef] [PubMed]
- Parish, M.; Massoud, G.; Hazimeh, D.; Segars, J.; Islam, M.S. Green Tea in Reproductive Cancers: Could Treatment Be as Simple? Cancers 2023, 15, 862. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Momeni, M.R. Epigallocatechin-3-gallate and its nanoformulation in cervical cancer therapy: The role of genes, MicroRNA and DNA methylation patterns. Cancer Cell Int. 2023, 23, 335. [Google Scholar] [CrossRef] [PubMed]
- Pandiella-Alonso, A.; Díaz-Rodríguez, E.; Sanz, E. Antitumoral Properties of the Nutritional Supplement Ocoxin Oral Solution: A Comprehensive Review. Nutrients 2020, 12, 2661. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, E.; Hernández-García, S.; Sanz, E.; Pandiella, A. Antitumoral effect of Ocoxin on acute myeloid leukemia. Oncotarget 2016, 7, 6231–6242. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, E.; El-Mallah, A.M.; Sanz, E.; Pandiella, A. Antitumoral effect of Ocoxin in hepatocellular carcinoma. Oncol. Lett. 2017, 14, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Hernández-SanMiguel, E.; Gargini, R.; Cejalvo, T.; Segura-Collar, B.; Núñez-Hervada, P.; Hortigüela, R.; Sepúlveda-Sánchez, J.M.; Hernández-Laín, A.; Pérez-Núñez, A.; Sanz, E.; et al. Ocoxin Modulates Cancer Stem Cells and M2 Macrophage Polarization in Glioblastoma. Oxid. Med. Cell Longev. 2019, 2019, 9719730. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Unzueta, I.; Benedicto, A.; Romayor, I.; Herrero, A.; Sanz, E.; Arteta, B.; Olaso, E.; Márquez, J. Ocoxin Oral Solution Exerts an Antitumoral Effect in Pancreatic Cancer and Reduces the Stromal-Mediated Chemoresistance. Pancreas 2019, 48, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Márquez, J.; Mena, J.; Hernandez-Unzueta, I.; Benedicto, A.; Sanz, E.; Arteta, B.; Olaso, E. Ocoxin® oral solution slows down tumor growth in an experimental model of colorectal cancer metastasis to the liver in Balb/c mice. Oncol. Rep. 2016, 35, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Peña, J.; Díaz-Rodríguez, E.; Sanz, E.; Pandiella, A. Central Role of Cell Cycle Regulation in the Antitumoral Action of Ocoxin. Nutrients 2019, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Pandiella, A.; Sanz, E.; Díaz-Rodríguez, E. Ocoxin oral solution demonstrates antiviral properties in cellular models. Exp. Ther. Med. 2021, 22, 1127. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahtab, M.; Akbar, S.M.; Khan, M.S.; Rahman, S. Increased survival of patients with end-stage hepatocellular carcinoma due to intake of ONCOXIN®, a dietary supplement. Indian. J. Cancer 2015, 52, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Fundora Ramos, M.I.; Maden, L.B.; Casanova, F.O.; Cruz, F.H.; Reyes, C.S.; Gato, A.H.; Lyncon, I.B.; González, E.V.; Morales, K.P.; Lence, J.J.; et al. Oncoxin-Viusid may improve quality of life and survival in patients with hormone-refractory prostate cancer undergoing onco-specific treatments. Mol. Clin. Oncol. 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Kaidarova, D.R.; Kopp, M.V.; Pokrovsky, V.S.; Dzhugashvili, M.; Akimzhanova, Z.M.; Abdrakhmanov, R.Z.; Babich, E.N.; Bilan, E.V.; Byakhov, A.V.; Gurov, S.N.; et al. Multicomponent nutritional supplement Oncoxin and its influence on quality of life and therapy toxicity in patients receiving adjuvant chemotherapy. Oncol. Lett. 2019, 18, 5644–5652. [Google Scholar] [CrossRef] [PubMed]
- Shumsky, A.; Bilan, E.; Sanz, E.; Petrovskiy, F. Oncoxin nutritional supplement in the management of chemotherapy- and/or radiotherapy-associated oral mucositis. Mol. Clin. Oncol. 2019, 10, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Giglia-Mari, G.; Zotter, A.; Vermeulen, W. DNA damage response. Cold Spring Harb. Perspect. Biol. 2011, 3, a000745. [Google Scholar] [CrossRef] [PubMed]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Carusillo, A.; Mussolino, C. DNA Damage: From Threat to Treatment. Cells 2020, 9, 1665. [Google Scholar] [CrossRef]
- John, P.C.; Mews, M.; Moore, R. Cyclin/Cdk complexes: Their involvement in cell cycle progression and mitotic division. Protoplasma 2001, 216, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Genta, S.; Aglietta, M.; Sapino, A.; Valabrega, G. PARP Inhibitors in Ovarian Cancer. Recent. Pat. Anticancer. Drug Discov. 2018, 13, 392–410. [Google Scholar] [CrossRef]
- Boyd, L.R.; Muggia, F.M. Carboplatin/Paclitaxel Induction in Ovarian Cancer: The Finer Points. Oncology 2018, 32, 418–420+422–424. [Google Scholar] [PubMed]
- Ma, L.; Rudert, W.; Harnaha, J.; Wright, M.; Machen, J.; Lakomy, R.; Qian, S.; Lu, L.; Robbins, P.D.; Trucco, M.; et al. Immunosuppressive Effects of Glucosamine. J. Biol. Chem. 2002, 277, 39343–39349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, H.; Jin, Y.; Liu, N.; Chen, J.; Yang, Y.; Dai, Z.; Wang, C.; Wu, G.; Wu, Z. Glycine Attenuates LPS-Induced Apoptosis and Inflammatory Cell Infiltration in Mouse Liver. J. Nutr. 2020, 150, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Okamoto, K.; Terashima, A.; Nitta, T.; Muro, R.; Negishi-Koga, T.; Kitamura, T.; Nakashima, T.; Takayanagi, H. Arginine methylation controls the strength of γc-family cytokine signaling in T cell maintenance. Nat. Immunol. 2018, 19, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R.; Rieckmann, J.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef] [PubMed]
- Šalamon, Š.; Kramar, B.; Marolt, T.; Poljšak, B.; Milisav, I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, K.; Iahida, N. Inhibitory effect of Glycyrrhizin on Polypeptide Phosporylation by Polypeptide-dependent Proteinase (Kinase P) in vitro. Biochem. Biophys. Res. Commun. 1988, 157, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Takei, M.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology 2003, 70, 229–236. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Krausse, R.; Ragazzi, E.; Pellati, D.; Armanini, D.; Bielenberg, J. Antiviral Effects of Glycyrrhiza species. Phytother. Res. 2008, 22, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Guerriero, E.; Capone, F.; Colonna, G.; Castello, G.; Costantini, S. Ascorbic Acid: Its Role in Immune System and Chronic Inflammation Diseases. Mini-Rev. Med. Chem. 2014, 14, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.; Maggini, S.; Hornig, D. Immune-Enhancing Role of Vitamin C and Zinc and Effect on Clinical Conditions. Ann. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.B.; Tian, Q.; Zhang, J.F.; Xiang, Y. Antitumor effects and molecular mechanisms of action of natural products in ovarian cancer. Oncol. Lett. 2020, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Xu, S.; Fang, J.; Jiang, H. The Protective Effect of Polyphenols for Colorectal Cancer. Front. Immunol. 2020, 11, 1407. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Stawny, M.; Mlynarczyk, D.T.; Muszalska-Kolos, I.; Goslinski, T.; Jelińska, A. Role of Curcumin and (-)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review. Cancers 2020, 12, 1801. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin. Cancer Biol. 2022, 80, 256–275. [Google Scholar] [CrossRef]
- Hogan, P. Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion. Cell Calcium 2017, 63, 66–69. [Google Scholar] [CrossRef]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef]
- Feske, S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007, 7, 690–702. [Google Scholar] [CrossRef]
- Hernroth, B.; Holm, I.; Gondikas, A.; Tassidis, H. Manganese Inhibits Viability of Prostate Cancer Cells. Anticancer Res. 2020, 38, 137–145. [Google Scholar]
- Liu, J.; Guo, W.; Li, J.; Li, X.; Geng, J.; Chen, Q.; Gao, J. Tumor-targeting novel manganese complex induces ROS-mediated apoptotic and autophagic cancer cell death. Int. J. Mol. Med. 2015, 35, 607–616. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Zhu, H.; Shi, Q.; Li, D.; Ju, X. Pyridoxal-5′-Phosphate Promotes Immunomodulatory Function of Adipose-Derived Mesenchymal Stem Cells through Indoleamine 2,3-Dioxygenase-1 and TLR4/NF-κB Pathway. Stem Cells Int. 2019, 2019, 3121246. [Google Scholar] [CrossRef]
- Sadeghi, S.; Davoodvandi, A.; Pourhanifeh, M.H.; Sharifi, N.; ArefNezhad, R.; Sahebnasagh, R.; Moghadam, S.A.; Sahebkar, A.; Mirzaei, H. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur. J. Med. Chem. 2019, 178, 131–140. [Google Scholar] [CrossRef]
- Samblas, M.; Martínez, J.; Milagro, F. Folic Acid Improves the Inflammatory Response in LPS-Activated THP-1 Macrophages. Mediat. Inflamm. 2018, 2018, 1312626. [Google Scholar] [CrossRef]
- Henry, C.; Nemkov, T.; Casás-Selves, M.; Bilousova, G.; Zaberezhnyy, V.; Higa, K.; Serkova, N.J.; Hansen, K.C.; D’alessandro, A.; DeGregori, J. Folate dietary insufficiency and folic acid supplementation similarly impair metabolism and compromise haematopoiesis. Haematologica 2017, 102, 1985–1994. [Google Scholar] [CrossRef]
- Calder, P.; Carr, A.; Gombart, A.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almaraz-Postigo, S.; Sanz, E.; Pandiella, A.; Díaz-Rodríguez, E. Ocoxin Oral Solution Triggers DNA Damage and Cell Death in Ovarian Cancer. Nutrients 2024, 16, 2416. https://doi.org/10.3390/nu16152416
Almaraz-Postigo S, Sanz E, Pandiella A, Díaz-Rodríguez E. Ocoxin Oral Solution Triggers DNA Damage and Cell Death in Ovarian Cancer. Nutrients. 2024; 16(15):2416. https://doi.org/10.3390/nu16152416
Chicago/Turabian StyleAlmaraz-Postigo, Sheila, Eduardo Sanz, Atanasio Pandiella, and Elena Díaz-Rodríguez. 2024. "Ocoxin Oral Solution Triggers DNA Damage and Cell Death in Ovarian Cancer" Nutrients 16, no. 15: 2416. https://doi.org/10.3390/nu16152416
APA StyleAlmaraz-Postigo, S., Sanz, E., Pandiella, A., & Díaz-Rodríguez, E. (2024). Ocoxin Oral Solution Triggers DNA Damage and Cell Death in Ovarian Cancer. Nutrients, 16(15), 2416. https://doi.org/10.3390/nu16152416




