Rise in Serum 25-Hydroxyvitamin D Levels during the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Quantitation of Serum 25-Hydroxyvitamin D
2.3. Statistical Analysis
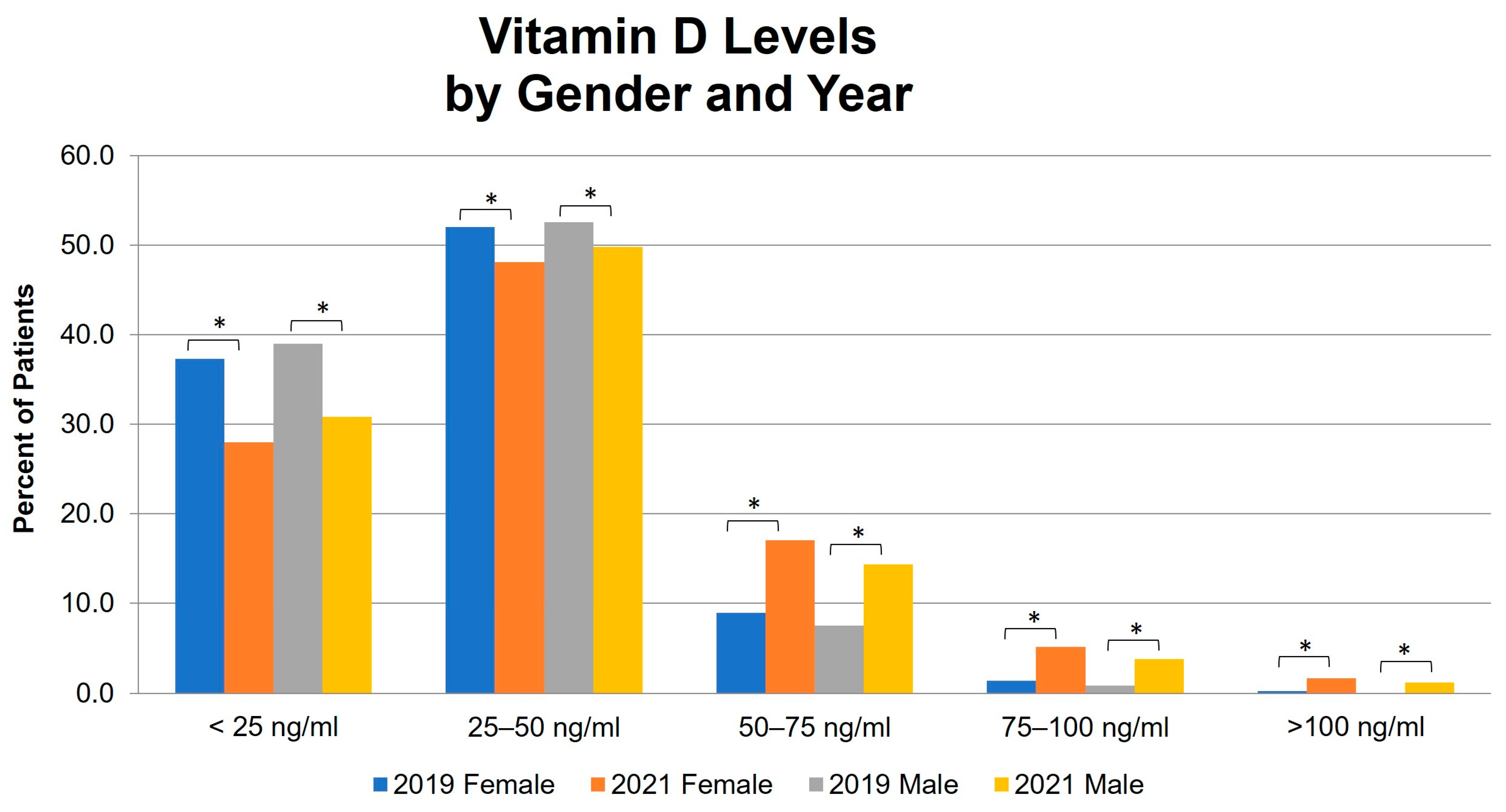
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heer, R.S.; Sandhu, P.; Wenban, C.; Mandal, A.K.J.; Missouris, C.G. Vitamin D in the news: A call for clear public health messaging during COVID-19. Nutr. Health 2022, 28, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Quinn, E.K.; Fenton, S.; Ford-Sahibzada, C.A.; Harper, A.; Marcon, A.R.; Caulfield, T.; Fazel, S.S.; Peters, C.E. COVID-19 and vitamin D misinformation on YouTube: Content analysis. JMIR Infodemiol. 2022, 2, e32452. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Simon, R.; Dubée, V.; Gonsard, J.; Parot-Schinkel, E. COVID-19 and high-dose Vitamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): Study protocol for a randomized controlled trial. Trials 2020, 21, 1031. [Google Scholar] [CrossRef] [PubMed]
- Bassatne, A.; Basbous, M.; Chakhtoura, M.; El Zein, O.; Rahme, M.; Fuleihan, G.E.-H. The link between COVID-19 and Vitamin D (VIVID): A systematic review and meta-analysis. Metabolism 2021, 119, 154753. [Google Scholar] [CrossRef] [PubMed]
- Aljadeed, R. The Rise and Fall of Hydroxychloroquine and Chloroquine in COVID-19. J. Pharm. Pract. 2022, 35, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Norton, R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009, 158, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; A Bischoff-Ferrari, H.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.; et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. Jama 2021, 325, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Brunvoll, S.H.; Nygaard, A.B.; Ellingjord-Dale, M.; Holland, P.; Istre, M.S.; Kalleberg, K.T.; Søraas, C.L.; Holven, K.B.; Ulven, S.M.; Hjartåker, A.; et al. Prevention of Covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: Quadruple blinded, randomised placebo controlled trial. BMJ 2022, 378, e071245. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, N.; Abou Warda, A.E.; Sarhan, R.M.; Boshra, M.S.; Mostafa-Hedeab, G.; Alruwaili, B.F.; Ibrahim, H.S.G.; Schaalan, M.F.; Fathy, S. Evidence for the efficacy of a high dose of vitamin D on the hyperinflammation state in moderate-to-severe COVID-19 patients: A randomized clinical trial. Medicina 2022, 58, 1358. [Google Scholar] [CrossRef] [PubMed]
- De Niet, S.; Trémège, M.; Coffiner, M.; Rousseau, A.F.; Calmes, D.; Frix, A.N.; Gester, F.; Delvaux, M.; Dive, A.F.; Guglielmi, E.; et al. Positive effects of vitamin D supplementation in patients hospitalized for COVID-19: A randomized, double-blind, placebo-controlled trial. Nutrients 2022, 14, 3048. [Google Scholar] [CrossRef]
- Karonova, T.L.; Golovatyuk, K.A.; Kudryavtsev, I.V.; Chernikova, A.T.; Mikhaylova, A.A.; Aquino, A.D.; Lagutina, D.I.; Zaikova, E.K.; Kalinina, O.V.; Golovkin, A.S.; et al. Effect of cholecalciferol supplementation on the clinical features and inflammatory markers in hospitalized COVID-19 patients: A randomized, open-label, single-center study. Nutrients 2022, 14, 2602. [Google Scholar] [CrossRef]
- Sartini, M.; Del Puente, F.; Oliva, M.; Carbone, A.; Bobbio, N.; Schinca, E.; Giribone, L.; Cristina, M.L. Preventive vitamin D supplementation and risk for COVID-19 infection: A systematic review and meta-analysis. Nutrients 2024, 16, 679. [Google Scholar] [CrossRef] [PubMed]
- Smaha, J.; Kužma, M.; Jackuliak, P.; Nachtmann, S.; Max, F.; Tibenská, E.; Binkley, N.; Payer, J. Serum 25-hydroxyvitamin D concentration significantly decreases in patients with COVID-19 pneumonia during the first 48 hours after hospital admission. Nutrients 2022, 14, 2362. [Google Scholar] [CrossRef]
- Hashemipour, S.; Ghobadi, A.; Hadizadeh Khairkhah, S.M.R.; Ranjbaran, M.; Badri, M.; Ghafelehbashi, S.H.; Gheraati, M. Association of weekly or biweekly use of 50 000 IU vitamin D3 with hypervitaminosis D. Br. J. Clin. Pharmacol. 2022, 88, 3506–3509. [Google Scholar] [CrossRef]
- McKenna, M.J.; Lyons, O.C.; Flynn, M.A.; Crowley, R.K.; Twomey, P.J.; Kilbane, M.T. COVID-19 pandemic and vitamin D: Rising trends in status and in daily amounts of vitamin D provided by supplements. BMJ Open 2022, 12, e059477. [Google Scholar] [CrossRef]
- Sempos, C.T.; Binkley, N. 25-Hydroxyvitamin D assay standardisation and vitamin D guidelines paralysis. Public Health Nutr. 2020, 23, 1153–1164. [Google Scholar] [CrossRef]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, sufficiency and toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D toxicity—A clinical perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Villasis-Keever, M.A.; López-Alarcón, M.G.; Miranda-Novales, G.; Zurita-Cruz, J.N.; Barrada-Vázquez, A.S.; González-Ibarra, J.; Martínez-Reyes, M.; Grajales-Muñiz, C.; E Santacruz-Tinoco, C.; Martínez-Miguel, B.; et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. A randomized clinical trial. Arch. Med. Res. 2022, 53, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R. Sorting out whether vitamin D deficiency raises COVID-19 risk. JAMA 2021, 325, 329–330. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; et al. Screening for vitamin D deficiency in adults: US preventive services task force recommendation statement. Jama 2021, 325, 1436–1442. [Google Scholar]
- Binkley, N.; Novotny, R.; Krueger, D.; Kawahara, T.; Daida, Y.G.; Lensmeyer, G.; Hollis, B.W.; Drezner, M.K. Low vitamin D status despite abundant sun exposure. J. Clin. Endocrinol. Metab. 2007, 92, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Nair-Shalliker, V.; Clements, M.; Fenech, M.; Armstrong, B.K. Personal sun exposure and serum 25-hydroxy vitamin D concentrations. Photochem. Photobiol. 2013, 89, 208–214. [Google Scholar] [CrossRef]
- Barger-Lux, M.J.; Heaney, R.P. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J. Clin. Endocrinol. Metab. 2002, 87, 4952–4956. [Google Scholar] [CrossRef]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Domínguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D supplementation and COVID-19 risk: A population-based, cohort study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.F.; Jude, E.B. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: A cross-sectional multi-centre observational study. Nutrients 2020, 12, 3799. [Google Scholar] [CrossRef] [PubMed]
- Ünsal, Y.A.; Gül, Ö.Ö.; Cander, S.; Ersoy, C.; Aydemir, E.; Ateş, C.; Uzun, Z.; Armağan, E.; Ünsal, O.; Ertürk, E. Retrospective analysis of vitamin D status on ınflammatory markers and course of the disease in patients with COVID-19 infection. J. Endocrinol. Investig. 2021, 44, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef] [PubMed]
| Total Patients n (%) 1 | Mean Serum 25(OH)D, ng/mL | p Value | |
|---|---|---|---|
| All patients | 35,556 (100%) | 35 ± 19 | |
| Age, mean years ± SD | 54.1 ± 20.7 | ||
| Gender 2 | ≤0.001 | ||
| Female | 22,203 (62.5%) | 35 ± 19 | |
| Male | 13,346 (37.5%) | 34 ± 18 | |
| Race 3 | ≤0.001 | ||
| White | 31,618 (93.5%) | 35 ± 19 | |
| Other 4 | 2200 (6.5%) | 29 ± 19 | |
| Serum 25(OH)D quintiles | ≤0.001 | ||
| <25 ng/mL | 11,687 (32.9%) | 18 ± 5 | |
| 25–50 ng/mL | 17,866 (50.3%) | 35 ± 7 | |
| 50–75 ng/mL | 4528 (12.7%) | 59 ± 7 | |
| 75–100 ng/mL | 1129 (3.2%) | 84 ± 7 | |
| >100 ng/mL | 346 (1.0%) | 112 ± 9 |
| 2019 (n = 15,318) n (%) 1 | 2021 (n = 20,238) n (%) 1 | p Value | |
|---|---|---|---|
| Age, mean years ± SD | 53.8 ± 21.5 | 54.3 ± 20.0 | 0.03 |
| Gender 2 | 0.6 | ||
| Female | 9587 (62.6%) | 7621 (37.7%) | |
| Male | 5725 (37.4%) | 12,616 (62.3%) | |
| Race 3 | 0.17 | ||
| White | 13,816 (93.7%) | 17,802 (93.3%) | |
| Other 4 | 928 (6.3%) | 1272 (6.7%) | |
| Serum 25(OH)D, mean ng/mL ± SD | 31 ± 15 | 37 ± 21 | ≤0.001 |
| Serum 25(OH)D quintile | ≤0.001 | ||
| <25 ng/mL | 5809 (37.9%) | 5878 (29.0%) | ≤0.001 |
| 25–50 ng/mL | 8001 (52.2%) | 9865 (48.7%) | ≤0.001 |
| 50–75 ng/mL | 1287 (8.4%) | 3241 (16.0%) | ≤0.001 |
| 75–100 ng/mL | 189 (1.2%) | 940 (4.6%) | ≤0.001 |
| >100 ng/mL | 32 (0.2%) | 314 (1.6%) | ≤0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durden, W.; Ezaldin, S.; Amos, J.; Kemper, S.; Campbell, J. Rise in Serum 25-Hydroxyvitamin D Levels during the COVID-19 Pandemic. Nutrients 2024, 16, 2449. https://doi.org/10.3390/nu16152449
Durden W, Ezaldin S, Amos J, Kemper S, Campbell J. Rise in Serum 25-Hydroxyvitamin D Levels during the COVID-19 Pandemic. Nutrients. 2024; 16(15):2449. https://doi.org/10.3390/nu16152449
Chicago/Turabian StyleDurden, Witt, Shady Ezaldin, Jessica Amos, Suzanne Kemper, and James Campbell. 2024. "Rise in Serum 25-Hydroxyvitamin D Levels during the COVID-19 Pandemic" Nutrients 16, no. 15: 2449. https://doi.org/10.3390/nu16152449
APA StyleDurden, W., Ezaldin, S., Amos, J., Kemper, S., & Campbell, J. (2024). Rise in Serum 25-Hydroxyvitamin D Levels during the COVID-19 Pandemic. Nutrients, 16(15), 2449. https://doi.org/10.3390/nu16152449






