Causal Relationship between Meat Intake and Biological Aging: Evidence from Mendelian Randomization Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Data Source
2.2. IVs Selection and Data Cleaning
2.3. MR Analysis
2.4. Sensitivity Analysis
3. Results
3.1. Causal Relationship between Meat Consumers and Aging-Related Phenotypes
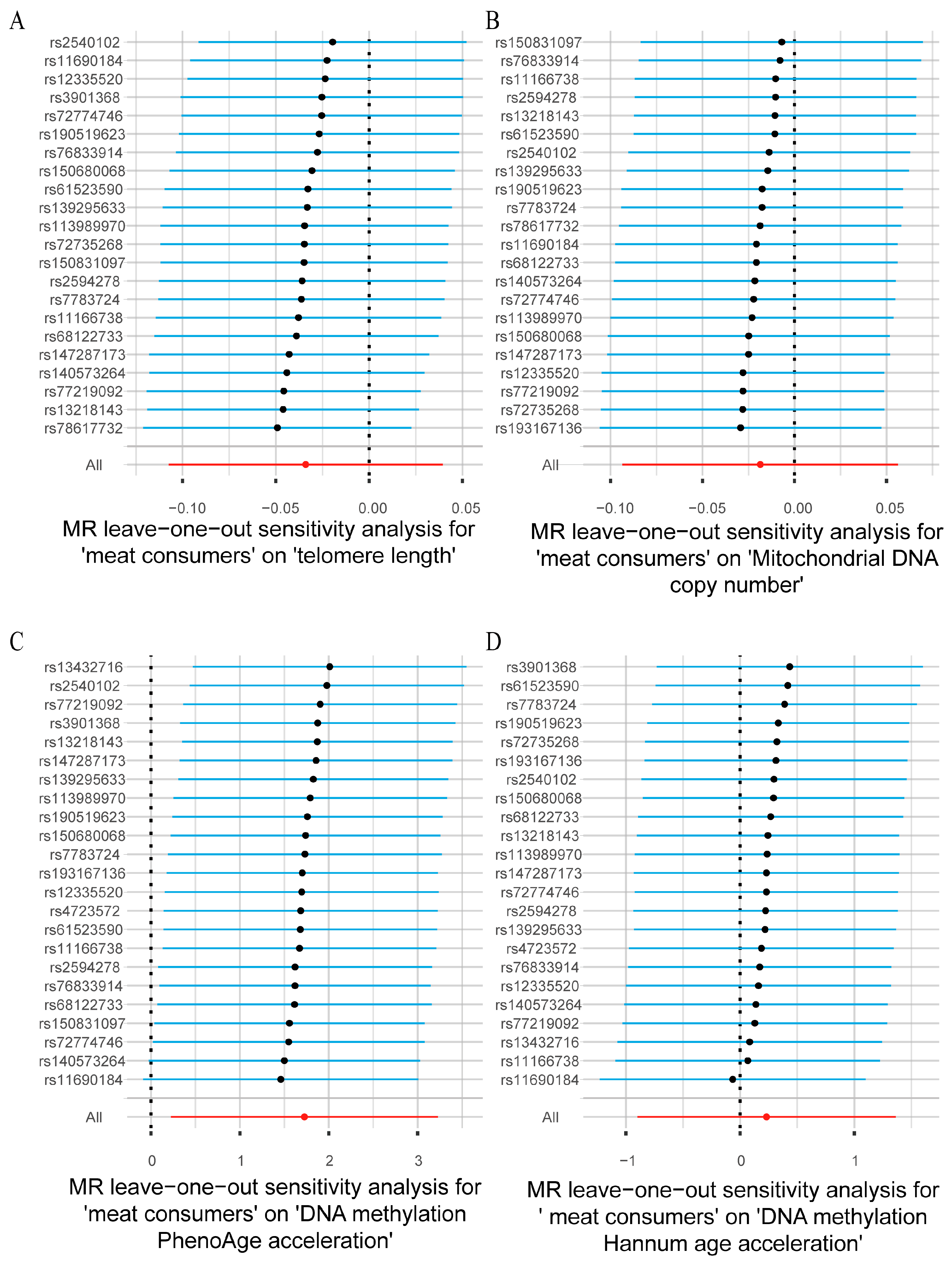
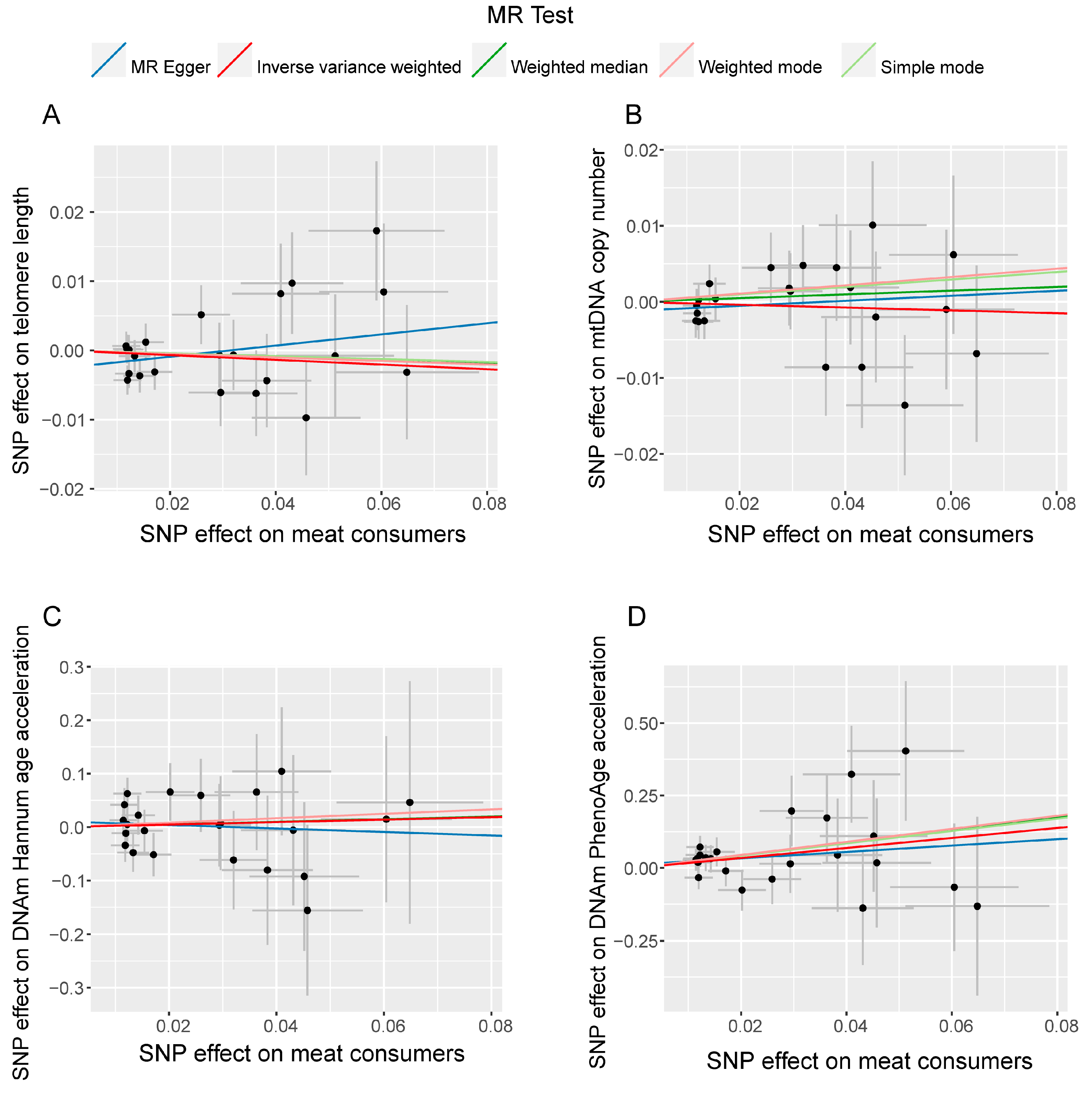
3.2. Sensitivity Analysis
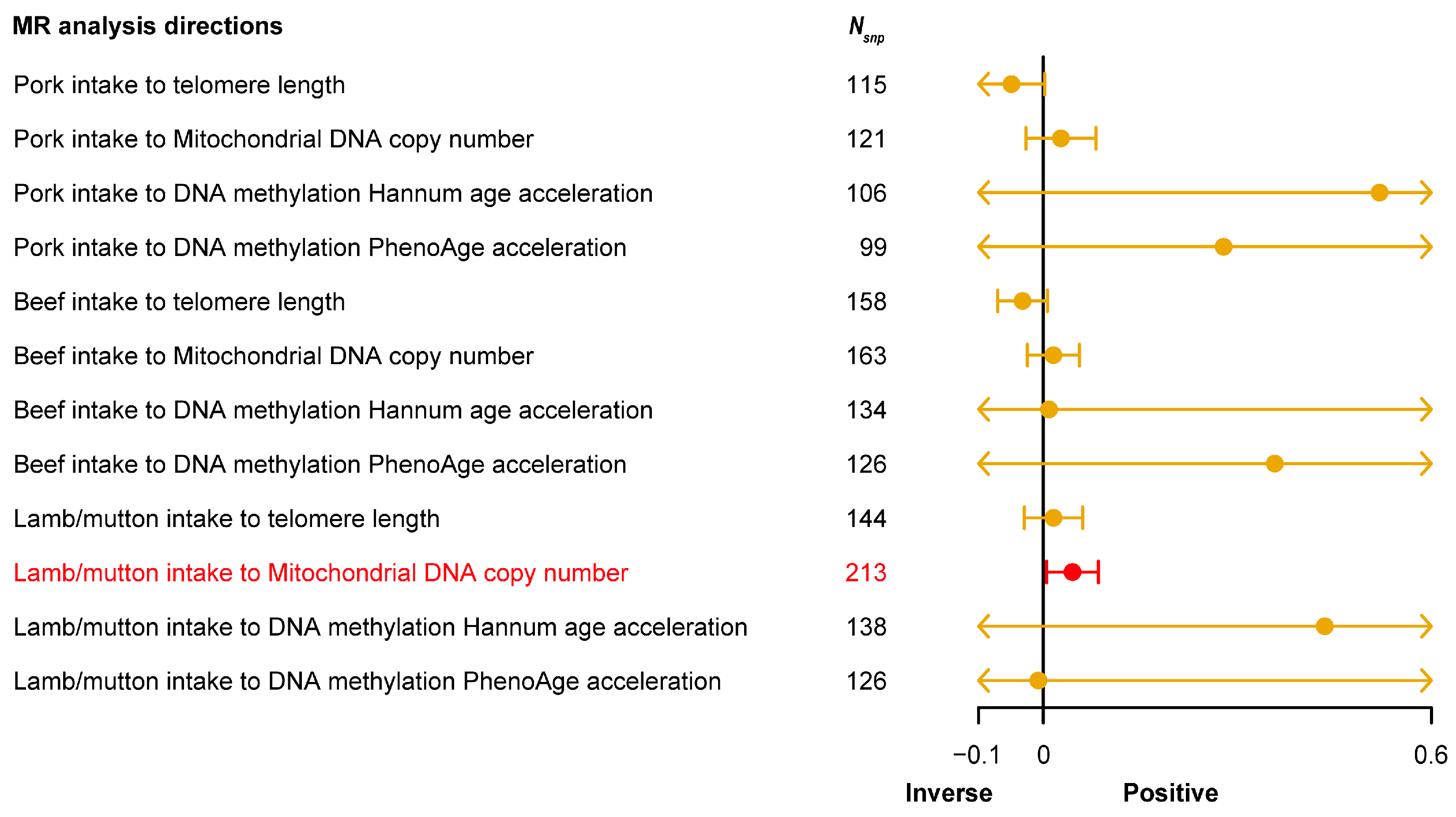
3.3. Causal Relationship between Red Meat Intake and Aging-Related Phenotypes
3.4. Causal Relationship between White Meat Intake and Aging-Related Phenotypes
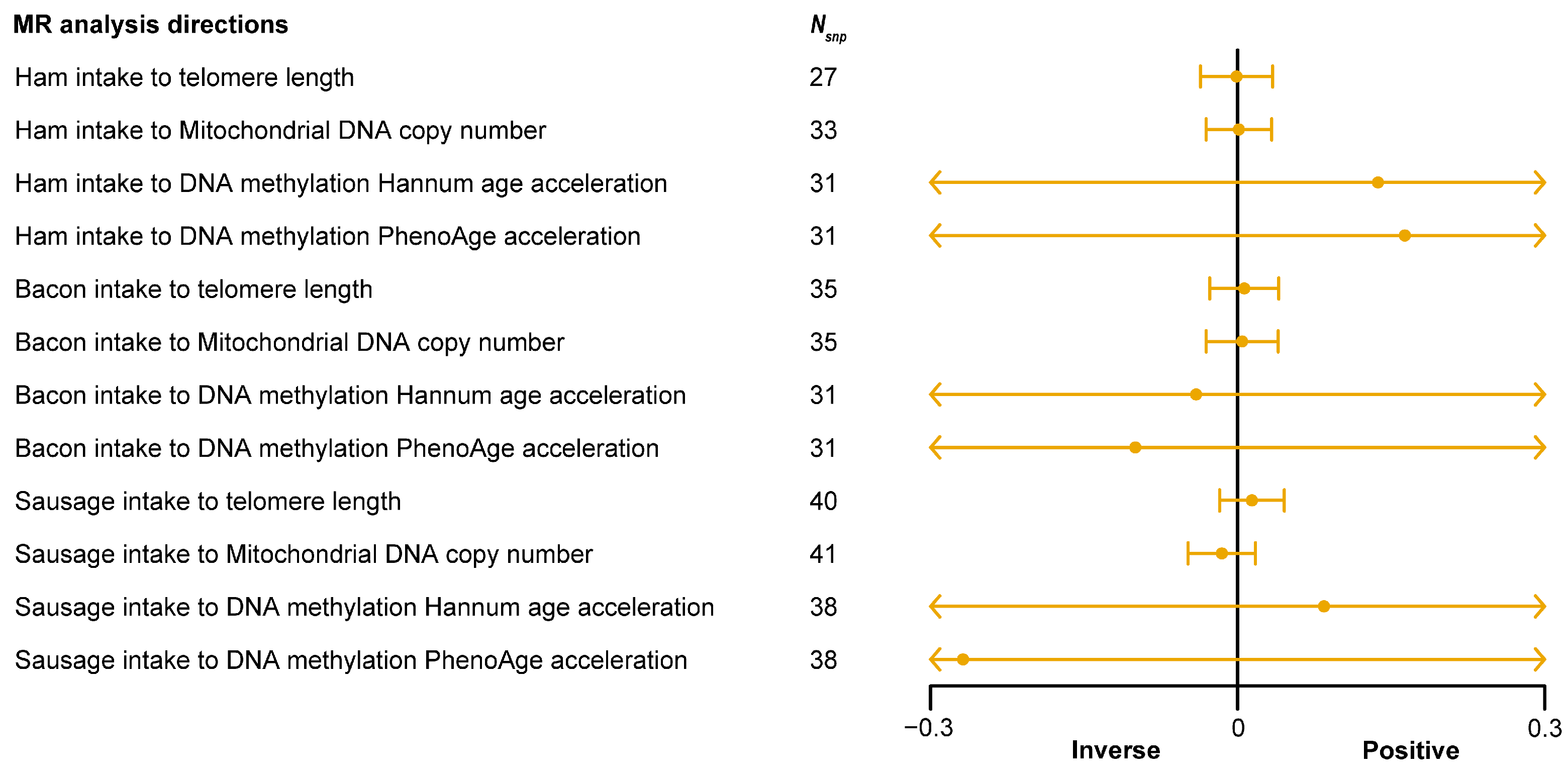
3.5. Causal Relationship between Processed Meat Intake and Aging-Related Phenotypes
3.6. Heterogeneity and Pleiotropy in Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talens, C.; Llorente, R.; Simó-Boyle, L.; Odriozola-Serrano, I.; Tueros, I.; Ibargüen, M. Hybrid Sausages: Modelling the Effect of Partial Meat Replacement with Broccoli, Upcycled Brewer’s Spent Grain and Insect Flours. Foods 2022, 11, 3396. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ning, J.; Cheng, X.; Lv, Q.; Teng, S.; Wang, W. Rapid and High-Throughput Determination of Sixteen β-agonists in Livestock Meat Using One-Step Solid-Phase Extraction Coupled with UHPLC-MS/MS. Foods 2022, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Spyrelli, E.D.; Doulgeraki, A.I.; Argyri, A.A.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.E. Implementation of Multispectral Imaging (MSI) for Microbiological Quality Assessment of Poultry Products. Microorganisms 2020, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Seo, J.W.; Lee, H.G.; Jung, W.K.; Park, Y.H.; Bae, H. Efficient Myogenic/Adipogenic Transdifferentiation of Bovine Fibroblasts in a 3D Bioprinting System for Steak-Type Cultured Meat Production. Adv. Sci. 2022, 9, e2202877. [Google Scholar] [CrossRef] [PubMed]
- Ivancovsky-Wajcman, D.; Fliss-Isakov, N.; Grinshpan, L.S.; Salomone, F.; Lazarus, J.V.; Webb, M.; Shibolet, O.; Kariv, R.; Zelber-Sagi, S. High Meat Consumption Is Prospectively Associated with the Risk of Non-Alcoholic Fatty Liver Disease and Presumed Significant Fibrosis. Nutrients 2022, 14, 3533. [Google Scholar] [CrossRef] [PubMed]
- Damigou, E.; Kosti, R.I.; Panagiotakos, D.B. White Meat Consumption and Cardiometabolic Risk Factors: A Review of Recent Prospective Cohort Studies. Nutrients 2022, 14, 5213. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chen, L.; Lv, J.; Pang, Y.; Guo, Y.; Pei, P.; Du, H.; Yang, L.; Millwood, I.Y.; Walters, R.G.; et al. Association of Red Meat Consumption, Metabolic Markers, and Risk of Cardiovascular Diseases. Front. Nutr. 2022, 9, 833271. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Pincay, R.; Salazar, D.; Flores, N.; Escolastico, C. Evaluation of the Quality and Lipid Content of Artisan Sausages Produced in Tungurahua, Ecuador. Foods 2023, 12, 4288. [Google Scholar] [CrossRef] [PubMed]
- Alegria-Lertxundi, I.; Bujanda, L.; Arroyo-Izaga, M. Role of Dairy Foods, Fish, White Meat, and Eggs in the Prevention of Colorectal Cancer: A Systematic Review of Observational Studies in 2018-2022. Nutrients 2022, 14, 3430. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Thun, M.J.; Connell, C.J.; McCullough, M.L.; Jacobs, E.J.; Flanders, W.D.; Rodriguez, C.; Sinha, R.; Calle, E.E. Meat consumption and risk of colorectal cancer. JAMA 2005, 293, 172–182. [Google Scholar] [CrossRef]
- Ma, H.; Qi, X. Red Meat Consumption and Cancer Risk: A Systematic Analysis of Global Data. Foods 2023, 12, 4164. [Google Scholar] [CrossRef] [PubMed]
- Van Blarigan, E.L.; Ou, F.S.; Bainter, T.M.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Hantel, A.; Benson, A.B., 3rd; et al. Associations Between Unprocessed Red Meat and Processed Meat With Risk of Recurrence and Mortality in Patients With Stage III Colon Cancer. JAMA Netw. Open 2022, 5, e220145. [Google Scholar] [CrossRef] [PubMed]
- Bučan Nenadić, D.; Radić, J.; Kolak, E.; Vučković, M.; Novak, I.; Selak, M.; Radić, M. Phase Angle Association with Dietary Habits and Metabolic Syndrome in Diabetic Hypertensive Patients: A Cross-Sectional Study. Nutrients 2022, 14, 5058. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, M.; Vahid, F.; Devaux, Y.; Bohn, T. Biomarkers of food intake and their relevance to metabolic syndrome. Food Funct. 2024, 15, 7271–7304. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Meat Consumption and Risk of Metabolic Syndrome: Results from the Korean Population and a Meta-Analysis of Observational Studies. Nutrients 2018, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.; Lau, R.; Aune, D.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE 2011, 6, e20456. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, R.; Kaaks, R.; Katzke, V. Food Sources of Animal Protein in Relation to Overall and Cause-Specific Mortality-Causal Associations or Confounding? An Analysis of the EPIC-Heidelberg Cohort. Nutrients 2023, 15, 3322. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Sinha, R.; Ward, M.H.; Graubard, B.I.; Inoue-Choi, M.; Dawsey, S.M.; Abnet, C.C. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: Population based cohort study. BMJ 2017, 357, j1957. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hyeon, J.; Lee, S.A.; Kwon, S.O.; Lee, H.; Keum, N.; Lee, J.K.; Park, S.M. Role of Total, Red, Processed, and White Meat Consumption in Stroke Incidence and Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005983. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Vitale, M.; Calabrese, I.; Giosuè, A.; Riccardi, G.; Vaccaro, O. White Meat Consumption, All-Cause Mortality, and Cardiovascular Events: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 13, 676. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Regulski, M.J. Cellular Senescence: What, Why, and How. Wounds 2017, 29, 168–174. [Google Scholar] [PubMed]
- Bao, H.; Cao, J.; Chen, M.; Chen, M.; Chen, W.; Chen, X.; Chen, Y.; Chen, Y.; Chen, Y.; Chen, Z.; et al. Biomarkers of aging. Sci. China Life Sci. 2023, 66, 893–1066. [Google Scholar] [CrossRef] [PubMed]
- Moqri, M.; Herzog, C.; Poganik, J.R.; Justice, J.; Belsky, D.W.; Higgins-Chen, A.; Moskalev, A.; Fuellen, G.; Cohen, A.A.; Bautmans, I.; et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell 2023, 186, 3758–3775. [Google Scholar] [CrossRef]
- Carey, A.; Niedernhofer, L.; Camell, C. Telomeres are a life-extending gift. Nat. Cell Biol. 2022, 24, 1449–1450. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, health, and hallmarks of aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef]
- Borghini, A.; Ndreu, R.; Canale, P.; Campolo, J.; Marinaro, I.; Mercuri, A.; Turchi, S.; Andreassi, M.G. Telomere Length, Mitochondrial DNA, and Micronucleus Yield in Response to Oxidative Stress in Peripheral Blood Mononuclear Cells. Int. J. Mol. Sci. 2024, 25, 1428. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Wang, C.; Hong, S.; Guan, X.; Meng, H.; Feng, Y.; Xiao, Y.; Zhou, Y.; Liu, C.; Zhong, G.; et al. Multiple metals exposure and blood mitochondrial DNA copy number: A cross-sectional study from the Dongfeng-Tongji cohort. Environ. Res. 2023, 216, 114509. [Google Scholar] [CrossRef]
- Li, R.; Chen, G.; Liu, X.; Pan, M.; Kang, N.; Hou, X.; Liao, W.; Dong, X.; Yuchi, Y.; Mao, Z.; et al. Aging biomarkers: Potential mediators of association between long-term ozone exposure and risk of atherosclerosis. J. Intern. Med. 2022, 292, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Field, A.E.; Robertson, N.A.; Wang, T.; Havas, A.; Ideker, T.; Adams, P.D. DNA Methylation Clocks in Aging: Categories, Causes, and Consequences. Mol. Cell 2018, 71, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Zhang, Z.; Zou, Z.; Lv, C.; Dong, Q.; He, Q.; Yi, Y.; Li, Y.; Wang, H.; Zhu, B. Kinetics and mechanisms of mitotic inheritance of DNA methylation and their roles in aging-associated methylome deterioration. Cell Res. 2020, 30, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, S.; Liu, Y.S. Roles and Mechanisms of DNA Methylation in Vascular Aging and Related Diseases. Front. Cell Dev. Biol. 2021, 9, 699374. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Wu, M.; Bondy, S.C. Telomere shortening during aging: Attenuation by antioxidants and anti-inflammatory agents. Mech. Ageing Dev. 2017, 164, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Codd, V.; Wang, Q.; Allara, E.; Musicha, C.; Kaptoge, S.; Stoma, S.; Jiang, T.; Hamby, S.E.; Braund, P.S.; Bountziouka, V.; et al. Polygenic basis and biomedical consequences of telomere length variation. Nat. Genet. 2021, 53, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Ashar, F.N.; Zhang, Y.; Longchamps, R.J.; Lane, J.; Moes, A.; Grove, M.L.; Mychaleckyj, J.C.; Taylor, K.D.; Coresh, J.; Rotter, J.I.; et al. Association of Mitochondrial DNA Copy Number With Cardiovascular Disease. JAMA Cardiol. 2017, 2, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Gavrilova, O.; Brown, A.L.; Soto, J.E.; Bremner, S.; Kim, J.; Xu, X.; Yang, S.; Um, J.H.; Koch, L.G.; et al. DNA-PK Promotes the Mitochondrial, Metabolic, and Physical Decline that Occurs During Aging. Cell Metab. 2017, 25, 1135–1146.e1137. [Google Scholar] [CrossRef]
- Yan, X.; Yang, P.; Li, Y.; Liu, T.; Zha, Y.; Wang, T.; Zhang, J.; Feng, Z.; Li, M. New insights from bidirectional Mendelian randomization: Causal relationships between telomere length and mitochondrial DNA copy number in aging biomarkers. Aging 2024, 16, 7387–7404. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Rey, F.; Carelli, S.; Cattaldo, S.; Fontana, J.M.; Goitre, I.; Ponzo, V.; Merlo, F.D.; Zuccotti, G.; Bertoli, S.; et al. Telomere Length and Mitochondrial DNA Copy Number Variations in Patients with Obesity: Effect of Diet-Induced Weight Loss-A Pilot Study. Nutrients 2022, 14, 4293. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, K.L.; Sisk, L.M.; Manczak, E.M.; Lin, J.; Gotlib, I.H. Depressive Symptoms Predict Change in Telomere Length and Mitochondrial DNA Copy Number Across Adolescence. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 1364–1370.e1362. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Y.J.; Sun, B.; Chen, H.G.; Mustieles, V.; Messerlian, C.; Sun, Y.; Meng, T.Q.; Lu, W.Q.; Pan, X.F.; et al. Blood trihalomethane concentrations in relation to sperm mitochondrial DNA copy number and telomere length among 958 healthy men. Environ. Res 2023, 216, 114737. [Google Scholar] [CrossRef]
- Vostatek, R.; Hohensinner, P.; Nopp, S.; Haider, P.; Englisch, C.; Pointner, J.; Pabinger, I.; Ay, C. Association of telomere length and mitochondrial DNA copy number, two biomarkers of biological aging, with the risk of venous thromboembolism. Thromb. Res. 2023, 223, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Becerra, C.Y.; Wells, R.K.; Kunihiro, B.P.; Lee, R.H.; Umeda, L.; Allan, N.P.; Rubas, N.C.; McCracken, T.A.; Nunokawa, C.K.L.; Lee, M.H.; et al. Examining the immunoepigenetic-gut microbiome axis in the context of self-esteem among Native Hawaiians and other Pacific Islanders. Front. Genet. 2023, 14, 1125217. [Google Scholar] [CrossRef] [PubMed]
- Chiavellini, P.; Canatelli-Mallat, M.; Lehmann, M.; Gallardo, M.D.; Herenu, C.B.; Cordeiro, J.L.; Clement, J.; Goya, R.G. Aging and rejuvenation—A modular epigenome model. Aging 2021, 13, 4734–4746. [Google Scholar] [CrossRef] [PubMed]
- Hernández Cordero, A.I.; Yang, C.X.; Yang, J.; Horvath, S.; Shaipanich, T.; MacIsaac, J.; Lin, D.T.S.; Kobor, M.S.; Guillemi, S.; Harris, M.; et al. Airway Aging and Methylation Disruptions in HIV-associated Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2022, 206, 150–160. [Google Scholar] [CrossRef] [PubMed]
- McCartney, D.L.; Min, J.L.; Richmond, R.C.; Lu, A.T.; Sobczyk, M.K.; Davies, G.; Broer, L.; Guo, X.; Jeong, A.; Jung, J.; et al. Genome-wide association studies identify 137 genetic loci for DNA methylation biomarkers of aging. Genome Biol. 2021, 22, 194. [Google Scholar] [CrossRef] [PubMed]
- Murach, K.A.; Dimet-Wiley, A.L.; Wen, Y.; Brightwell, C.R.; Latham, C.M.; Dungan, C.M.; Fry, C.S.; Watowich, S.J. Late-life exercise mitigates skeletal muscle epigenetic aging. Aging Cell 2022, 21, e13527. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Gao, X.; Cao, Y.; Chen, Y.; Cui, Q.; Deng, F.; Yang, B.; Lin, E.Z.; Fang, J.; Li, T.; et al. Personal airborne chemical exposure and epigenetic ageing biomarkers in healthy Chinese elderly individuals: Evidence from mixture approaches. Environ. Int. 2022, 170, 107614. [Google Scholar] [CrossRef] [PubMed]
- Andreo-López, M.C.; Contreras-Bolívar, V.; Muñoz-Torres, M.; García-Fontana, B.; García-Fontana, C. Influence of the Mediterranean Diet on Healthy Aging. Int. J. Mol. Sci. 2023, 24, 4491. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Hernández, V.; Montaño, L.M.; Caldelas, I.; Marmolejo-Valencia, A. A High-Fat and High-Carbohydrate Diet Promotes Reminiscent Hallmarks of an Aging Ovary in the Rabbit Model. Biomedicines 2022, 10, 3068. [Google Scholar] [CrossRef] [PubMed]
- Grande de França, N.A.; Rolland, Y.; Guyonnet, S.; de Souto Barreto, P. The role of dietary strategies in the modulation of hallmarks of aging. Ageing Res. Rev. 2023, 87, 101908. [Google Scholar] [CrossRef]
- Longo, V.D.; Anderson, R.M. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell 2022, 185, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.C.; Conti, D.V. Commentary: The concept of ‘Mendelian Randomization’. Int. J. Epidemiol. 2004, 33, 21–25. [Google Scholar] [CrossRef]
- Gurung, R.L.; Dorajoo, R.; Liu, S.; M, Y.; Liu, J.J.; Wang, L.; Guo, L.; Yu, X.; Liu, J.J.; Lim, S.C. Genetic markers for urine haptoglobin is associated with decline in renal function in type 2 diabetes in East Asians. Sci. Rep. 2018, 8, 5109. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Gu, Q.; Hu, Y.; Li, H.; Wu, Z.; Liu, W.; Zhu, Z.; Yuan, P.; Duan, L.; Zhou, Y.; et al. Genetic Analysis of a Collection of Rice Germplasm (Oryza sativa L.) through High-Density SNP Array Provides Useful Information for Further Breeding Practices. Genes 2022, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Small, D.S.; Thompson, S.G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017, 26, 2333–2355. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Pirastu, N.; McDonnell, C.; Grzeszkowiak, E.J.; Mounier, N.; Imamura, F.; Merino, J.; Day, F.R.; Zheng, J.; Taba, N.; Concas, M.P.; et al. Using genetic variation to disentangle the complex relationship between food intake and health outcomes. PLoS Genet. 2022, 18, e1010162. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Nan, M.; Li, X.; Liu, Z.; Xu, J.; Du, X.; Dong, Q.; Hou, L. Processed meat, red meat, white meat, and digestive tract cancers: A two-sample Mendelian randomization study. Front. Nutr. 2023, 10, 1078963. [Google Scholar] [CrossRef] [PubMed]
- Allaire, P.; He, J.; Mayer, J.; Moat, L.; Gerstenberger, P.; Wilhorn, R.; Strutz, S.; Kim, D.S.L.; Zeng, C.; Cox, N.; et al. Genetic and clinical determinants of telomere length. HGG Adv. 2023, 4, 100201. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.; Mohammadi-Shemirani, P.; Perrot, N.; Nelson, W.; Morton, R.; Narula, S.; Lali, R.; Khan, I.; Khan, M.; Judge, C.; et al. GWAS and ExWAS of blood mitochondrial DNA copy number identifies 71 loci and highlights a potential causal role in dementia. eLife 2022, 11, e70382. [Google Scholar] [CrossRef] [PubMed]
- Glymour, M.M.; Tchetgen Tchetgen, E.J.; Robins, J.M. Credible Mendelian randomization studies: Approaches for evaluating the instrumental variable assumptions. Am. J. Epidemiol. 2012, 175, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wu, H.; Wang, X.; Huang, T.; Jia, J. Shared Genetic Basis and Causal Relationship Between Television Watching, Breakfast Skipping and Type 2 Diabetes: Evidence From a Comprehensive Genetic Analysis. Front. Endocrinol. 2022, 13, 836023. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Byrska-Bishop, M.; Evani, U.S.; Zhao, X.; Basile, A.O.; Abel, H.J.; Regier, A.A.; Corvelo, A.; Clarke, W.E.; Musunuri, R.; Nagulapalli, K.; et al. High-coverage whole-genome sequencing of the expanded 1000 Genomes Project cohort including 602 trios. Cell 2022, 185, 3426–3440.e3419. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, X.; Pan, X.F.; Zhou, T.; Zhu, C.; Li, F.; Fan, J.G.; Targher, G.; Zhao, J. The hepato-ovarian axis: Genetic evidence for a causal association between non-alcoholic fatty liver disease and polycystic ovary syndrome. BMC Med. 2023, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Brown, D.W.; Machiela, M.J. LDtrait: An Online Tool for Identifying Published Phenotype Associations in Linkage Disequilibrium. Cancer Res. 2020, 80, 3443–3446. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Spiller, W.; Del Greco M, F.; Sheehan, N.; Thompson, J.; Minelli, C.; Davey Smith, G. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int. J. Epidemiol. 2018, 47, 1264–1278. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Wu, P.F.; Lu, H.; Zhou, X.; Liang, X.; Li, R.; Zhang, W.; Li, D.; Xia, K. Assessment of causal effects of physical activity on neurodegenerative diseases: A Mendelian randomization study. J. Sport Health Sci. 2021, 10, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Knoblauch, N.; Marcus, J.H.; Stephens, M.; He, X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat. Genet. 2020, 52, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Foley, C.N.; Allara, E.; Staley, J.R.; Howson, J.M.M. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat. Commun. 2020, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Dudbridge, F.; Thompson, S.G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 2016, 35, 1880–1906. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 2016, 37, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Von Hinke Kessler Scholder, S.; Smith, G.D.; Lawlor, D.A.; Propper, C.; Windmeijer, F. Mendelian randomization: The use of genes in instrumental variable analyses. Health Econ. 2011, 20, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Pingault, J.B.; O’Reilly, P.F.; Schoeler, T.; Ploubidis, G.B.; Rijsdijk, F.; Dudbridge, F. Using genetic data to strengthen causal inference in observational research. Nat. Rev. Genet. 2018, 19, 566–580. [Google Scholar] [CrossRef]
- Van Kippersluis, H.; Rietveld, C.A. Pleiotropy-robust Mendelian randomization. Int. J. Epidemiol. 2018, 47, 1279–1288. [Google Scholar] [CrossRef]
- Rees, J.M.B.; Wood, A.M.; Dudbridge, F.; Burgess, S. Robust methods in Mendelian randomization via penalization of heterogeneous causal estimates. PLoS ONE 2019, 14, e0222362. [Google Scholar] [CrossRef] [PubMed]
- Feskens, E.J.; Sluik, D.; van Woudenbergh, G.J. Meat consumption, diabetes, and its complications. Curr. Diabetes Rep. 2013, 13, 298–306. [Google Scholar] [CrossRef]
- Laskowski, W.; Górska-Warsewicz, H.; Kulykovets, O. Meat, Meat Products and Seafood as Sources of Energy and Nutrients in the Average Polish Diet. Nutrients 2018, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.M.; Mir, S.; Alqahtani, S.A.; Younossi, Y.; Ong, J.P.; Younossi, Z.M. Dietary Risks for Liver Mortality in NAFLD: Global Burden of Disease Data. Hepatol. Commun. 2022, 6, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ma, S.; Farooq, U.; Niu, J.; Li, F.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; Shi, Y. Pasture intake protects against commercial diet-induced lipopolysaccharide production facilitated by gut microbiota through activating intestinal alkaline phosphatase enzyme in meat geese. Front. Immunol. 2022, 13, 1041070. [Google Scholar] [CrossRef] [PubMed]
- Neuffer, J.; González-Domínguez, R.; Lefèvre-Arbogast, S.; Low, D.Y.; Driollet, B.; Helmer, C.; Du Preez, A.; de Lucia, C.; Ruigrok, S.R.; Altendorfer, B.; et al. Exploration of the Gut-Brain Axis through Metabolomics Identifies Serum Propionic Acid Associated with Higher Cognitive Decline in Older Persons. Nutrients 2022, 14, 4688. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.; Lew, L.C.; Hor, Y.Y.; Liong, M.T. Probiotics: The Next Dietary Strategy against Brain Aging. Prev. Nutr. Food Sci. 2022, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yaskolka Meir, A.; Keller, M.; Hoffmann, A.; Rinott, E.; Tsaban, G.; Kaplan, A.; Zelicha, H.; Hagemann, T.; Ceglarek, U.; Isermann, B.; et al. The effect of polyphenols on DNA methylation-assessed biological age attenuation: The DIRECT PLUS randomized controlled trial. BMC Med. 2023, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ortega, E.F.; Meydani, S.N.; Adkins, Y.; Stephensen, C.B.; Thompson, B.; Zwickey, H. Nutrition, Immunosenescence, and Infectious Disease: An Overview of the Scientific Evidence on Micronutrients and on Modulation of the Gut Microbiota. Adv. Nutr. 2022, 13, S1–S26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, W.; Hu, X.; Yu, X.; Guo, C.; Jin, X.; Wang, W. Targeting the blood-brain barrier to delay aging-accompanied neurological diseases by modulating gut microbiota, circadian rhythms, and their interplays. Acta Pharm. Sin. B 2023, 13, 4667–4687. [Google Scholar] [CrossRef] [PubMed]
- Warman, D.J.; Jia, H.; Kato, H. The Potential Roles of Probiotics, Resistant Starch, and Resistant Proteins in Ameliorating Inflammation during Aging (Inflammaging). Nutrients 2022, 14, 747. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Pan, J.; Guo, M.; Duan, H.; Zhang, H.; Narbad, A.; Zhai, Q.; Tian, F.; Chen, W. Gut microbiota and anti-aging: Focusing on spermidine. Crit. Rev. Food Sci. Nutr. 2023, 1–19, Online ahead of print. [Google Scholar] [CrossRef]
- Zeng, X.; Li, X.; Li, X.; Wei, C.; Shi, C.; Hu, K.; Kong, D.; Luo, Q.; Xu, Y.; Shan, W.; et al. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation. Blood 2023, 141, 1691–1707. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, D.Y.; Kang, J.H.; Kim, J.H.; Jeong, J.W.; Kim, H.W.; Oh, D.H.; Yoon, S.H.; Hur, S.J. Relationship between gut microbiota and colorectal cancer: Probiotics as a potential strategy for prevention. Food Res. Int. 2022, 156, 111327. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, H.; Tian, L.; Shi, C.; Zheng, Y.; Wang, J.; Tan, Y.; Luo, Y.; Hong, H. Gut microbiota and metabolic profile as affected by Maillard reaction products derived from bighead carp meat hydrolysates with galactose and galacto-oligosaccharides during in vitro pig fecal fermentation. Food Chem. 2023, 398, 133905. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Wang, H.; Wei, X.; Lu, M.; Yang, W.; Aalim, H.; Capanoglu, E.; Zou, X.; Battino, M.; Zhang, D. Cooking-Induced Oxidation and Structural Changes in Chicken Protein: Their Impact on In Vitro Gastrointestinal Digestion and Intestinal Flora Fermentation Characteristics. Foods 2023, 12, 4322. [Google Scholar] [CrossRef] [PubMed]
- De La Pomélie, D.; Santé-Lhoutellier, V.; Gatellier, P. Mechanisms and kinetics of heme iron nitrosylation in an in vitro gastro-intestinal model. Food Chem. 2018, 239, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Jia, J.; Peng, H.; Qian, Q.; Pan, Z.; Liu, D. Nitrite and nitrate in meat processing: Functions and alternatives. Curr. Res. Food Sci. 2023, 6, 100470. [Google Scholar] [CrossRef] [PubMed]
- James, K.L.; Gertz, E.R.; Cervantes, E.; Bonnel, E.L.; Stephensen, C.B.; Kable, M.E.; Bennett, B.J. Diet, Fecal Microbiome, and Trimethylamine N-Oxide in a Cohort of Metabolically Healthy United States Adults. Nutrients 2022, 14, 1376. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.C.; Hullar, M.A.J.; Randolph, T.W.; Franke, A.A.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Shepherd, J.A.; Madeleine, M.M.; Le Marchand, L.; et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 2020, 111, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.B.; Nolin, T.D.; et al. Serum Trimethylamine-N-Oxide is Elevated in CKD and Correlates with Coronary Atherosclerosis Burden. J. Am. Soc. Nephrol. JASN 2016, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Telomere length and chronic kidney disease: Cause or consequence? Kidney Int. 2021, 100, 980–983. [Google Scholar] [CrossRef]
- Hernández-Martín, M.; Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Bocanegra, A.; Redondo-Castillejo, R.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Silicon-enriched meat positively improves plasma lipidaemia and lipoproteinaemia, LDLr, and insulin capability and the signalling pathway induced by an atherogenic diet in late-stage type 2 diabetes mellitus rats. Food Funct. 2024, 15, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.M.; Toomey, S.; McBride, R.; McMonagle, J.; Morine, M.J.; Belton, O.; Moloney, A.P.; Roche, H.M. Divergent effects of a CLA-enriched beef diet on metabolic health in ApoE-/- and ob/ob mice. J. Nutr. Biochem. 2013, 24, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D. Red meat and type 2 diabetes mellitus. JAMA Intern. Med. 2014, 174, 646. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D. Red meat and type 2 diabetes: Do methodological advancements sufficiently increase the certainty of evidence? Am. J. Clin. Nutr. 2023, 118, 1075–1076. [Google Scholar] [CrossRef]
- Lescinsky, H.; Afshin, A.; Ashbaugh, C.; Bisignano, C.; Brauer, M.; Ferrara, G.; Hay, S.I.; He, J.; Iannucci, V.; Marczak, L.B.; et al. Health effects associated with consumption of unprocessed red meat: A Burden of Proof study. Nat. Med. 2022, 28, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Abdi, S.; Sabico, S.; Alnaami, A.M.; Wani, K.A.; Ansari, M.G.A.; Khattak, M.N.K.; Khan, N.; Tripathi, G.; Chrousos, G.P.; et al. Gut-Derived Endotoxin and Telomere Length Attrition in Adults with and without Type 2 Diabetes. Biomolecules 2021, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Demircan, K.; Hybsier, S.; Chillon, T.S.; Vetter, V.M.; Rijntjes, E.; Demuth, I.; Schomburg, L. Sex-specific associations of serum selenium and selenoprotein P with type 2 diabetes mellitus and hypertension in the Berlin Aging Study II. Redox Biol. 2023, 65, 102823. [Google Scholar] [CrossRef] [PubMed]
- Induri, S.N.R.; Kansara, P.; Thomas, S.C.; Xu, F.; Saxena, D.; Li, X. The Gut Microbiome, Metformin, and Aging. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yue, R. Aging adipose tissue, insulin resistance, and type 2 diabetes. Biogerontology 2024, 25, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giovinazzo, S.; Barbalace, M.C.; Cristani, M.; Alibrandi, A.; Vicchio, T.M.; Giuffrida, G.; Aguennouz, M.H.; Malaguti, M.; Angeloni, C.; et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid Off. J. Am. Thyroid Assoc. 2021, 31, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Fedacko, J.; Pella, D.; Fatima, G.; Elkilany, G.; Moshiri, M.; Hristova, K.; Jakabcin, P.; Vaňova, N. High Exogenous Antioxidant, Restorative Treatment (Heart) for Prevention of the Six Stages of Heart Failure: The Heart Diet. Antioxidants 2022, 11, 1464. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.; Boonekamp, J. Does oxidative stress shorten telomeres in vivo? A meta-analysis. Ageing Res. Rev. 2023, 85, 101854. [Google Scholar] [CrossRef] [PubMed]
- De Vos-Houben, J.M.; Ottenheim, N.R.; Kafatos, A.; Buijsse, B.; Hageman, G.J.; Kromhout, D.; Giltay, E.J. Telomere length, oxidative stress, and antioxidant status in elderly men in Zutphen and Crete. Mech. Ageing Dev. 2012, 133, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Jitjumnong, M.; Chalermkitpanit, P.; Suantawee, T.; Dechsupa, S.; Vajarintarangoon, L.; Honsawek, S. Telomere Shortening and Increased Oxidative Stress in Lumbar Disc Degeneration. Int. J. Mol. Sci. 2022, 23, 10125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, L.; Cui, X.; Feng, L.; Zhao, X.; He, S.; Ping, F.; Li, W.; Li, Y. Influence of diet on leukocyte telomere length, markers of inflammation and oxidative stress in individuals with varied glucose tolerance: A Chinese population study. Nutr. J. 2016, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Testa, H.; Greenwood, T.; Kostek, M.; Haushalter, K.; Kris-Etherton, P.M.; Petersen, K.S. The effect of herbs and spices on risk factors for cardiometabolic diseases: A review of human clinical trials. Nutr. Rev. 2022, 80, 400–427. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Nagayach, A.; Lu, Y.; Peng, H.; Duong, Q.A.; Pham, N.B.; Vuong, C.A.; Bazan, N.G. A high fat, sugar, and salt Western diet induces motor-muscular and sensory dysfunctions and neurodegeneration in mice during aging: Ameliorative action of metformin. CNS Neurosci. Ther. 2021, 27, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Lastuvkova, H.; Faradonbeh, F.A.; Schreiberova, J.; Hroch, M.; Mokry, J.; Faistova, H.; Nova, Z.; Hyspler, R.; Igreja Sa, I.C.; Nachtigal, P.; et al. Atorvastatin Modulates Bile Acid Homeostasis in Mice with Diet-Induced Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2021, 22, 6468. [Google Scholar] [CrossRef] [PubMed]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 2014, 2, 4172. [Google Scholar] [CrossRef] [PubMed]
- Reljic, D.; Koller, A.; Herrmann, H.J.; Ekici, A.B.; Neurath, M.F.; Zopf, Y. Differential Effects of Very-Low-Volume Exercise Modalities on Telomere Length, Inflammation, and Cardiometabolic Health in Obese Metabolic Syndrome Patients: A Subanalysis from Two Randomized Controlled Trials. Antioxidants 2023, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Charlebois, E.; Pantopoulos, K. Nutritional Aspects of Iron in Health and Disease. Nutrients 2023, 15, 2441. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.C.; Saadoun, A. An overview of the nutritional value of beef and lamb meat from South America. Meat Sci. 2014, 98, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Sandanger, T.M.; Brustad, M. Selected vitamins and essential elements in meat from semi-domesticated reindeer (Rangifer tarandus tarandus L.) in mid- and northern Norway: Geographical variations and effect of animal population density. Nutrients 2012, 4, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Vahid, F.; Hatami, M.; Sadeghi, M.; Ameri, F.; Faghfoori, Z.; Davoodi, S.H. The association between the Index of Nutritional Quality (INQ) and breast cancer and the evaluation of nutrient intake of breast cancer patients: A case-control study. Nutrition 2018, 45, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, Y.; Fu, Z.; Liao, J.; Liu, H.; Zhou, M. Exploring the Causal Effects of Mineral Metabolism Disorders on Telomere and Mitochondrial DNA: A Bidirectional Two-Sample Mendelian Randomization Analysis. Nutrients 2024, 16, 1417. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Yin, Y.; Han, M.; Guo, L.; Duan, Y.; Guo, Q.; Yin, J.; Li, F. Dietary leucine and fish oil cooperatively regulate skeletal myofiber type transformation via the CaMKII signaling pathway of pigs. Food Funct. 2023, 14, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Kyrou, I.; Tan, B.K.; Dimitriadis, G.K.; Ramanjaneya, M.; Tripathi, G.; Patel, V.; James, S.; Kawan, M.; Chen, J.; et al. Short-Chain Fatty Acid Acetate Stimulates Adipogenesis and Mitochondrial Biogenesis via GPR43 in Brown Adipocytes. Endocrinology 2016, 157, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Mancusso, R.; Gregorio, G.G.; Liu, Q.; Wang, D.N. Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter. Nature 2012, 491, 622–626. [Google Scholar] [CrossRef]
- Polak-Szczybyło, E.; Tabarkiewicz, J. IL-17A, IL-17E and IL-17F as Potential Biomarkers for the Intensity of Low-Grade Inflammation and the Risk of Cardiovascular Diseases in Obese People. Nutrients 2022, 14, 643. [Google Scholar] [CrossRef]



| Directions and Methods | Nsnp | B | SE | p-Value |
|---|---|---|---|---|
| Meat consumers to telomere length | ||||
| MR-Egger | 22 | 0.08 | 0.07 | 0.28 |
| Weighted median | 22 | −0.02 | 0.05 | 0.67 |
| Inverse-variance weighted | 22 | −0.03 | 0.04 | 0.36 |
| Simple mode | 22 | −0.02 | 0.11 | 0.85 |
| Weighted mode | 22 | −0.03 | 0.10 | 0.79 |
| Meat consumers to mitochondrial DNA copy number | ||||
| MR-Egger | 22 | 0.03 | 0.07 | 0.66 |
| Weighted median | 22 | 0.02 | 0.05 | 0.63 |
| Inverse-variance weighted | 22 | −0.02 | 0.04 | 0.63 |
| Simple mode | 22 | 0.05 | 0.10 | 0.63 |
| Weighted mode | 22 | 0.05 | 0.10 | 0.61 |
| Meat consumers to DNA methylation Hannum age acceleration | ||||
| MR-Egger | 23 | −0.33 | 1.33 | 0.81 |
| Weighted median | 23 | 0.24 | 0.82 | 0.77 |
| Inverse-variance weighted | 23 | 0.23 | 0.58 | 0.69 |
| Simple mode | 23 | 0.41 | 1.67 | 0.81 |
| Weighted mode | 23 | 0.41 | 1.62 | 0.80 |
| Meat consumers to DNA methylation PhenoAge acceleration | ||||
| MR-Egger | 23 | 1.10 | 1.79 | 0.55 |
| Weighted median | 23 | 2.20 | 1.07 | 0.04 |
| Inverse-variance weighted | 23 | 1.73 | 0.77 | 0.02 * |
| Simple mode | 23 | 2.12 | 2.12 | 0.33 |
| Weighted mode | 23 | 2.25 | 1.93 | 0.26 |
| Outcomes | Pleiotropy | Heterogeneity | ||||
|---|---|---|---|---|---|---|
| Intercept * | SE | p-Value | Q Test | Q_df | p-Value | |
| Telomere length | −0.003 | 0.001 | 0.083 | 22.953 | 21 | 0.347 |
| mtDNA copy number | −0.001 | 0.001 | 0.431 | 14.716 | 21 | 0.837 |
| DNAm Hannum age acceleration | 0.011 | 0.022 | 0.644 | 17.088 | 22 | 0.759 |
| DNAm PhenoAge acceleration | 0.012 | 0.030 | 0.701 | 17.166 | 22 | 0.754 |
| Directions and Methods | Nsnp | B | SE | p-Value |
|---|---|---|---|---|
| Processed meat consumers to telomere length | ||||
| MR-Egger | 71 | −0.33 | 0.37 | 0.37 |
| Weighted median | 71 | −0.19 | 0.12 | 0.12 |
| Inverse-variance weighted | 71 | −0.19 | 0.09 | 0.03 * |
| Simple mode | 71 | −0.35 | 0.33 | 0.30 |
| Weighted mode | 71 | −0.33 | 0.31 | 0.28 |
| Processed meat consumers to mitochondrial DNA copy number | ||||
| MR-Egger | 76 | −0.11 | 0.40 | 0.78 |
| Weighted median | 76 | −0.15 | 0.13 | 0.26 |
| Inverse-variance weighted | 76 | 0.01 | 0.09 | 0.89 |
| Simple mode | 76 | −0.20 | 0.35 | 0.56 |
| Weighted mode | 76 | −0.21 | 0.34 | 0.54 |
| Processed meat consumers to DNA methylation Hannum age acceleration | ||||
| MR-Egger | 67 | −0.63 | 7.63 | 0.94 |
| Weighted median | 67 | 1.44 | 1.88 | 0.44 |
| Inverse-variance weighted | 67 | 1.41 | 1.38 | 0.31 |
| Simple mode | 67 | −4.23 | 4.79 | 0.38 |
| Weighted mode | 67 | −3.91 | 4.48 | 0.39 |
| Processed meat consumers to DNA methylation PhenoAge acceleration | ||||
| MR-Egger | 64 | 1.25 | 9.43 | 0.89 |
| Weighted median | 64 | 2.32 | 2.46 | 0.35 |
| Inverse-variance weighted | 64 | 0.97 | 1.83 | 0.59 |
| Simple mode | 64 | 4.78 | 5.99 | 0.43 |
| Weighted mode | 64 | 5.75 | 6.05 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Deng, Y.; Liu, H.; Fu, Z.; Wang, Y.; Zhou, M.; Feng, Z. Causal Relationship between Meat Intake and Biological Aging: Evidence from Mendelian Randomization Analysis. Nutrients 2024, 16, 2433. https://doi.org/10.3390/nu16152433
Liu S, Deng Y, Liu H, Fu Z, Wang Y, Zhou M, Feng Z. Causal Relationship between Meat Intake and Biological Aging: Evidence from Mendelian Randomization Analysis. Nutrients. 2024; 16(15):2433. https://doi.org/10.3390/nu16152433
Chicago/Turabian StyleLiu, Shupeng, Yinyun Deng, Hui Liu, Zhengzheng Fu, Yinghui Wang, Meijuan Zhou, and Zhijun Feng. 2024. "Causal Relationship between Meat Intake and Biological Aging: Evidence from Mendelian Randomization Analysis" Nutrients 16, no. 15: 2433. https://doi.org/10.3390/nu16152433
APA StyleLiu, S., Deng, Y., Liu, H., Fu, Z., Wang, Y., Zhou, M., & Feng, Z. (2024). Causal Relationship between Meat Intake and Biological Aging: Evidence from Mendelian Randomization Analysis. Nutrients, 16(15), 2433. https://doi.org/10.3390/nu16152433







