Carbohydrate Intake Levels and the Risk of Metabolic Syndrome in Korean Populations: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
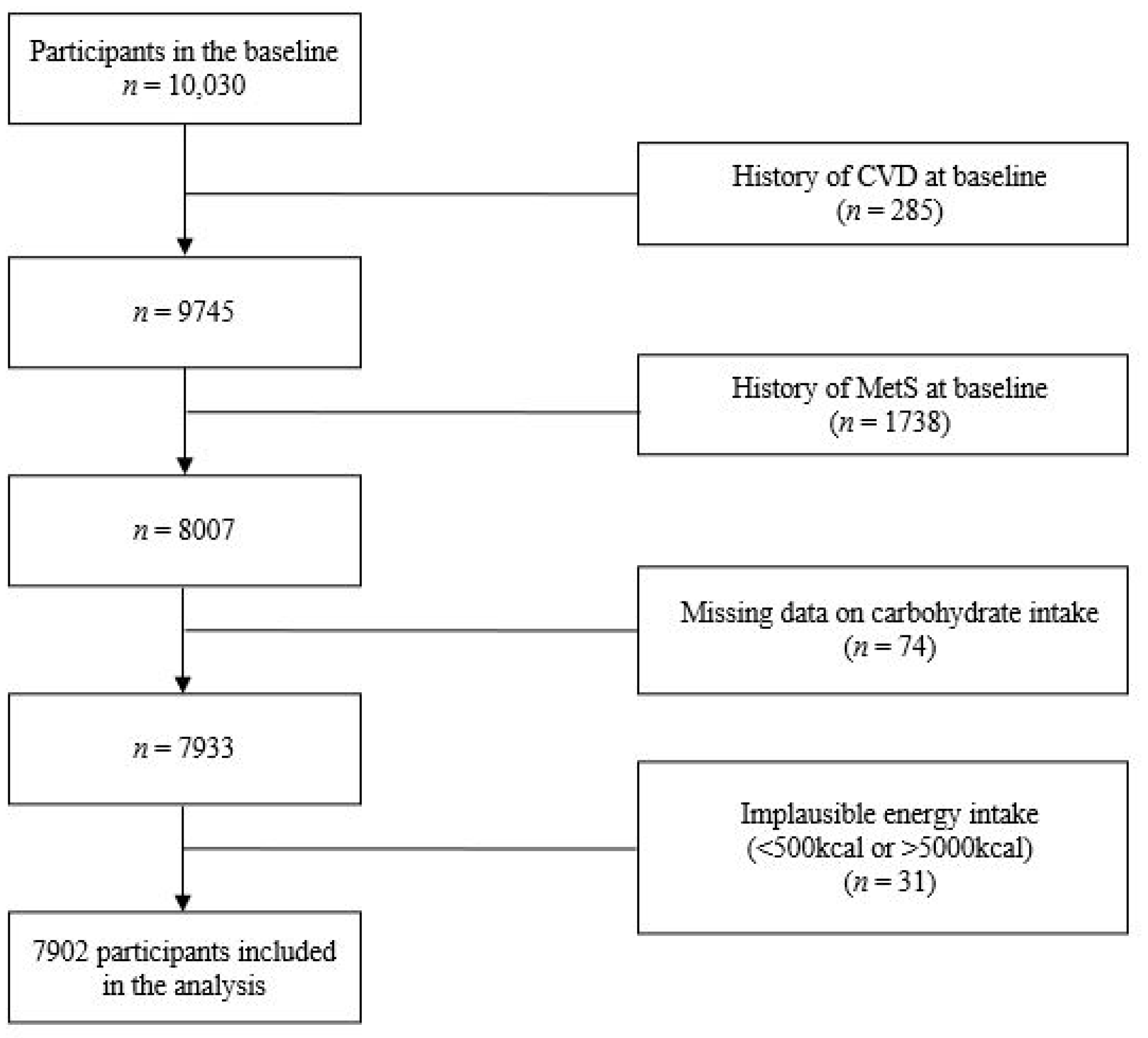
2.1. Data Source and Participants
2.2. Demographic and Lifestyle Information
2.3. Dietary Information
2.4. Blood Sample Collection and Analysis
2.5. Definition of MetS and Its Components
- -
- Hypertriglyceridemia: Triglyceride levels ≥ 200 mg/dL.
- -
- Hyperglycemia: Fasting glucose ≥ 100 mg/dL or use of diabetes medication/insulin
- -
- High Blood Pressure: Systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg, or use of antihypertensive medication
- -
- Abdominal Obesity: Waist circumference > 90 cm in men and > 85 cm in women
- -
- Hypo-high density lipoprotein (HDL) Cholesterolemia: HDL cholesterol < 40 mg/dL
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of the Participants According to P_CARB
3.2. Association between P_CARB and MetS
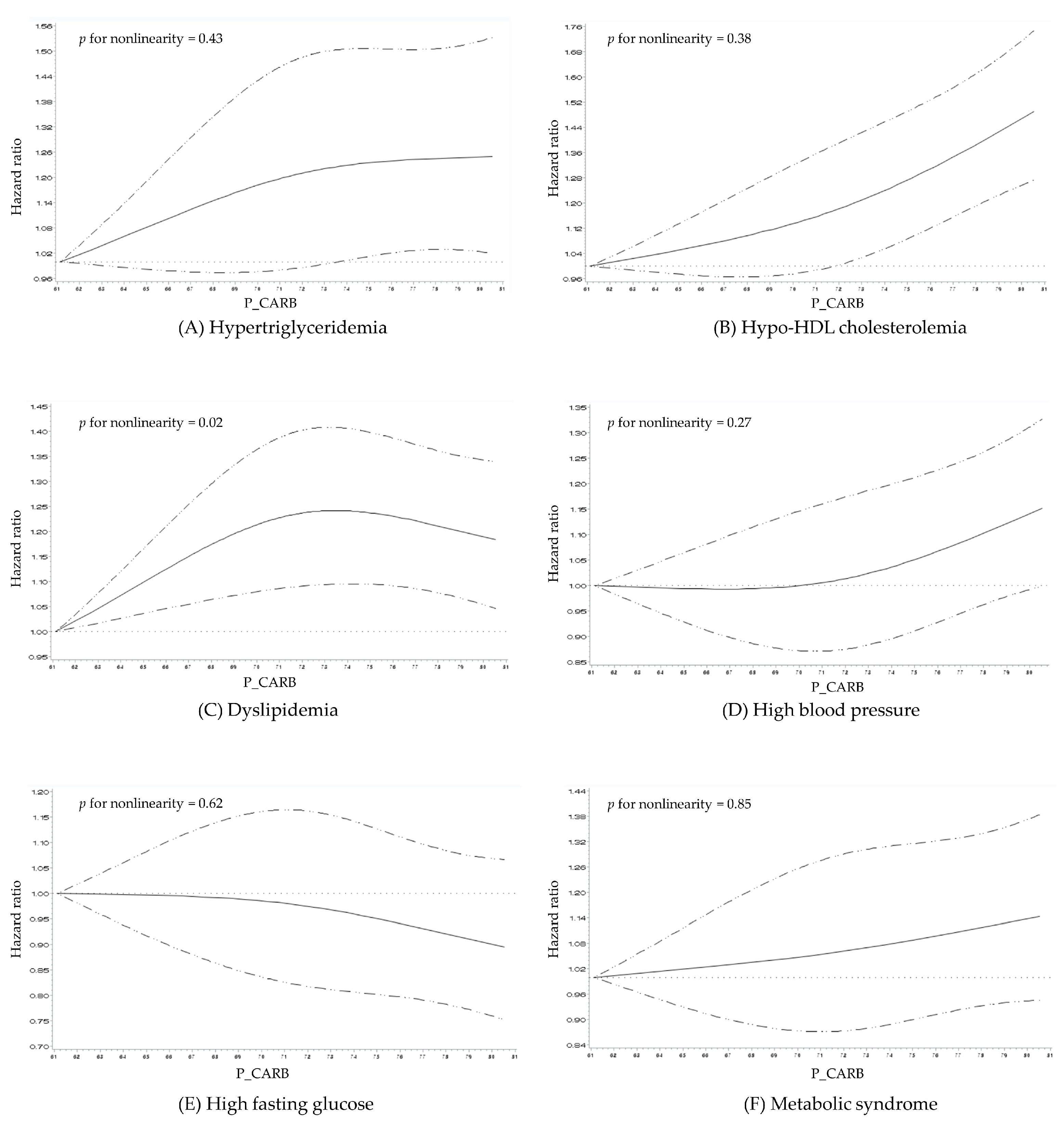
3.3. Dose–Response Relationship between Proportion of Total Energy from Carbohydrate Intake and the Risk of MetS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huh, J.H.; Kang, D.R.; Kim, J.Y.; Koh, K.K. Metabolic Syndrome Fact Sheet 2021: Executive Report. CardioMetabolic Syndr. J. 2021, 1, 125–134. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.S.; Lee, M.S.; Park, Y.S.; Lee, H.J.; Kang, S.-a.; Lee, H.S.; Lee, K.-E.; Yang, H.J.; Kim, M.J. Korean diet: Characteristics and historical background. J. Ethn. Foods 2016, 3, 26–31. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. 2020 Dietary Reference Intakes for Koreans. Available online: https://www.kns.or.kr/fileroom/FileRoom_view.asp?mode=mod&restring=%252FFileRoom%252FFileRoom%252Easp%253Fxsearch%253D1%253D%253Dcn%255Fsearch%253D2020%253D%253Dxrow%253D10%253D%253DBoardID%253DKdr%253D%253Dpage%253D2&idx=108&page=2&BoardID=Kdr&xsearch=1&cn_search=2020 (accessed on 9 May 2023).
- Soh, S.M.; Chung, S.-J.; Yoon, J. Dietary and health characteristics of Korean adults according to the level of energy intake from carbohydrate: Analysis of the 7th (2016–2017) Korea National Health and Nutrition Examination Survey Data. Nutrients 2020, 12, 429. [Google Scholar] [CrossRef]
- Song, S.; Song, Y. Three types of a high-carbohydrate diet are differently associated with cardiometabolic risk factors in Korean adults. Eur. J. Nutr. 2019, 58, 3279–3289. [Google Scholar] [CrossRef]
- Park, H.; Kityo, A.; Kim, Y.; Lee, S.A. Macronutrient Intake in Adults Diagnosed with Metabolic Syndrome: Using the Health Examinee (HEXA) Cohort. Nutrients 2021, 13, 4457. [Google Scholar] [CrossRef]
- Moon, H.; Ha, K.; Song, Y. High fiber and high carbohydrate intake and its association with the metabolic disease using the data of KNHANES 2013~2017. J. Nutr. Health 2019, 52, 540–551. [Google Scholar] [CrossRef]
- Cho, Y.-A.; Choi, J.-H. Association between carbohydrate intake and the prevalence of metabolic syndrome in Korean women. Nutrients 2021, 13, 3098. [Google Scholar] [CrossRef]
- Ha, K.; Kim, K.; Chun, O.K.; Joung, H.; Song, Y. Differential association of dietary carbohydrate intake with metabolic syndrome in the US and Korean adults: Data from the 2007–2012 NHANES and KNHANES. Eur. J. Clin. Nutr. 2018, 72, 848–860. [Google Scholar] [CrossRef]
- Yoo, J.S.; Choe, E.Y.; Kim, Y.M.; Kim, S.H.; Won, Y.J. Predictive costs in medical care for Koreans with metabolic syndrome from 2009 to 2013 based on the National Health Insurance claims dataset. Korean J. Intern. Med. 2020, 35, 936–945. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. Guidelines for Food Frequency Questionnaire of the Korean Genome and Epidemiology Study. Available online: https://nih.go.kr/contents.es?mid=a40504100200 (accessed on 29 April 2023).
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R., Jr.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S., Jr. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. Manual of Method of Anthropometry Measurements and Examination Investigation. Available online: https://nih.go.kr/contents.es?mid=a40504100200 (accessed on 2 November 2023).
- Ahn, Y.; Kwon, E.; Shim, J.; Park, M.; Joo, Y.; Kimm, K.; Park, C.; Kim, D. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Ahn, Y.-O.; Paik, H.-Y.; Aim, Y.; Tokudome, Y.; Hamajima, N.; Inouc, M.; Tajima, K.; Ahn, Y. Development of a food frequency questionnaire in Koreans. Asia Pac. J. Clin. Nutr. 2003, 12, 243–250. [Google Scholar]
- Korea Disease Control and Prevention Agency. Guideline to the Use of Total Data for Korean Genome and Epidemiology. Available online: https://nih.go.kr/contents.es?mid=a40504100300 (accessed on 29 April 2023).
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- Korean Society of Cardiometabolic Syndrome. Metabolic Syndrome Treatment Guidelines 2021; Korean Society of Cardiometabolic Syndrome: Seoul, Republic of Korea, 2021. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Shan, Z.; Rehm, C.D.; Rogers, G.; Ruan, M.; Wang, D.D.; Hu, F.B.; Mozaffarian, D.; Zhang, F.F.; Bhupathiraju, S.N. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999–2016. JAMA 2019, 322, 1178–1187. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Z.; Liu, M.; Zhang, Y.; Li, H.; He, P.; Li, Q.; Liu, C.; Qin, X. Dietary carbohydrate intake and new-onset diabetes: A nationwide cohort study in China. Metabolism 2021, 123, 154865. [Google Scholar] [CrossRef]
- Saito, A.; Imai, S.; Htun, N.C.; Okada, E.; Yoshita, K.; Yoshiike, N.; Takimoto, H. The trends in total energy, macronutrients and sodium intake among Japanese: Findings from the 1995–2016 National Health and Nutrition Survey. Br. J. Nutr. 2018, 120, 424–434. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Lee, H.S.; Park, J.-Y.; Lee, J.-W. Associating intake proportion of carbohydrate, fat, and protein with all-cause mortality in Korean adults. Nutrients 2020, 12, 3208. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Wu, Q.-J.; Xia, Y.; Zhang, J.-Y.; Jiang, Y.-T.; Chang, Q.; Zhao, Y.-H. Carbohydrate intake and risk of metabolic syndrome: A dose–response meta-analysis of observational studies. Nutr. Metab. 2019, 29, 1288–1298. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.E.; Rebello, T. Carbohydrates and blood pressure. Ann. Intern. Med. 1983, 98, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R.; Standley, P.R.; Ram, J.L.; Zemel, M.B.; Resnick, L.M. Insulin Resistance, Carbohydrate Metabolism, and Hypertension. Am. J. Hypertens. 1991, 4, 466S–472S. [Google Scholar] [CrossRef]
- Gambardella, S.; Frontoni, S.; Pellegrinotti, M.; Testa, G.; Spallone, V.; Menzinger, G. Carbohydrate Metabolism in Hypertension: Influence of Treatment. J. Cardiovasc. Pharmacol. 1993, 22, 87–97. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Kashirskikh, D.A.; Sukhorukov, V.N.; Kalmykov, V.; Omelchenko, A.V.; Orekhov, A.N. Cholesterol Transport Dysfunction and Its Involvement in Atherogenesis. Int. J. Mol. Sci. 2022, 23, 1332. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J. Effect of dietary carbohydrate on triglyceride metabolism in humans. J. Nutr. 2001, 131, 2772S–2774S. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J.; Hellerstein, M.K. Carbohydrate-induced hypertriacylglycerolemia: Historical perspective and review of biological mechanisms. Am. J. Clin. Nutr. 2000, 71, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, itc81–itc96. [Google Scholar] [CrossRef]
- Chan, Q.; Stamler, J.; Griep, L.M.; Daviglus, M.L.; Horn, L.V.; Elliott, P. An Update on Nutrients and Blood Pressure. J. Atheroscler. Thromb. 2016, 23, 276–289. [Google Scholar] [CrossRef]
- Ascherio, A.; Hennekens, C.; Willett, W.C.; Sacks, F.; Rosner, B.; Manson, J.; Witteman, J.; Stampfer, M.J. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension 1996, 27, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Okuda, N.; Okamura, T.; Kadota, A.; Miyagawa, N.; Hayakawa, T.; Kita, Y.; Fujiyoshi, A.; Nagai, M.; Takashima, N.; et al. Low-carbohydrate diets and cardiovascular and total mortality in Japanese: A 29-year follow-up of NIPPON DATA80. Br. J. Nutr. 2014, 112, 916–924. [Google Scholar] [CrossRef] [PubMed]
| Quartile of P_CARB | p Value 1 | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| No. of participants | 1975 | 1976 | 1976 | 1975 | |
| P_CARB (%) | 63.57 ± 0.05 | 69.95 ± 0.05 | 73.79 ± 0.05 | 78.74 ± 0.05 | |
| Sex | <0.001 | ||||
| Men | 1081 (54.73) | 1031 (52.18) | 886 (44.84) | 658 (33.32) | |
| Women | 894 (45.27) | 945 (47.82) | 1090 (55.16) | 1317 (66.68) | |
| Age | <0.001 | ||||
| 40–49 | 1300 (65.82) | 1118 (56.58) | 951 (48.13) | 616 (31.19) | |
| 50–59 | 420 (21.27) | 497 (25.15) | 519 (26.27) | 545 (27.59) | |
| ≥60 | 255 (12.91) | 361 (18.27) | 506 (25.60) | 814 (41.22) | |
| Household income (KRW) 2 | <0.001 | ||||
| Low or mid-low | 657 (33.62) | 770 (39.43) | 1008 (51.91) | 1404 (72.82) | |
| Mid-high or high | 1297 (66.38) | 1183 (60.57) | 934 (48.09) | 524 (27.18) | |
| Smoking status | <0.001 | ||||
| Smokers | 631 (32.41) | 543 (27.69) | 444 (22.65) | 369 (19.02) | |
| Non-smokers | 1316 (67.59) | 1418 (72.31) | 1516 (77.35) | 1571 (80.98) | |
| Alcohol consumption | <0.001 | ||||
| Drinkers | 1163 (59.16) | 1050 (53.46) | 918 (46.72) | 631 (32.31) | |
| Non-drinkers | 803 (40.84) | 914 (46.54) | 1047 (53.28) | 1322 (67.69) | |
| Physical activity levels 3 | <0.001 | ||||
| Low | 676 (34.65) | 701 (35.69) | 649 (33.10) | 572 (29.47) | |
| Moderate | 724 (37.11) | 730 (37.17) | 664 (33.86) | 499 (25.71) | |
| High | 551 (28.24) | 533 (27.14) | 648 (33.04) | 870 (44.82) | |
| Total energy intake (kcal/day) | 2129.95 ± 11.23 | 1919.94 ± 11.23 | 1787.22 ± 11.23 | 1640.29 ± 11.23 | <0.001 |
| Body mass index (kg/m2) | 24.08 ± 0.07 | 24.08 ± 0.07 | 24.03 ± 0.07 | 24.04 ± 0.07 | 0.9 |
| Serum total cholesterol (mg/dL) | 192.44 ± 0.79 | 191.30 ± 0.79 | 187.26 ± 0.79 | 187.42 ± 0.79 | <0.001 |
| Serum HDL cholesterol (mg/dL) | 47.05 ± 0.22 | 46.34 ± 0.22 | 46.03 ± 0.22 | 45.72 ± 0.22 | <0.001 |
| Serum triglycerides (mg/dL) | 130.57 ± 1.87 | 129.05 ± 1.87 | 131.37 ± 1.87 | 132.95 ± 1.87 | 0.5 |
| LDL cholesterol (mg/dL) 4 | 119.32 ± 0.73 | 119.15 ± 0.73 | 114.95 ± 0.73 | 115.11 ± 0.73 | <0.001 |
| Quartile of P_CARB | p for Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Hypertriglyceridemia | |||||
| No. of cases (%) | 584 (29.57) | 620 (31.38) | 595 (30.11) | 611 (30.94) | |
| Model 1 | 1 | 1.04 (0.93–1.17) | 0.99 (0.88–1.11) | 1.03 (0.92–1.16) | 0.8 |
| Model 2 | 1 | 1.05 (0.94–1.18) | 1.04 (0.93–1.17) | 1.15 (1.02–1.29) | 0.04 |
| Model 3 | 1 | 1.11 (0.98–1.25) | 1.14 (1.00–1.29) | 1.25 (1.09–1.44) | 0.002 |
| Hypo-HDL cholesterolemia | |||||
| No. of cases (%) | 895 (45.32) | 930 (47.06) | 1021 (51.67) | 1046 (52.96) | |
| Model 1 | 1 | 1.06 (0.97–1.17) | 1.20 (1.09–1.31) | 1.28 (1.17–1.40) | <0.001 |
| Model 2 | 1 | 1.07 (0.98–1.18) | 1.24 (1.13–1.35) | 1.36 (1.24–1.50) | <0.001 |
| Model 3 | 1 | 1.06 (0.97–1.17) | 1.19 (1.07–1.31) | 1.28 (1.15–1.43) | <0.001 |
| Dyslipidemia | |||||
| No. of cases (%) | 1545 (78.23) | 1589 (80.41) | 1610 (81.48) | 1606 (81.32) | |
| Model 1 | 1 | 1.06 (0.99–1.14) | 1.08 (1.01–1.16) | 1.10 (1.03–1.18) | 0.006 |
| Model 2 | 1 | 1.05 (0.98–1.13) | 1.06 (0.99–1.14) | 1.07 (0.99–1.15) | 0.08 |
| Model 3 | 1 | 1.08 (1.01–1.17) | 1.13 (1.05–1.22) | 1.14 (1.04–1.24) | 0.002 |
| High blood pressure | |||||
| No. of cases (%) | 1095 (55.44) | 1115 (56.43) | 1210 (61.23) | 1347 (68.20) | |
| Model 1 | 1 | 1.01 (0.93–1.10) | 1.16 (1.07–1.26) | 1.45 (1.34–1.57) | <0.001 |
| Model 2 | 1 | 0.95 (0.87–1.03) | 1.03 (0.95–1.12) | 1.13 (1.04–1.23) | 0.001 |
| Model 3 | 1 | 0.96 (0.88–1.05) | 1.01 (0.92–1.10) | 1.14 (1.03–1.25) | 0.01 |
| High fasting glucose | |||||
| No. of cases (%) | 818 (41.42) | 827 (41.85) | 786 (39.78) | 810 (41.01) | |
| Model 1 | 1 | 0.99 (0.90–1.09) | 0.90 (0.82–1.00) | 0.94 (0.85–1.04) | 0.1 |
| Model 2 | 1 | 0.99 (0.90–1.09) | 0.90 (0.82–1.00) | 0.94 (0.85–1.04) | 0.1 |
| Model 3 | 1 | 0.96 (0.87–1.06) | 0.92 (0.83–1.03) | 0.92 (0.81–1.03) | 0.1 |
| Metabolic Syndrome | |||||
| No. of cases (%) | 644 (32.61) | 664 (33.60) | 706 (35.73) | 771 (39.04) | |
| Model 1 | 1 | 1.01 (0.91–1.12) | 1.10 (0.99–1.23) | 1.29 (1.16–1.43) | <0.001 |
| Model 2 | 1 | 0.98 (0.88–1.09) | 1.05 (0.94–1.17) | 1.16 (1.04–1.30) | 0.005 |
| Model 3 | 1 | 1.01 (0.90–1.13) | 1.08 (0.96–1.22) | 1.17 (1.02–1.33) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.; Jo, U.; Park, K. Carbohydrate Intake Levels and the Risk of Metabolic Syndrome in Korean Populations: A Prospective Study. Nutrients 2024, 16, 2440. https://doi.org/10.3390/nu16152440
Yoo H, Jo U, Park K. Carbohydrate Intake Levels and the Risk of Metabolic Syndrome in Korean Populations: A Prospective Study. Nutrients. 2024; 16(15):2440. https://doi.org/10.3390/nu16152440
Chicago/Turabian StyleYoo, Hyeonji, Unhui Jo, and Kyong Park. 2024. "Carbohydrate Intake Levels and the Risk of Metabolic Syndrome in Korean Populations: A Prospective Study" Nutrients 16, no. 15: 2440. https://doi.org/10.3390/nu16152440
APA StyleYoo, H., Jo, U., & Park, K. (2024). Carbohydrate Intake Levels and the Risk of Metabolic Syndrome in Korean Populations: A Prospective Study. Nutrients, 16(15), 2440. https://doi.org/10.3390/nu16152440







