Dietary Fiber’s Physicochemical Properties and Gut Bacterial Dysbiosis Determine Fiber Metabolism in the Gut
Abstract
:1. Introduction
2. Materials and Methods
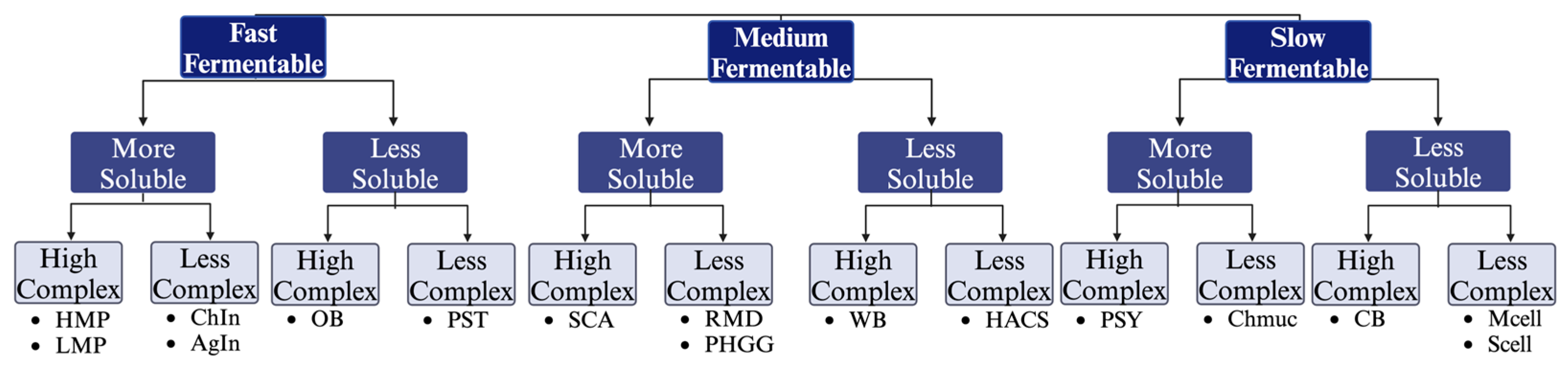
2.1. Experimental Design
2.2. Dietary Fibers
2.3. Solubility Measurements of Dietary Fibers
2.4. In Vitro Upper Gastrointestinal Digestion
2.5. In Vitro Human Fecal Fermentation
2.6. Short-Chain Fatty Acid Quantification
2.7. Bacterial Sequencing
2.8. Statistical Analysis
3. Results
3.1. Dietary Fiber Solubility Measurements
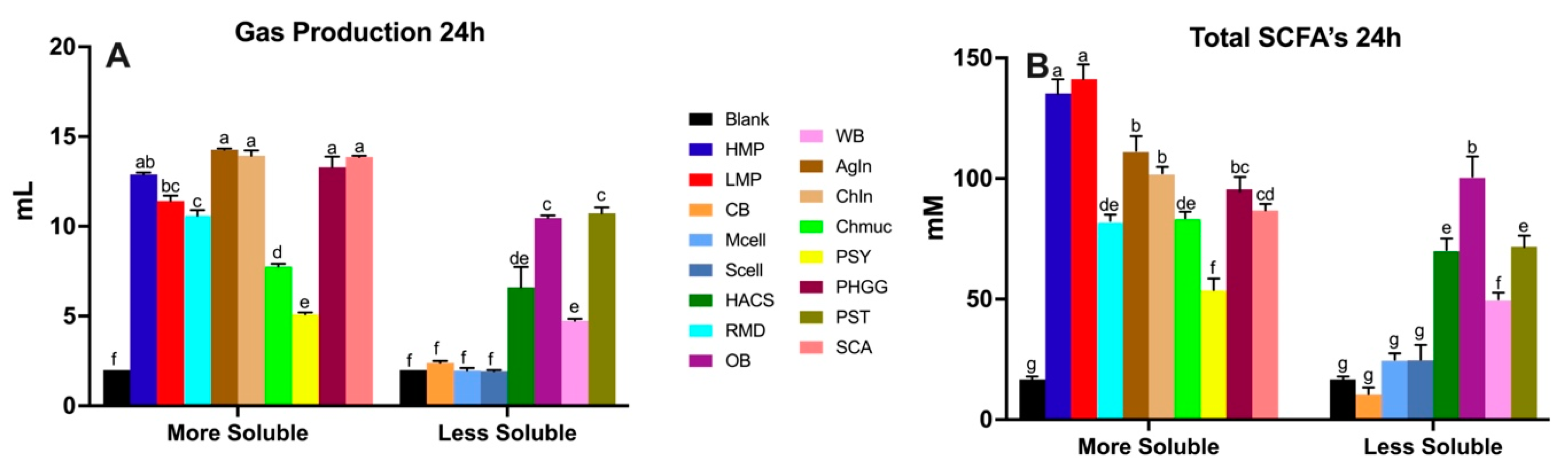
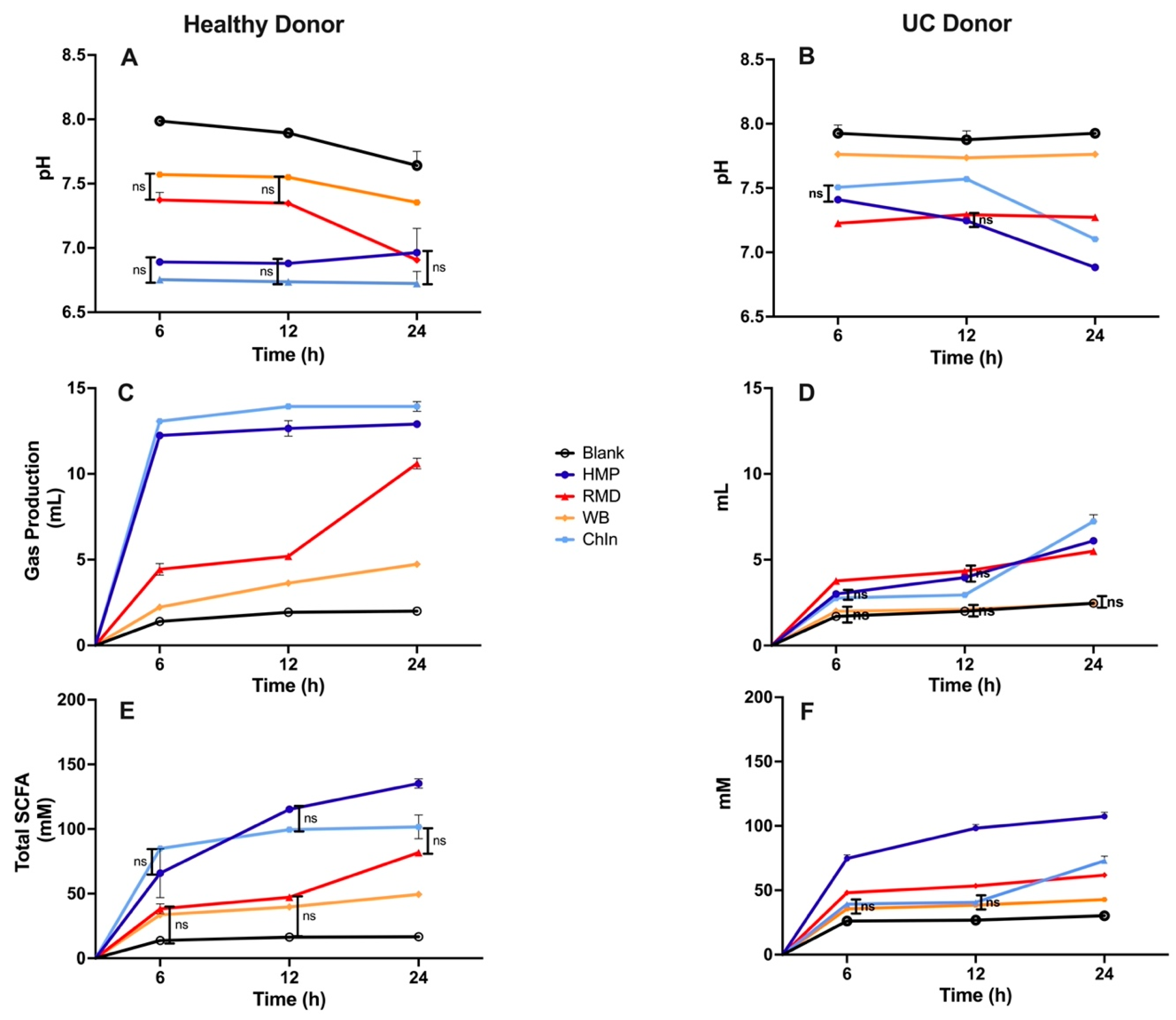
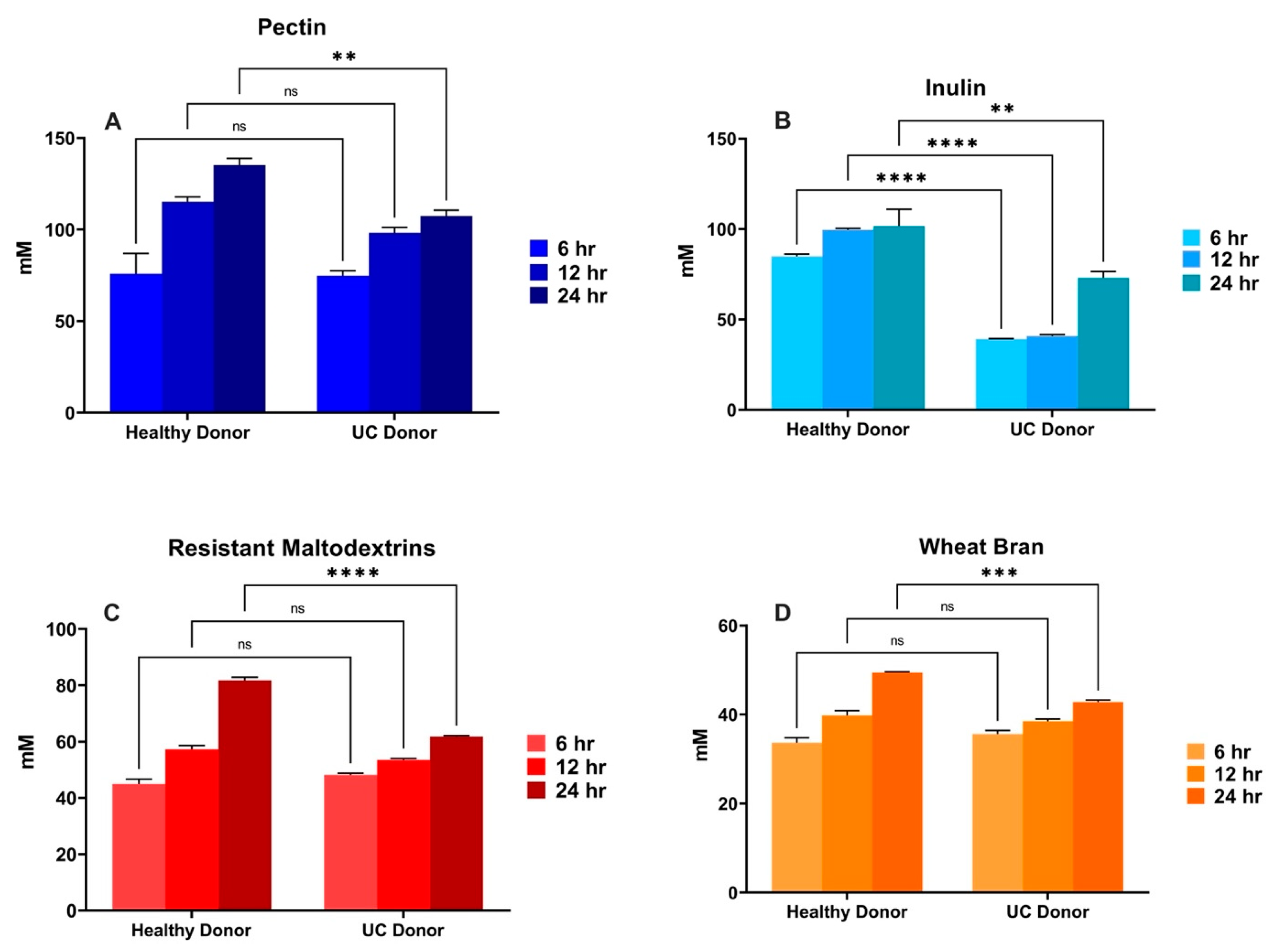
3.2. In Vitro Fermentation of Dietary Fibers Inoculated with a Healthy Human Fecal Sample
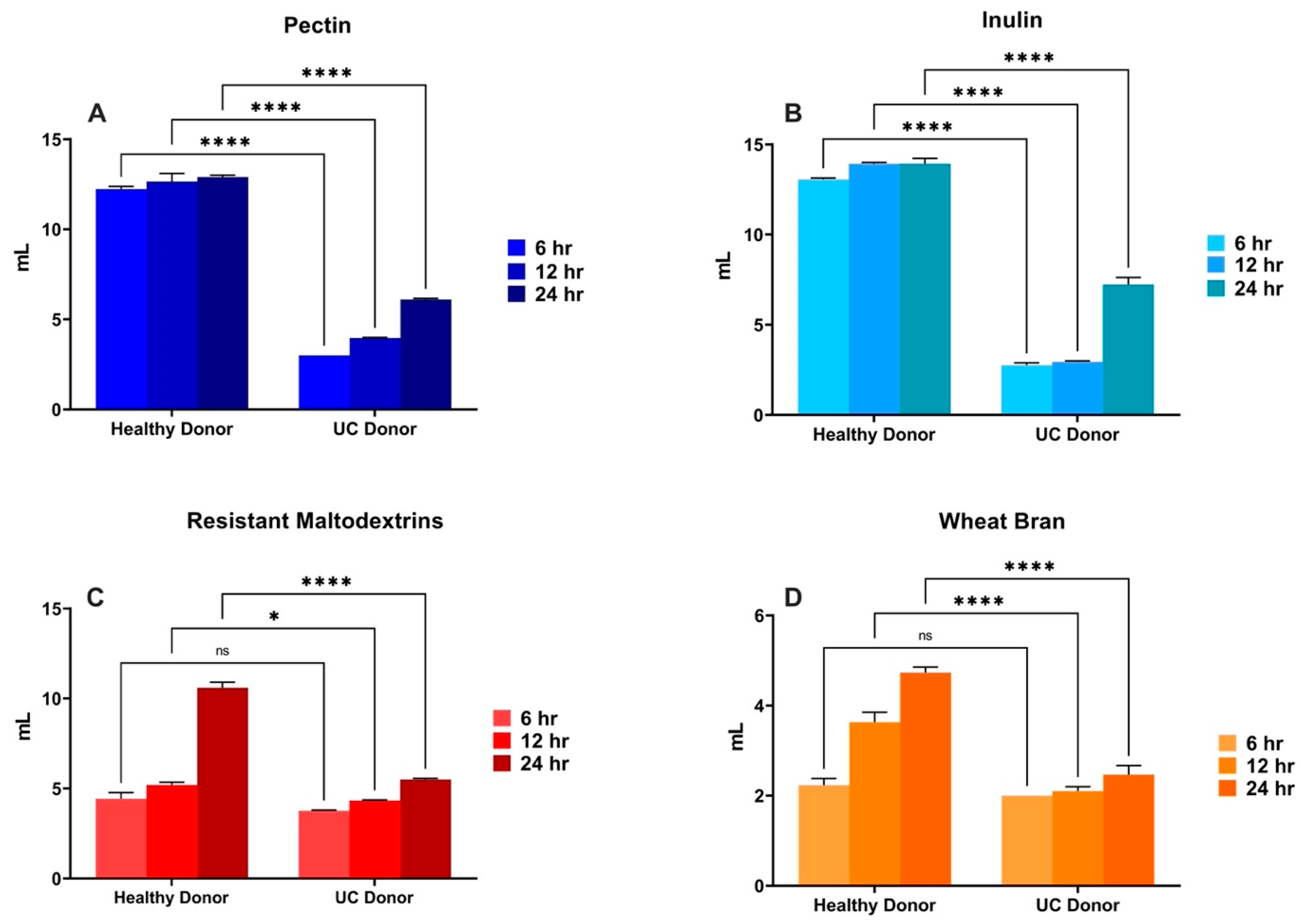
3.3. In Vitro Fermentation of Dietary Fibers Inoculated with UC Patient Fecal Sample-Role of Gut Bacterial Dysbiosis
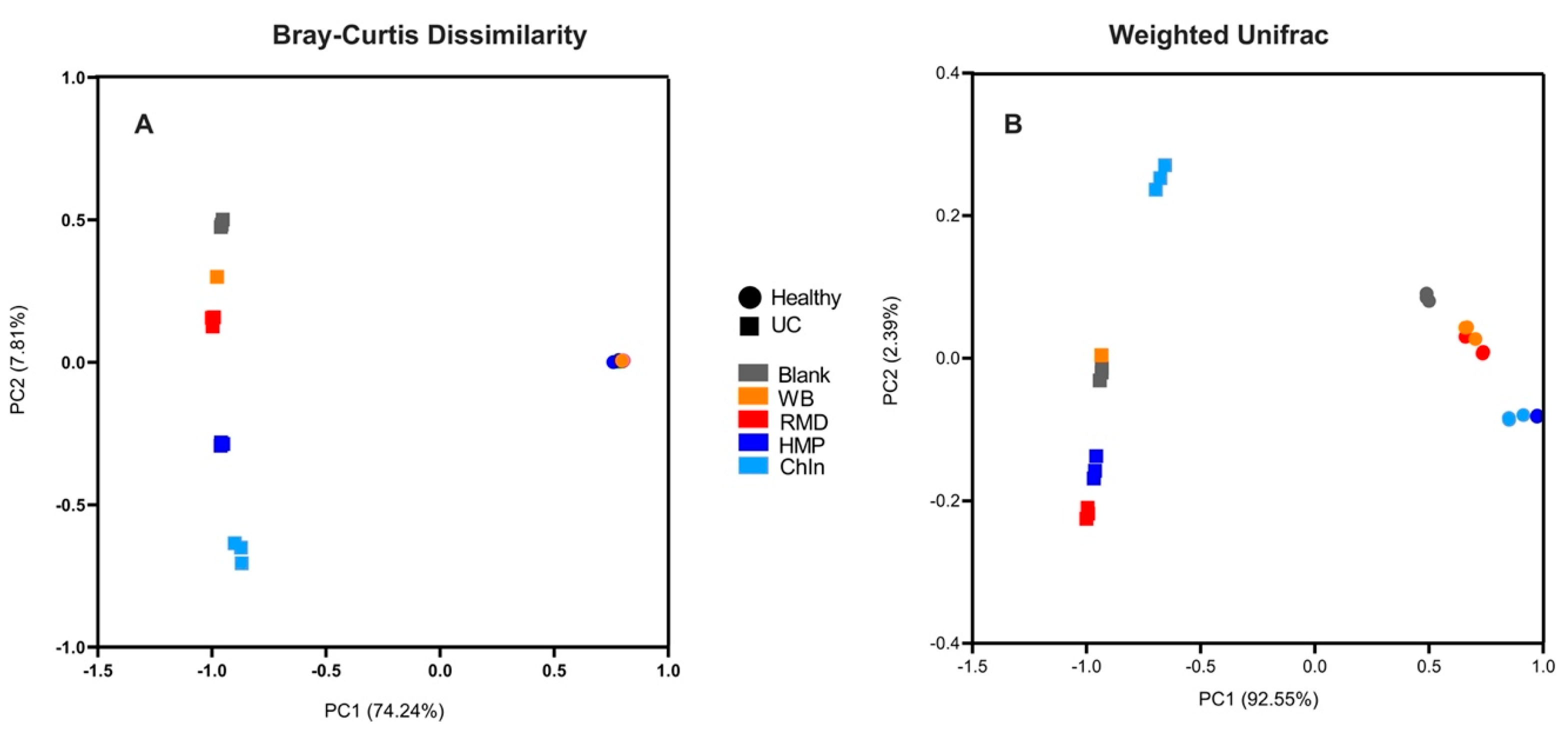
3.4. Gut Bacterial Sequencing-Shifts during Healthy and UC Donor In Vitro Fermentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgart, D.C.; Carding, S.R. Gastroenterology 1 Inflammatory Bowel Disease: Cause and Immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The Microbiome and Inflammatory Bowel Disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary Fiber and Body Weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Klosterbuer, A.; Roughead, Z.F.; Slavin, J. Benefits of Dietary Fiber in Clinical Nutrition. Nutr. Clin. Pract. 2011, 26, 625–635. [Google Scholar] [CrossRef]
- Kuang, R.; Binion, D.G. Should High-Fiber Diets Be Recommended for Patients with Inflammatory Bowel Disease? Curr. Opin. Gastroenterol. 2022, 38, 168–172. [Google Scholar] [CrossRef]
- Pi, Y.; Hu, J.; Bai, Y.; Wang, Z.; Wu, Y.; Ye, H.; Zhang, S.; Tao, S.; Xiao, Y.; Han, D.; et al. Effects of Dietary Fibers with Different Physicochemical Properties on Fermentation Kinetics and Microbial Composition by Fecal Inoculum from Lactating Sows in Vitro. J. Sci. Food Agric. 2021, 101, 907–917. [Google Scholar] [CrossRef]
- Chutkan, R.; Fahey, G.; Wright, W.L.; McRorie, J. Viscous versus Nonviscous Soluble Fiber Supplements: Mechanisms and Evidence for Fiber-Specific Health Benefits. J. Am. Acad. Nurse Pract. 2012, 24, 476–487. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Wichienchot, S.; Huang, Q.; Dhital, S. Dietary Fibers: Structural Aspects and Nutritional Implications. In Food Hydrocolloids; Springer: Singapore, 2021; pp. 505–524. [Google Scholar]
- Rose, D.J.; DeMeo, M.T.; Keshavarzian, A.; Hamaker, B.R. Influence of Dietary Fiber on Inflammatory Bowel Disease and Colon Cancer: Importance of Fermentation Pattern. Nutr. Rev. 2008, 65, 51–62. [Google Scholar] [CrossRef]
- Bosaeus, I. Fibre Effects on Intestinal Functions (Diarrhoea, Constipation and Irritable Bowel Syndrome). Clin. Nutr. Suppl. 2004, 1, 33–38. [Google Scholar] [CrossRef]
- Muir, J. An Overview of Fiber and Fiber Supplements for Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2019, 15, 387–389. [Google Scholar]
- Hamaker, B.R.; Tuncil, Y.E. A Perspective on the Complexity of Dietary Fiber Structures and Their Potential Effect on the Gut Microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Solvang, M.; Duncan, S.H.; Walker, A.W.; Mukhopadhya, I. Dietary Fibre Complexity and Its Influence on Functional Groups of the Human Gut Microbiota. Proc. Nutr. Soc. 2021, 80, 386–397. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary Fibre in Gastrointestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.; San Martin-González, M.F.; Garcia-Bravo, J.; López Malo Vigil, A.; Liceaga, A.M. Physicochemical Characteristics of Chia Seed (Salvia hispanica) Protein Hydrolysates Produced Using Ultrasonication Followed by Microwave-Assisted Hydrolysis. Food Hydrocoll. 2019, 97, 105187. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Kaur, A.; Rose, D.J.; Rumpagaporn, P.; Patterson, J.A.; Hamaker, B.R. In Vitro Batch Fecal Fermentation Comparison of Gas and Short-Chain Fatty Acid Production Using “Slowly Fermentable” Dietary Fibers. J. Food Sci. 2011, 76, H137–H142. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary Fiber and Health Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and Lifestyle Recommendations Revision 2006. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary Fiber Intake and Mortality in the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis Jr, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Marlett, J.A.; McBurney, M.I.; Slavin, J.L. Position of the American Dietetic Association. J. Am. Diet. Assoc. 2002, 102, 993–1000. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Rigottier-Gois, L. Dysbiosis in Inflammatory Bowel Diseases: The Oxygen Hypothesis. ISME J. 2013, 7, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Seksik, P. Alterations of the Dominant Faecal Bacterial Groups in Patients with Crohn’s Disease of the Colon. Gut 2003, 52, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Seksik, P.; Rigottier-Gois, L.; Lay, C.; Lepage, P.; Podglajen, I.; Marteau, P.; Doré, J. Specificities of the Fecal Microbiota in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2006, 12, 106–111. [Google Scholar] [CrossRef]
- Swidsinski, A.; Loening-Baucke, V.; Theissig, F.; Engelhardt, H.; Bengmark, S.; Koch, S.; Lochs, H.; Dorffel, Y. Comparative Study of the Intestinal Mucus Barrier in Normal and Inflamed Colon. Gut 2007, 56, 343–350. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary Fiber in Irritable Bowel Syndrome (Review). Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Andoh, A.; Bamba, T.; Sasaki, M. Physiological and Anti-Inflammatory Roles of Dietary Fiber and Butyrate in Intestinal Functions. J. Parenter. Enter. Nutr. 1999, 23, S70–S73. [Google Scholar] [CrossRef]
- Galvez, J.; Rodríguez-Cabezas, M.E.; Zarzuelo, A. Effects of Dietary Fiber on Inflammatory Bowel Disease. Mol. Nutr. Food Res. 2005, 49, 601–608. [Google Scholar] [CrossRef]
- Rodríguez-Cabezas, M.E.; Gálvez, J.; Camuesco, D.; Lorente, M.D.; Concha, A.; Martinez-Augustin, O.; Redondo, L.; Zarzuelo, A. Intestinal Anti-Inflammatory Activity of Dietary Fiber (Plantago Ovata Seeds) in HLA-B27 Transgenic Rats. Clin. Nutr. 2003, 22, 463–471. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P. Effectiveness of Fiber Supplementation for Constipation, Weight Loss, and Supporting Gastrointestinal Function: A Narrative Review of Meta-Analyses. J. Chiropr. Med. 2020, 19, 58–64. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, H.; Gu, L.; Nie, Y.; Ding, C.; Ge, X.; Yang, B.; Gong, J.; Li, N. Long-Term Follow-up of the Effects of Fecal Microbiota Transplantation in Combination with Soluble Dietary Fiber as a Therapeutic Regimen in Slow Transit Constipation. Sci. China Life Sci. 2018, 61, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Ogura, Y.; Satake, S.; Ito, I.; Iguchi, A.; Takagi, K.; Nabeshima, T. Usefulness of Soluble Dietary Fiber for the Treatment of Diarrhea during Enteral Nutrition in Elderly Patients. Nutrition 2002, 18, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Patil, G.R. Soluble Dietary Fiber. Compr. Rev. Food Sci. Food Saf. 2010, 9, 178–196. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- O’May, G.A.; Reynolds, N.; Macfarlane, G.T. Effect of PH on an In Vitro Model of Gastric Microbiota in Enteral Nutrition Patients. Appl. Environ. Microbiol. 2005, 71, 4777–4783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory Role of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Cell Commun. Signal. 2022, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The Role of Short-Chain Fatty Acids in Intestinal Barrier Function, Inflammation, Oxidative Stress, and Colonic Carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Manichanh, C. Reduced Diversity of Faecal Microbiota in Crohn’s Disease Revealed by a Metagenomic Approach. Gut 2006, 55, 205–211. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-Mediated Inflammation Disrupts the Intestinal Microbiota and Promotes the Overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 204. [Google Scholar] [CrossRef]
- Khorsand, B.; Asadzadeh Aghdaei, H.; Nazemalhosseini-Mojarad, E.; Nadalian, B.; Nadalian, B.; Houri, H. Overrepresentation of Enterobacteriaceae and Escherichia Coli Is the Major Gut Microbiome Signature in Crohn’s Disease and Ulcerative Colitis; a Comprehensive Metagenomic Analysis of IBDMDB Datasets. Front. Cell Infect. Microbiol. 2022, 12, 1015890. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]



| Dietary Fiber | Source |
|---|---|
| Chicory Inulin (ChIn) | Sigma Aldrich I2255 (St. Louis, MO, USA) |
| Agave Inulin (AgIn) | Bought from local supermarket |
| High Methoxyl Pectin (HMP) | Sigma Aldrich 93854 (St. Louis, MO, USA) |
| Low Methoxyl Pectin (LMP) | Sigma Aldrich P9135 (St. Louis, MO, USA) |
| High Amylose Corn Starch (HACS) | Ingredion Hi-Maize 260 (Hamburg, Germany) |
| Resistant Maltodextrins (RMD) | ADM Fibersol-2 (Chicago, IL, USA) |
| Chia Mucilage (Chmuc) | Extracted [18] |
| Psyllium Husk (PSY) | Bought from local supermarket |
| Potato Starch (PST) | Sigma Aldrich S4251 (St. Louis, MO, USA) |
| Soluble Corn Arabinoxylans (SCA) | Extracted and donated by collaborating lab |
| Partially Hydrolyzed Guar Gum (PHGG) | Bought from local supermarket |
| Corn Bran (CB) | Bought from local supermarket |
| Oat Bran (OB) | Bought from local supermarket |
| Wheat Bran (WB) | Bought from local supermarket |
| Small-sized Cellulose (Scell) | Sigma Aldrich 310697 (St. Louis, MO, USA) |
| Medium-sized Cellulose (Mcell) | Sigma Aldrich C6288 (St. Louis, MO, USA) |
| Dietary Fiber | SDF (%) | IDF (%) |
|---|---|---|
| LMP | 89.54 | 10.46 |
| HMP | 81.39 | 18.61 |
| SCA | 72.31 | 27.69 |
| PHGG | 71.84 | 28.16 |
| AgIn | 71.29 | 28.71 |
| Chmuc | 70.13 | 29.87 |
| RMD | 66.82 | 33.18 |
| PSY | 65.83 | 34.17 |
| ChIn | 64.89 | 35.11 |
| OB | 37.01 | 62.99 |
| HACS | 28.74 | 71.26 |
| PST | 23.79 | 76.21 |
| Mcell | 16.19 | 83.81 |
| Scell | 8.68 | 91.32 |
| WB | 5.97 | 94.03 |
| CB | 5.65 | 94.35 |
| Dietary Fiber | Total SCFA | 24 h Gas Production | 6 h Fermentation Rate | Scenario Classification |
|---|---|---|---|---|
| RMD | Medium | Medium | Medium | Scenario 1 |
| PHGG | Medium | High | Medium | |
| SCA | Medium | High | Medium | |
| HMP | High | High | Fast | Scenario 2 |
| LMP | High | High | Fast | |
| AgIn | High | High | Fast | |
| ChIn | High | High | Fast | |
| OB | High | Medium | Medium | |
| Chmuc | Medium | Medium | Slow | |
| CB | Low | Low | Slow | Scenario 3 |
| Mcell | Low | Low | Slow | |
| Scell | Low | Low | Slow | |
| HACS | Low | Low | Medium | |
| WB | Low | Low | Slow | |
| PSY | Low | Low | Slow |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncada, E.; Bulut, N.; Li, S.; Johnson, T.; Hamaker, B.; Reddivari, L. Dietary Fiber’s Physicochemical Properties and Gut Bacterial Dysbiosis Determine Fiber Metabolism in the Gut. Nutrients 2024, 16, 2446. https://doi.org/10.3390/nu16152446
Moncada E, Bulut N, Li S, Johnson T, Hamaker B, Reddivari L. Dietary Fiber’s Physicochemical Properties and Gut Bacterial Dysbiosis Determine Fiber Metabolism in the Gut. Nutrients. 2024; 16(15):2446. https://doi.org/10.3390/nu16152446
Chicago/Turabian StyleMoncada, Edward, Nuseybe Bulut, Shiyu Li, Timothy Johnson, Bruce Hamaker, and Lavanya Reddivari. 2024. "Dietary Fiber’s Physicochemical Properties and Gut Bacterial Dysbiosis Determine Fiber Metabolism in the Gut" Nutrients 16, no. 15: 2446. https://doi.org/10.3390/nu16152446
APA StyleMoncada, E., Bulut, N., Li, S., Johnson, T., Hamaker, B., & Reddivari, L. (2024). Dietary Fiber’s Physicochemical Properties and Gut Bacterial Dysbiosis Determine Fiber Metabolism in the Gut. Nutrients, 16(15), 2446. https://doi.org/10.3390/nu16152446







