Influence of Carbohydrate Intake on Caprylic Acid (C8:0)-Induced Ketogenesis—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy, Selection Criteria and Screening
2.2. Data Extraction
2.3. Data Synthesis
2.4. Quality Assessment
2.5. Meta-Analysis
3. Results
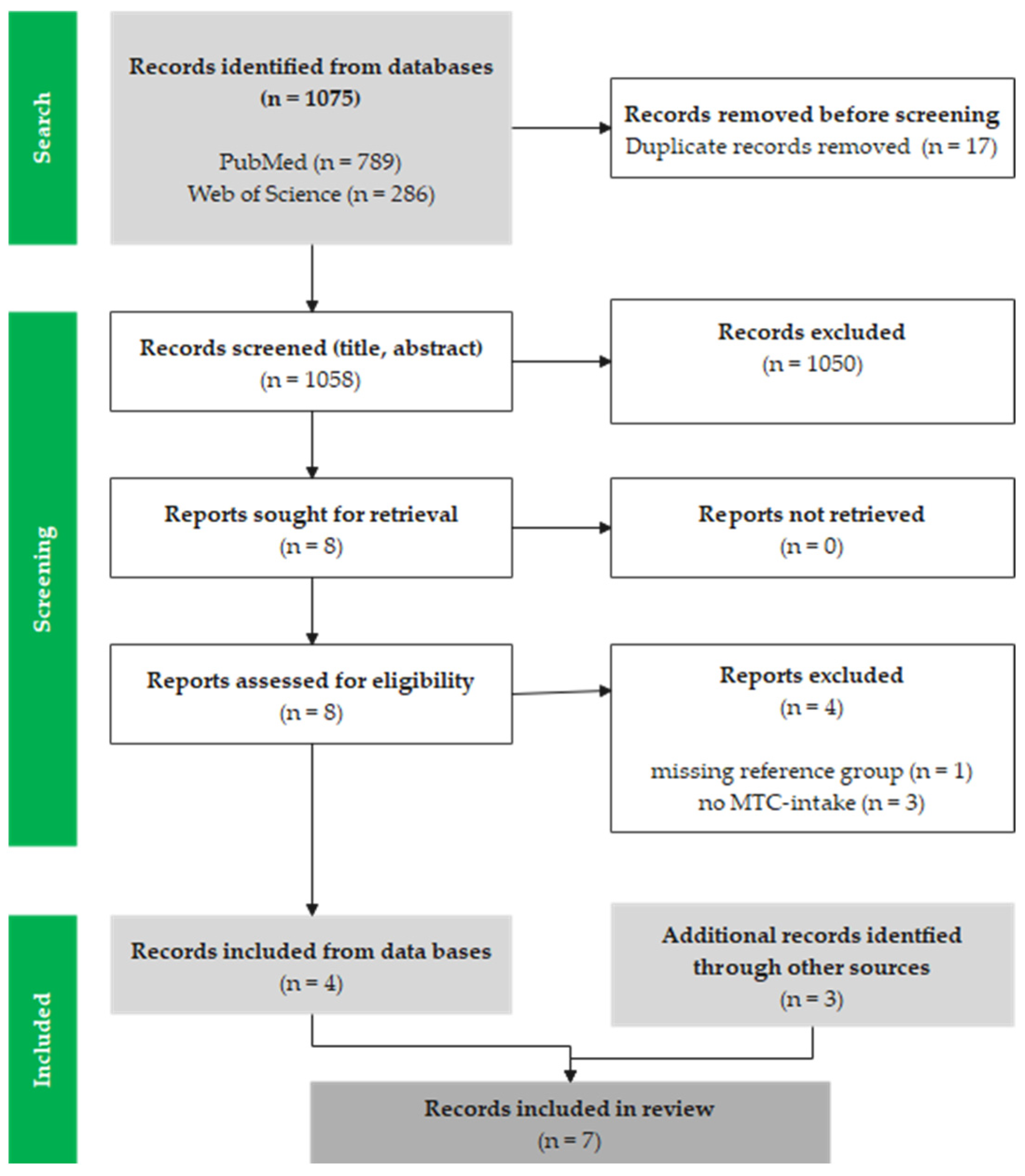
3.1. Study Selection
3.2. Characteristics of the Studies
3.3. Interventions and Controls
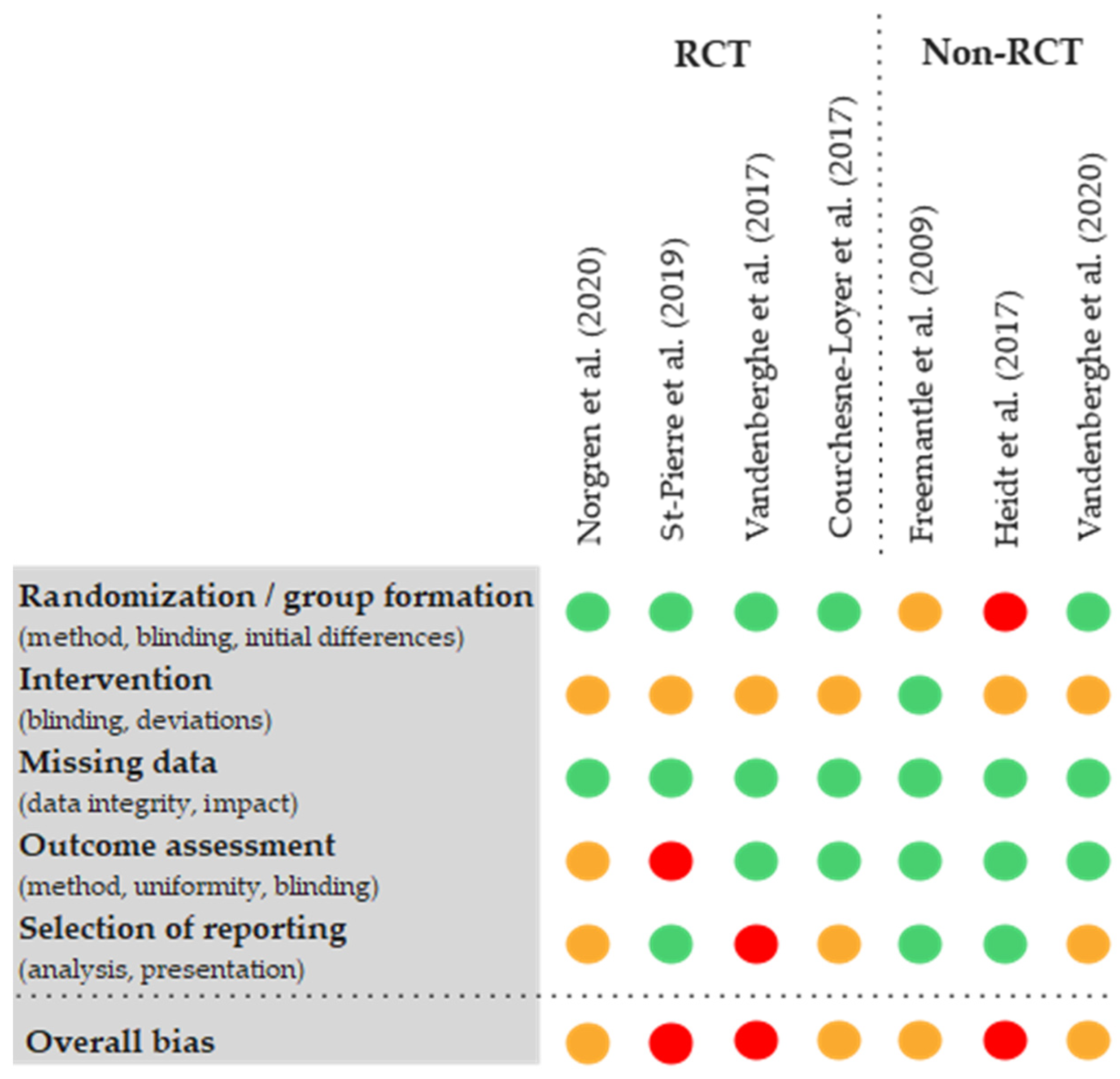
3.4. Risk of Bias Assessments
3.5. Conflicts of Interest
3.6. Effect of Interventions
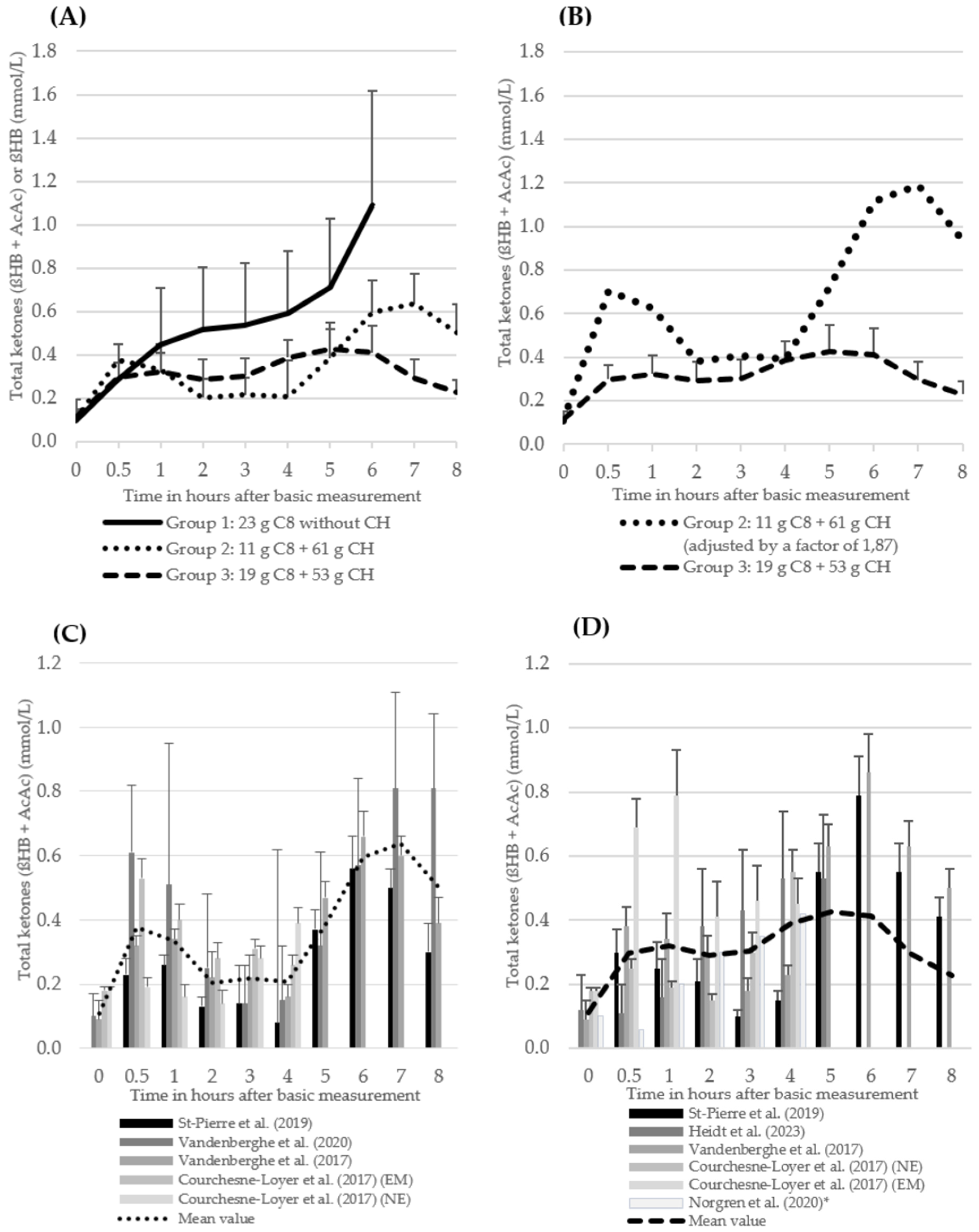
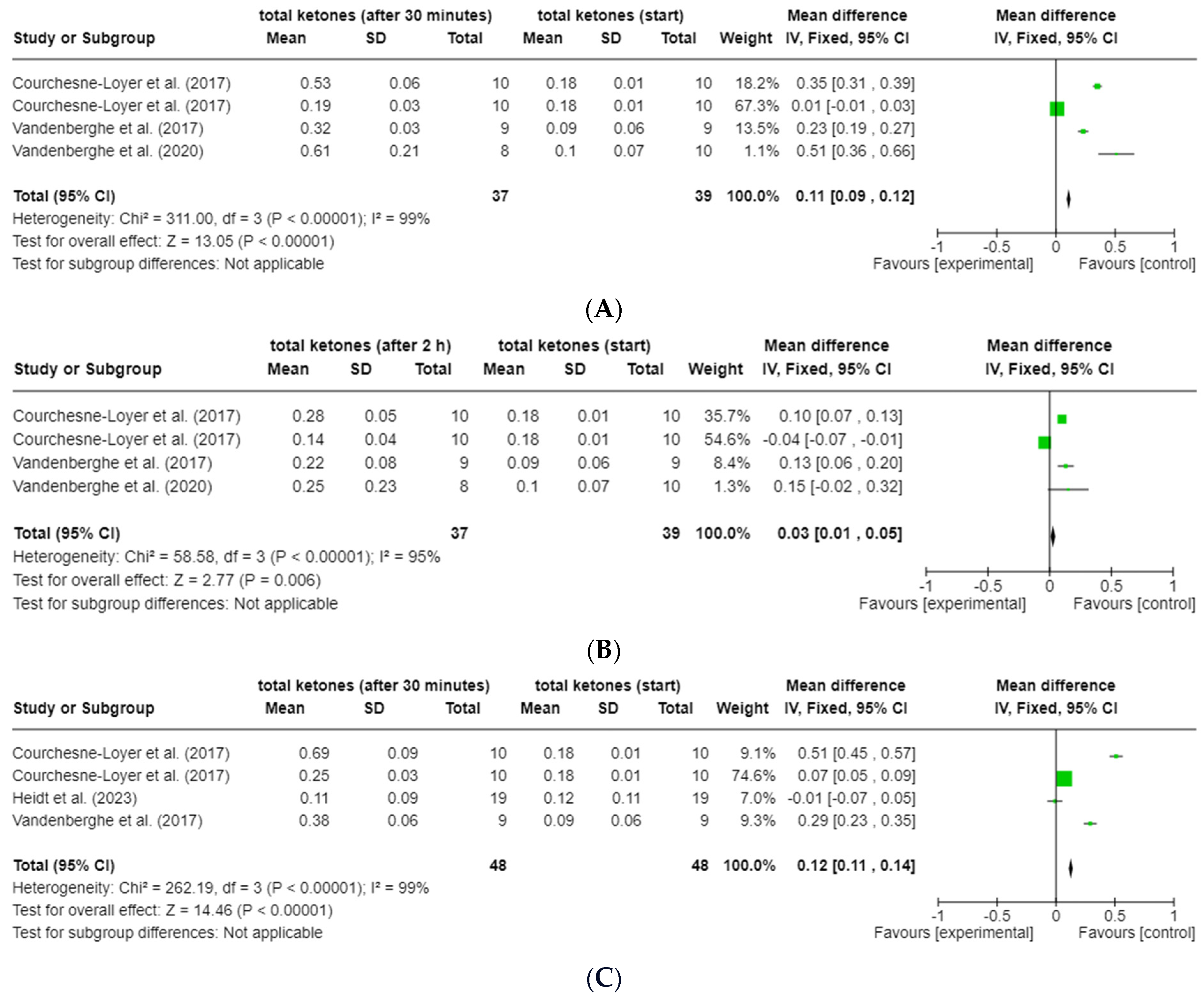
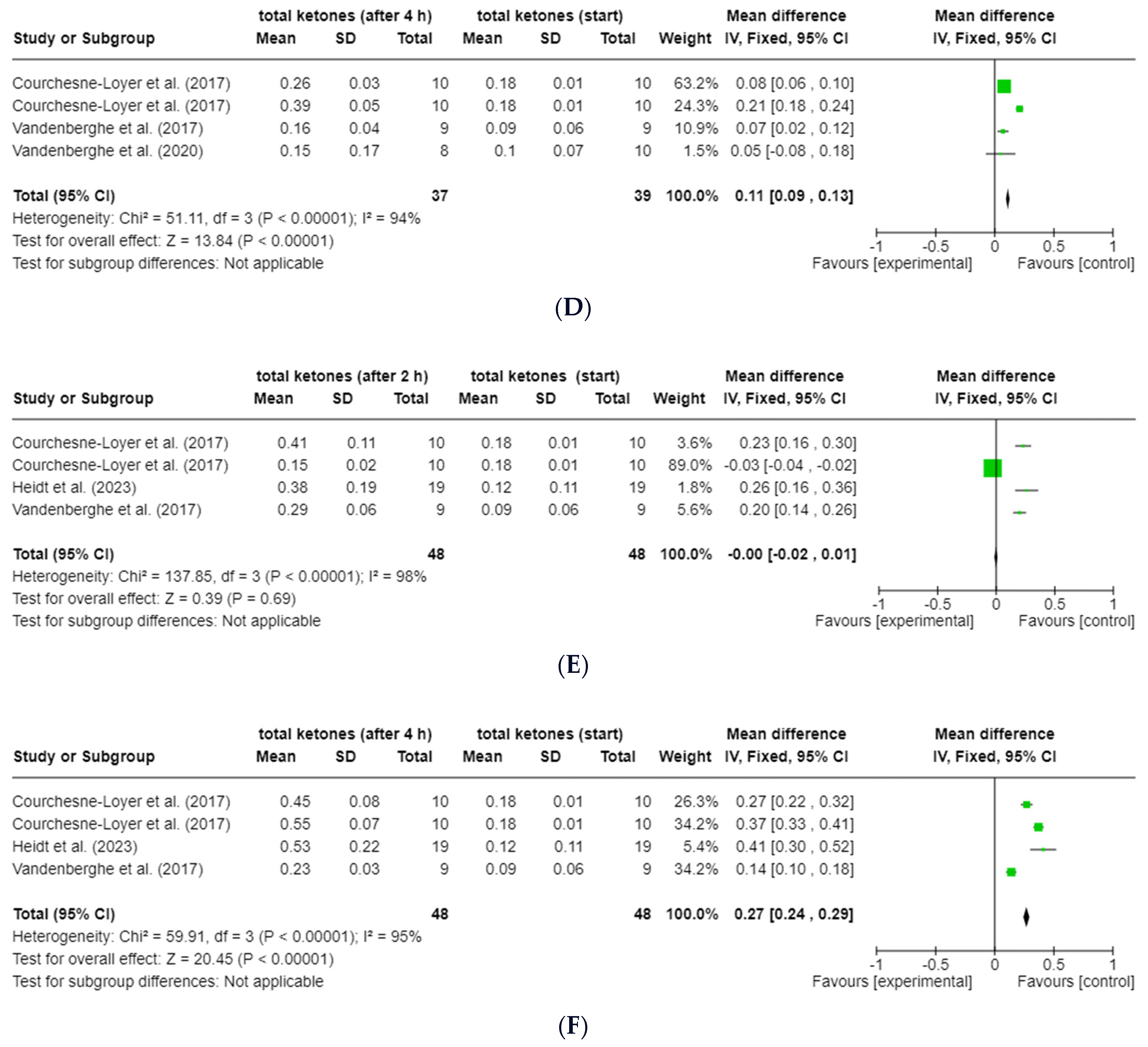
3.6.1. ßHB and Total Ketones
3.6.2. Plasma Glucose
3.6.3. Area under the Curve (AUC)
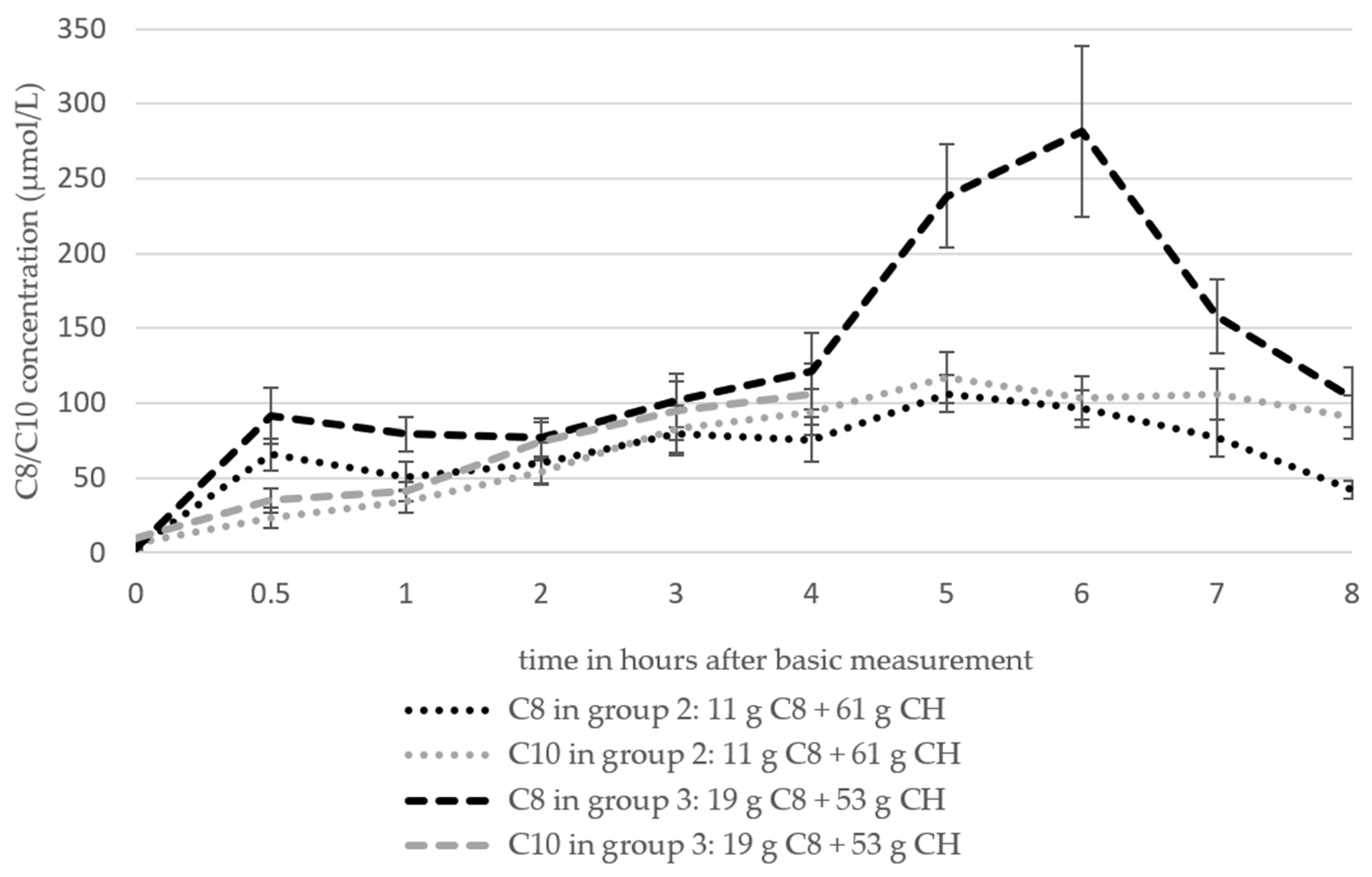
3.6.4. Octanoat (C8) and Decanoat (C10)
3.6.5. Side Effects of MCT Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Data Base | Search incl. Filter |
|---|---|
| PubMed | ((“carbohydrat*”[Text Word] OR “carbohydrate intake”[Text Word] OR “carbohydrate supply”[Text Word] OR “glucos*”[Text Word] OR “dextros*”[Text Word] OR “sugar*”[Text Word] OR “Carbohydrates”[MeSH Terms] OR “Sugars”[MeSH Terms]) AND (“MCT”[Text Word] OR “mct s*”[Text Word] OR “medium chain triglycerid*”[Text Word] OR “medium chain triglycerid*”[Text Word] OR “caprylic acid*”[Text Word] OR “capric acid*”[Text Word] OR “octanoic acid*”[Text Word] OR “decanoic acid*”[Text Word] OR “Triglycerides”[MeSH Terms] OR “Fatty Acids”[MeSH Terms]) AND (“ketogen*”[Text Word] OR “ketogenic diet”[Text Word] OR “Ketone Bodies”[Text Word] OR “low carb*”[Text Word] OR “low carb*”[Text Word] OR “beta-hydroxybutyrate”[Text Word] OR “Ketone Bodies”[MeSH Terms] OR “diet, carbohydrate restricted”[MeSH Terms])) AND ((casereports[Filter] OR clinicalstudy[Filter] OR clinicaltrial[Filter] OR clinicaltrialphasei[Filter] OR clinicaltrialphaseii[Filter] OR clinicaltrialphaseiii[Filter] OR clinicaltrialphaseiv[Filter] OR clinicaltrialprotocol[Filter] OR comparativestudy[Filter] OR controlledclinicaltrial[Filter] OR multicenterstudy[Filter] OR observationalstudy[Filter] OR pragmaticclinicaltrial[Filter] OR randomizedcontrolledtrial[Filter] OR twinstudy[Filter] OR validationstudy[Filter]) AND (humans[Filter]) AND (english[Filter])) |
| Web of Science | Carbohydrat* OR “carbohydrate intake” OR “carbohydrate supply” OR glucos* OR dextros* OR sugar* OR sucros* (Topic) and MCT OR MCTs* OR “medium-chain triglycerid*” OR “medium chain triglycerid*“ OR “caprylic acid*” OR “capric acid*” OR “octanoic acid*” OR “decanoic acid*” (Topic) and ketogen* OR “ketogenic diet” OR “ketone bodies” OR “low-carb*” OR “low carb*” OR “beta-hydroxybutyrate” OR “carbohydrate restricted*” OR “carbohydrate-restricted” (Topic) and Preprint Citation Index (Exclude–Database) and English (Languages) and Article or Other or Dissertation Thesis or Clinical Trial or Abstract or Meeting or Case Report or Unspecified or Early Access (Document Types) |
Appendix B
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Studies from which intervention arms were taken | Freemantle et al. (2009) [22] Heidt et al. (2023) [42] Norgren et al. (2020) [20] | Courchesne-Loyer et al. (2017) [25] St-Pierre et al. (2019) [21] Vandenberghe et al. (2017) [24] Vandenberghe et al. (2020) [43] | Courchesne-Loyer et al. (2017) [25] Heidt et al. (2023) [42] Norgren et al. (2020) [20] St-Pierre et al. (2019) [21] Vandenberghe et al. (2017) [24] |
| Interventions: CH (⌀) (mean values ± standard deviation) | 1.4 ± 1.2 g | 61.1 ± 6.1 g | 53.3 ± 18.8 g |
| Interventions: MCT (⌀) (mean values ± standard deviation) | C8: 22.5 ± 4.1 g C10: 18.1 ± 17.4 g C12: 0.4 ± 0.5 g MCT: 41.0 ± 21.8 g | C8: 11.4 ± 1.0 g C10: 7.6 ± 0.5 g C12: 0.4 ± 0.7 g MCT: 19.2 ± 0.9 g | C8: 18.6 ± 0.9 g C10: 5.0 ± 6.1 g C12: 0.2 ± 0.4 g MCT: 23.8 ± 5.9 g |
| Ratio g CH/g C8 | 5.36 | 2.87 |
References
- Roehl, K.; Sewak, S.L. Practice Paper of the Academy of Nutrition and Dietetics: Classic and Modified Ketogenic Diets for Treatment of Epilepsy. J. Acad. Nutr. Diet. 2017, 117, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Bier, D.M.; Brosnan, J.T.; Flatt, J.P.; Hanson, R.W.; Heird, W.; Hellerstein, M.K.; Jéquier, E.; Kalhan, S.; Koletzko, B.; MacDonald, I.; et al. Report of the IDECG Working Group on lower and upper limits of carbohydrate and fat intake. Eur. J. Clin. Nutr. 1999, 53, s177–s178. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.M. The effect of ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307–308. [Google Scholar]
- Freeman, J.M.; Vining, E.P.; Pillas, D.J.; Pyzik, P.L.; Casey, J.C.; Kelly, L.M. The efficacy of the ketogenic diet-1998: A prospective evaluation of intervention in 150 children. Pediatrics 1998, 102, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; McGrogan, J.R. Worldwide use of the ketogenic diet. Epilepsia 2005, 46, 280–289. [Google Scholar] [CrossRef]
- Freeman, J.M.; Kossoff, E.H.; Hartman, A.L. The ketogenic diet: One decade later. Pediatrics 2007, 119, 535–543. [Google Scholar] [CrossRef]
- Schoeler, N.E.; Cross, J.H. Ketogenic dietary therapies in adults with epilepsy: A practical guide. Pract. Neurol. 2016, 16, 208–214. [Google Scholar] [CrossRef]
- Kossoff, E.H.; McGrogan, J.R.; Bluml, R.M.; Pillas, D.J.; Rubenstein, J.E.; Vining, E.P. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia 2006, 47, 421–424. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Cervenka, M.C.; Henry, B.J.; Haney, C.A.; Turner, Z. A decade of the modified Atkins diet (2003–2013): Results, insights, and future directions. Epilepsy Behav. 2013, 29, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, H.H.; Thiele, E.A. Low-glycemic-index treatment: A liberalized ketogenic diet for treatment of intractable epilepsy. Neurology 2005, 65, 1810–1812. [Google Scholar] [CrossRef] [PubMed]
- Sourbron, J.; Klinkenberg, S.; van Kuijk, S.M.J.; Lagae, L.; Lambrechts, D.; Braakman, H.M.H.; Majoie, M. Ketogenic diet for the treatment of pediatric epilepsy: Review and meta-analysis. Child’s Nerv. Syst. 2020, 36, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Lopes Neri, L.d.C.; Guglielmetti, M.; Fiorini, S.; Pasca, L.; Zanaboni, M.P.; de Giorgis, V.; Tagliabue, A.; Ferraris, C. Adherence to ketogenic dietary therapies in epilepsy: A systematic review of literature. Nutr. Res. 2024, 126, 67–87. [Google Scholar] [CrossRef] [PubMed]
- McNamara, N.A.; Carbone, L.A.; Shellhaas, R.A. Epilepsy characteristics and psychosocial factors associated with ketogenic diet success. J. Child Neurol. 2013, 28, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, P.R.; Wilbourn, A.J.; Signore, J.M. Medium-chain triglycerides as a Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971, 21, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C. Medium-chain triglyceride (MCT) ketogenic therapy. Epilepsia 2008, 49 (Suppl. S8), 33–36. [Google Scholar] [CrossRef]
- Huttenlocher, P.R. Ketonemia and seizures: Metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr. Res. 1976, 10, 536–540. [Google Scholar] [CrossRef]
- Norgren, J.; Sindi, S.; Sandebring-Matton, A.; Kareholt, I.; Daniilidou, M.; Akenine, U.; Nordin, K.; Rosenborg, S.; Ngandu, T.; Kivipelto, M. Ketosis after intake of coconut oil and caprylic acid—With and without glucose: A cross-over study in healthy older adults. Front. Nutr. 2020, 7, 40. [Google Scholar] [CrossRef]
- St-Pierre, V.; Vandenberghe, C.; Lowry, C.-M.; Fortier, M.; Castellano, C.-A.; Wagner, R.; Cunnane, S.C. Plasma ketone and medium chain fatty acid response in humans consuming different medium chain triglycerides during a metabolic study day. Front. Nutr. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Freemantle, E.; Vandal, M.; Tremblay Mercier, J.; Plourde, M.; Poirier, J.; Cunnane, S.C. Metabolic response to a ketogenic breakfast in the healthy elderly. J. Nutr. Health Aging 2009, 13, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Vitaflo. K. Quik-Datenblatt. Available online: https://www.nestlehealthscience.de/sites/default/files/2024-04/DataCard_K.Quik_1122.pdf (accessed on 29 April 2024).
- Vandenberghe, C.; St-Pierre, V.; Pierotti, T.; Fortier, M.; Castellano, C.-A.; Cunnane, S.C. Tricaprylin alone increases plasma ketone response more than coconut oil or other medium-chain triglycerides: An acute crossover study in healthy adults. Curr. Dev. Nutr. 2017, 1, e000257. [Google Scholar] [CrossRef] [PubMed]
- Courchesne-Loyer, A.; Lowry, C.-M.; St-Pierre, V.; Vandenberghe, C.; Fortier, M.; Castellano, C.-A.; Wagner, J.R.; Cunnane, S.C. Emulsification increases the acute ketogenic effect and bioavailability of medium-chain triglycerides in humans. Curr. Dev. Nutr. 2017, 1, e000851. [Google Scholar] [CrossRef]
- Dr. Schär AG. Kanso MCT Öl 100%. Available online: https://www.kanso.com/de/ketogene-diaet/keto-produkte/detail/product/kanso-mct-oel-100 (accessed on 2 May 2024).
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Nimbkar, S.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Medium chain triglycerides (MCT): State-of-the-art on chemistry, synthesis, health benefits and applications in food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 843–867. [Google Scholar] [CrossRef] [PubMed]
- Dayrit, F.M. The properties of lauric acid and their significance in coconut oil. J. Americ Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009, 50, 1109–1117. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Tang, T.-K.; Chan, E.-S.; Phuah, E.-T.; Lai, O.-M.; Tan, C.-P.; Wang, Y.; Ab Karim, N.A.; Mat Dian, N.H.; Tan, J.S. Medium chain triglyceride and medium-and long chain triglyceride: Metabolism, production, health impacts and its applications—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4169–4185. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.d.C.; Schofield, G.M.; Williden, M.; McQuillan, J.A. The effect of medium chain triglycerides on time to nutritional ketosis and symptoms of keto-induction in healthy adults: A randomised controlled clinical trial. J. Nutr. Metab. 2018, 2018, 2630565. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Liu, H.-W.; Hung, T.-M. The ketogenic effect of medium-chain triacylglycerides. Front. Nutr. 2021, 8, 747284. [Google Scholar] [CrossRef] [PubMed]
- Schultz, L.H.; Smith, V.R.; Lardy, H.A. The effect of the administration of various fatty acids on the blood ketone levels of ruminants. J. Dairy Sci. 1949, 32, 817–822. [Google Scholar] [CrossRef]
- Thevenet, J.; de Marchi, U.; Domingo, J.S.; Christinat, N.; Bultot, L.; Lefebvre, G.; Sakamoto, K.; Descombes, P.; Masoodi, M.; Wiederkehr, A. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. Faseb J. 2016, 30, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook/current (accessed on 21 February 2024).
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V.; Thomas, J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In Cochrane Handbook for Systematic Reviews; Wiley Online Library: Hoboken, NJ, USA, 2019. [Google Scholar]
- Lefebvre, G.; Glanville, J.; Briscoe, S.; Featherstone, R.; Littlewood, A.; Marshall, C.; Metzendorf, M.-I.; Noel-Storr, A.H.; Paynter, R.; Rader, T. Chapter 4: Searching for and selecting studies. In Cochrane Handbook for Systematic Reviews; Wiley Online Library: Hoboken, NJ, USA, 2019. [Google Scholar]
- Tianhing, L.; Higgins, J.P.T.; Deeks, J.J. Chapter 5: Collecting data. In Cochrane Handbook for Systematic Reviews; Wiley Online Library: Hoboken, NJ, USA, 2019. [Google Scholar]
- Kraft Chemical. Safety Data Sheet MCT Oil. Available online: https://greenfield.com/wp-content/uploads/2018/11/MCT-Oil-SDS.pdf (accessed on 20 December 2023).
- Heidt, C.; Fobker, M.; Newport, M.; Feldmann, R.; Fischer, T.; Marquardt, T. Beta-Hydroxybutyrate (BHB), glucose, insulin, octanoate (C8), and decanoate (C10) responses to a medium-chain triglyceride (MCT) oil with and without glucose: A single-center study in healthy adults. Nutrients 2023, 15, 1148. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, C.; St-Pierre, V.; Fortier, M.; Castellano, C.-A.; Cuenoud, B.; Cunnane, S.C. Medium chain triglycerides modulate the ketogenic effect of a metabolic switch. Front. Nutr. 2020, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Sterne, J.A.C. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2). 2019. Available online: https://drive.google.com/file/d/19R9savfPdCHC8XLz2iiMvL_71lPJERWK/view (accessed on 21 February 2024).
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews; Wiley Online Library: Hoboken, NJ, USA, 2019. [Google Scholar]
- McPherson, H.T.; Werk, E.E.; Myers, J.D.; Engel, F.L. Studies on ketone metabolism in man. II. The effect of glucose, insulin, cortisone and hypoglycemia on splanchnic ketone production. J. Clin. Investig. 1958, 37, 1379–1393. [Google Scholar] [CrossRef]
- Nakamura, K.; Hagihara, K.; Nagai, N.; Egashira, R.; Takeuchi, M.; Nakano, M.; Saito, H.; Moriguchi, M.; Tonari, S.; Fujii, H.; et al. Ketogenic effects of medium chain triglycerides containing formula and its correlation to breath acetone in healthy volunteers: A randomized, double-blinded, placebo-controlled, single dose-response study. Front. Nutr. 2023, 10, 1224740. [Google Scholar] [CrossRef]
- Bergen, S.S.; Hashim, S.A.; van Itallie, T.B. Hyperketonemia induced in man by medium-chain triglyceride. Diabetes 1966, 15, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Courchesne-Loyer, A.; Vandenberghe, C.; St-Pierre, V.; Fortier, M.; Hennebelle, M.; Croteau, E.; Bocti, C.; Fulop, T.; Castellano, C.-A. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of alzheimer’s disease. Front. Mol. Neurosci. 2016, 9, 53. [Google Scholar] [CrossRef]
- Xin, L.; Ipek, Ö.; Beaumont, M.; Shevlyakova, M.; Christinat, N.; Masoodi, M.; Greenberg, N.; Gruetter, R.; Cuenoud, B. Nutritional ketosis increases NAD+/NADH Rrtio in healthy human brain: An in vivo study by 31P-MRS. Front. Nutr. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Schön, H.; Gelpke, W.; Lippach, I. Stoffwechseluntersuchungen mit einem Mischglycerid der Fettsäuren mittlerer Kettenlänge. Gastroeneterologia 1959, 91, 89–108. [Google Scholar] [CrossRef]
- Vandenberghe, C.; St-Pierre, V.; Courchesne-Loyer, A.; Hennebelle, M.; Castellano, C.-A.; Cunnane, S.C. Caffeine intake increases plasma ketones: An acute metabolic study in humans. Can. J. Physiol. Pharmacol. 2017, 95, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Bellet, S.; Kershbaum, A.; Finck, E.M. Response of free fatty acids to coffee and caffeine. Metabolism 1968, 17, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, A.; Gardemann, J.; Fobker, M.; Spiegler, V.; Fischer, T. Short-term influence of caffeine and medium-chain triglycerides on ketogenesis: A controlled double-blind intervention study. J. Nutr. Metab. 2021, 2021, 1861567. [Google Scholar] [CrossRef]
- Hopman, W.P.; Jansen, J.B.; Rosenbusch, G.; Lamers, C.B. Effect of equimolar amounts of long-chain triglycerides and medium-chain triglycerides on plasma cholecystokinin and gallbladder contraction. Am. J. Clin. Nutr. 1984, 39, 356–359. [Google Scholar] [CrossRef]
- Trauner, D.A. Medium-chain triglyceride (MCT) diet in intractable seizure disorders. Neurology 1985, 35, 237–238. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Aldred, S. Fat supplementation, health, and endurance performance. Nutrition 2004, 20, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, H.; Liu, Y.; Zhang, P.; Zhang, Y.; Zhang, X.; Liu, Y. Medium-chain triglycerides reduce diarrhea with improved immune status and gut microbiomics in tunnel workers in China. Asia Pac. J. Clin. Nutr. 2022, 31, 229–241. [Google Scholar] [CrossRef]
- Schwartz, R.H.; Eaton, J.; Bower, B.D.; Aynsley-Green, A. Ketogenic diets in the treatment of epilepsy: Short-term clinical effects. Dev. Med. Child Neurol. 1989, 31, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Juby, A.G.; Brocks, D.R.; Jay, D.A.; Davis, C.M.J.; Mager, D.R. Assessing the impact of factors that influence the ketogenic response to varying doses of medium chain triglyceride (MCT) oil. JPAD-J. Prev. Alzheimers Dis. 2021, 8, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.; Sampson, A.; Goslawska, M.; Pangua-Irigaray, C.; Shafat, A.; Clegg, M.E. Food intake and satiety response after medium-chain triglycerides ingested as solid or liquid. Nutrients 2019, 11, 1638. [Google Scholar] [CrossRef]
- Shcherbakova, K.; Schwarz, A.; Apryatin, S.; Karpenko, M.; Trofimov, A. Supplementation of regular diet with medium-chain triglycerides for procognitive effects: A narrative review. Front. Nutr. 2022, 9, 934497. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. 2009, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.; Sailer, D. Effect of fat emulsions containing medium-chain triglycerides and glucose on ketone body production and excretion. JPEN J. Parenter. Enteral Nutr. 1984, 8, 285–289. [Google Scholar] [CrossRef]
- Ingle, D.L.; Driedger, A.; Traul, K.A.; Nakhasi, D.K. Dietary energy value of medium-chain triglycerides. J. Food Sci. 1999, 64, 960–963. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Bourque, C.; Jones, P.J.H.; Ross, R.; Parsons, W.E. Medium- versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int. J. Obes. 2003, 27, 95–102. [Google Scholar] [CrossRef]
- Rego Costa, A.C.; Rosado, E.L.; Soares-Mota, M. Influence of the dietary intake of medium chain triglycerides on body composition, energy expenditure and satiety: A systematic review. Nutr. Hosp. 2012, 27, 103–108. [Google Scholar] [PubMed]
- European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. ANNEX XIV, L304/18; Official Journal of the European Union, 22 October 2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:en:PDF (accessed on 21 February 2024).

| Unit | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Healthy people (regardless of age, gender, ethnicity) | In vitro studies, animal studies, existing diseases (e.g., diabetes mellitus, insulin resistance, metabolic diseases, epilepsy), people on a KD, professional athletes |
| Intervention | Dietary intervention with intake or complementation of MCT- or MCFA-containing products with C8 content with simultaneous oral CH intake | Intervention without MCT or MCFA supply or with supply of exogenous ketone bodies, no accompanying use of CH |
| Comparison | Completed and published studies, controlled and uncontrolled, as well as randomised and non-randomised intervention studies | Ongoing and unpublished studies, poster abstracts, observational studies, reviews, meta-analyses |
| Outcome | Essential: ßHB (plasma ßHB or total ketones (ßHB and AcAc)); optional: acetoacetate, acetone, plasma C8/C10/C12, plasma glucose, AUC 1 ßHB/total ketones, side effects | Ketone concentration measured only in the form of acetone or acetoacetate |
| Author, Year, Country | Age (Ø Years) m/f (%) | N | Blinding | Dropout Rate (%) |
|---|---|---|---|---|
| RCT | ||||
| Courchesne-Loyer et al. (2017) [25] Canada | 31.0 ± 3.0 60/40 | 10 | Single | 0 |
| Norgren et al. (2020) [20] Sweden | 69.2 ± 2.4 47/53 | 15 | Single | One subject (n = 1) dropped out and was replaced by another from the waiting list. This participant went through all intervention arms. |
| St-Pierre et al. (2019) [21] Canada | 34.0 ± 12.0 78/22 | 9 | Single | One subject (n = 1) did not take part in one of the interventions. The available data were analysed. |
| Vandenberghe et al. (2017) [24] Canada, | 34.0 ± 12.0 78/22 | 9 | Single | 0 |
| Controlled cross-over study | ||||
| Heidt et al. (2023) [42] Germany | 25.0 (20.0–27.0) 37/63 | 19 | Single | 0 |
| Controlled intervention study | ||||
| Vandenberghe et al. (2020) [43] Canada | 28.0 ± 7.0 1 40/60 1 | 10 1 | - | Four subjects (n = 4) did not carry out all interventions. The available data were analysed. |
| Uncontrolled intervention study | ||||
| Freemantle et al. (2009) [22] Canada | 48.0 ± 1.3 -/- | 32 | - | 0 |
| Study | Intervention | Nutrient Composition | Measurement Parameters Considered | |
|---|---|---|---|---|
| Point of Time | Components | |||
| Within 15 min after the basic measurement | Standard breakfast: 2 slices of toast, jam + | |||
| 1. 280 mL lactose-free, low-fat milk, 12 g C8, 8 g C10 (emulsion) | Carbohydrates: 64.5 g Protein: 14.3 g | |||
| Courchesne-Loyer et al. (2017) [25] | 2. 280 mL lactose-free, low-fat milk, 12 g C8, 8 g C10 (non-emulsion) 3. 270 mL lactose-free, low-fat milk, 18 g C8, 12 g C10 (emulsion) 4. 270 mL lactose-free, low-fat milk, 18 g C8, 12 g C10 (non-emulsion) | Fat: 26.4 g Total MCT: 20.0 g 2 C8: 12.0 g C10: 8.0 g Energy: 544.2 kcal Carbohydrates: 64.2 g Protein: 13.9 g Fat: 36.2 g Total MCT: 30.0 g 2 C8: 18.0 g C10: 12.0 g Energy: 623.5 kcal | Total ketones 1, AUC (ßHB), Plasma C8/10/12 | |
| Freemantle et al. (2009) [22] | Within 30 min after the baseline measurement | Ketogenic breakfast drink: 100 g cream, 25 g protein powder (milk), 46 g water, 71 g MCTs | Carbohydrates: 3.0 g Protein: 25.0 g Fat: 110.0 g Total MCT: 71.0 g 2 C8: 28.3 g C10: 41.6 g C12: 1.1 g Energy: 1.012.0 kcal | Plasma glucose, ßHB |
| Heidt et al. (2023) [42] | < 1 min after the baseline measurement | 1. 200 mL water, 0.5 g/kg KG MCT oil 2. 200 mL water, 0.5 g/kg KG MCT oil, 0.2 g/kg KG glucose On average: 32 (30.0–35.5) g MCTs (19.2 g C8, 12.8 g C10) 12.7 (11.8–13.8) g Glucose | Carbohydrates: 0.0 g Protein: 0.0 g Fat: 32.0 g Total MCT: 32.0 g 2 C8: 19.2 g C10: 12.8 g Energy: 266.0 kcal Carbohydrates: 12.7 g Protein: 0.0 g Fat: 32.0 g Total MCT: 32.0 g 2 C8: 19.2 g C10: 12.8 g Energy: 317.5 kcal | Plasma glucose, ßHB, total ketones 1, AUC (ßHB), plasma C8/C10 |
| Norgren et al. (2020) [20] | Within 5–7 min after the basic measurement | 2.5 dL coffee (approx. 170 mg caffeine), 15 g cream + 1. 10 g sunflower oil + 20 g C8 2. 10 g sunflower oil + 20 g C8 + 50 g glucose | Carbohydrates: 1.2 g Protein: 0.9 g Fat: 34.8 g Total MCT: 20.0 g 2 C8: 20.0 g Energy: 305.6 kcal Carbohydrates: 51.1 g Protein: 0.9 g Fat: 34.8 g Total MCT: 20.0 g 2 C8: 20.0 g Energy: 508.4 kcal | Plasma glucose, ßHB, AUC (ßHB) |
| St-Pierre et al. (2019) [21] | Breakfast after the baseline measurement, test drink after the baseline measurement and 4 h (t4) after the baseline measurement | Standard breakfast: 2 slices of toast, raspberry jam, 1 piece of cheese, 2 scrambled eggs + 1. 250 mL lactose-free, low-fat milk + 17.3 g C8, 1.0 g C12 2. 250 mL lactose-free, low-fat milk + 9.5 g C8, 6.7 g C10, 1.7 g C12 | Carbohydrates: 62.0 g Protein: 32.5 g Fat: 41.5 g Total MCT: 18.2 g 2 C8: 17.3 g C12: 1.0 g Energy: 718.1 kcal Carbohydrates: 62.0 g Protein: 32.5 g Fat: 41.2 g Total MCT: 17.9 g 2 C8: 9.5 g C10: 6.7 g C12: 1.7 g Energy: 715.6 kcal | ßHB, total ketones 1, AUC (ßHB), plasma C8/C10/C12 |
| Vandenberghe et al. (2017) [24] | Breakfast after the baseline measurement, test drink after the baseline measurement and 4 h (t4) after the baseline measurement | Standard breakfast: 2 slices of toast, jam, 1 piece of cheese, 2 scrambled eggs + 1. 250 mL lactose-free, low-fat milk + 11.4 g C8 & 7.6 g C10 2. 250 mL lactose-free, low-fat milk + 19.0 g C8 | Carbohydrates: 65.4 g Protein: 34.9 g Fat: 44.6 g Total MCT: 19.0 g 2 C8: 11.4 g C10: 7.6 g Energy: 794.6 kcal Carbohydrates: 65.4 g Protein: 34.9 g Fat: 44.6 g Total MCT: 19.0 g 2 C8: 19.0 g Energy: 794.6 kcal | Total ketones 1, AUC (ßHB) |
| Vandenberghe et al. (2020) [43] | Breakfast after the baseline measurement, test drink after the baseline measurement and 4 h after the baseline measurement (t4) | Standard breakfast: 2 slices of wholegrain toast, strawberry jam, 1 slice of cheese, 2 scrambled eggs + 100 mL water, emulsifier, sweetener, 12 g C8, 8 g C10 | Carbohydrates: 49.0 g Protein: 23.2 g Fat: 39.5 g Total MCT: 20.0 g 2 C8: 12.0 g C10: 8.0 g Energy: 626.0 kcal | Plasma glucose, total ketones 1, AUC (ßHB) |
| Study | Caffeine Intake | Dispersion Type |
|---|---|---|
| Group 1: 23 g C8 without CH | ||
| Freemantle et al. (2009) [22] | No | Mixture |
| Heidt et al. (2023) [42] | No | Mixture |
| Norgren et al. (2020) [20] | Yes | Mixture |
| Group 2: 11 g C8 + 61 g CH | ||
| Courchesne-Loyer et al. (2017) [25] | No | Emulsion |
| Courchesne-Loyer et al. (2017) [25] | No | Mixture |
| St-Pierre et al. (2019) [21] | No | Emulsion |
| Vandenberghe et al. (2020) [43] | No | Emulsion |
| Vandenberghe et al. (2017) [24] | No | Emulsion |
| Group 3: 19 g C8 + 53 g CH | ||
| Courchesne-Loyer et al. (2017) [25] | No | Emulsion |
| Courchesne-Loyer et al. (2017) [25] | No | Mixture |
| Heidt et al. (2023) [42] | No | Mixture |
| Norgren et al. (2020) [20] | Yes | Mixture |
| St-Pierre et al. (2019) [21] | No | Emulsion |
| Vandenberghe et al. (2017) [24] | No | Emulsion |
| 23 g C8 without CH (Group 1) | 11 g C8 + 61 g CH (Group 2) | 19 g C8 + 53 g CH (Group 3) | ||||
|---|---|---|---|---|---|---|
| AUC t0–t4 [µmol × h]/L | AUC t5–t8 [µmol × h]/L | AUC t0–t4 [µmol × h]/L | AUC t5–t8 [µmol × h]/L | AUC t0–t4 [µmol × h]/L | AUC t5–t8 [µmol × h]/L | |
| Heidt et al. (2023) [42] (ßHB) 1 | 1022.37 ± 331.18 | - | 1033.92 ± 403.35 | - | - | - |
| Norgren et al. (2020) [20] (ßHB) | 450.00 ± 190.00 | - | - | - | 280.00 ± 120.00 | - |
| St-Pierre et al. (2019) [21] (ßHB + AcAc) | - | - | 339.29 ± 65.47 | 1005.95 ± 89.29 | 407.74 ± 83.33 | 1217.26 ± 127.97 |
| Vandenberghe et al. (2017) [24] (ßHB + AcAc) | - | - | 621.95 ± 97.56 | 1548.78 ± 134.15 | 780.49 ± 152.44 | 1878.05 ± 274.39 |
| Mean value ± standard deviation | 736.19 ± 286.24 | - | 665.05 ± 285.18 | 1277.37 ± 271.42 | 489.41 ± 212.47 | 1547.66 ± 330.40 |
| t0 | t0.5 | t1 | t2 | t3 | t4 | t5 | |
|---|---|---|---|---|---|---|---|
| C8 (µmol/L) | 0.00853 ± 0.00038 | 0.0341 ± 0.0385 | 0.0742 ± 0.0711 | 0.1102 ± 0.0672 | 0.1167 ± 0.0572 | 0.1153 ± 0.0591 | 0.1148 ± 0.0535 |
| C10 (µmol/L) | 0.0045 ± 0.00087 | 0.009 ± 0.0105 | 0.0099 ± 0.0078 | 0.0197 ± 0.0149 | 0.0233 ± 0.0193 | 0.0279 ± 0.0194 | 0.0278 ± 0.0176 |
| Intervention | Dropout Rate (%) | Nausea (%) | Gastric Reflux (%) | Vomiting (%) | Diarrhoea (%) | Stomach Discomfort (%) | Abdominal Discomfort/Pain (%) | |
|---|---|---|---|---|---|---|---|---|
| Courchesne-Loyer et al. (2017) [25] | MCT emulsion with CH | 0 | 20 (20 g) 1 10 (30 g) 1 | 10 (20 g)1 | 0 | 0 | 0 | 20 (10 g) 1 20 (20 g) 1 40 (30 g) 1 |
| MCT non-emulsion with CH | 0 | 0 | 10 (30 g)1 | 0 | 10 (10 g) 1 20 (20 g) 1 50 (30 g) 1 | 0 | 10 (10g) 1 30 (20 g) 1 40 (30 g) 1 | |
| Control (meal without MCT intake) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Heidt et al. (2023) [42] | MCT with glucose | 0 | 0 | 0 | 0 | 11 | 21 | 21 |
| MCT | 0 | 26 | 0 | 5 | 11 | 21 | 21 | |
| Control (water) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frenser, M.; Fischer, T.; Albrecht, I.; Marquardt, T. Influence of Carbohydrate Intake on Caprylic Acid (C8:0)-Induced Ketogenesis—A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2456. https://doi.org/10.3390/nu16152456
Frenser M, Fischer T, Albrecht I, Marquardt T. Influence of Carbohydrate Intake on Caprylic Acid (C8:0)-Induced Ketogenesis—A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(15):2456. https://doi.org/10.3390/nu16152456
Chicago/Turabian StyleFrenser, Marius, Tobias Fischer, Isabel Albrecht, and Thorsten Marquardt. 2024. "Influence of Carbohydrate Intake on Caprylic Acid (C8:0)-Induced Ketogenesis—A Systematic Review and Meta-Analysis" Nutrients 16, no. 15: 2456. https://doi.org/10.3390/nu16152456





