Effects of an Acute Dose of Zinc Monomethionine Asparate and Magnesium Asparate (ZMA) on Subsequent Sleep and Next-Day Morning Performance (Countermovement Jumps, Repeated Sprints and Stroop Test)
Abstract
:1. Introduction
2. Materials and Methods
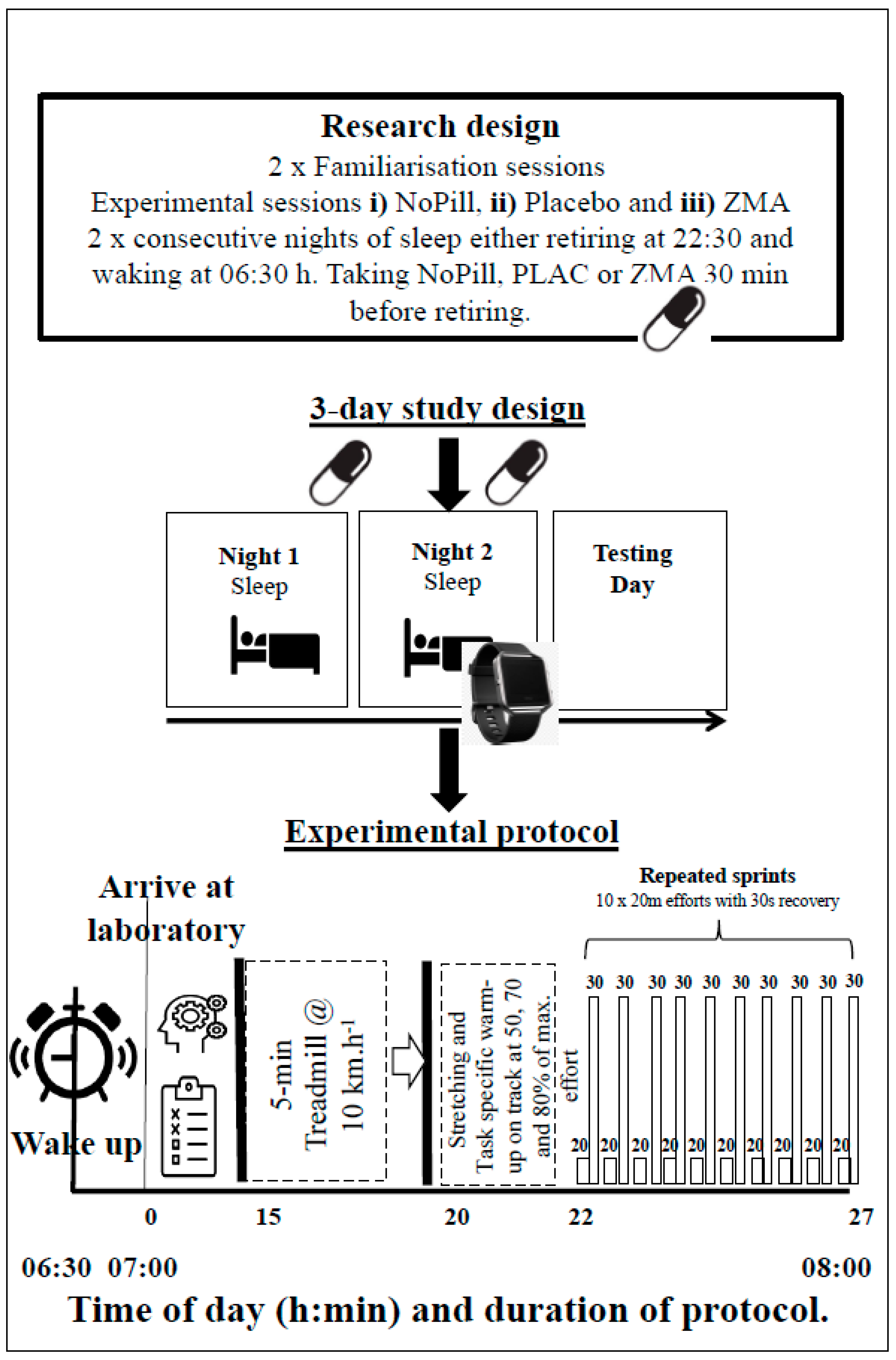
2.1. Research Design
2.2. Protocol
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Actimetry and Subjective Sleep Variables
3.2. Performance
3.2.1. Counter Movement Jump
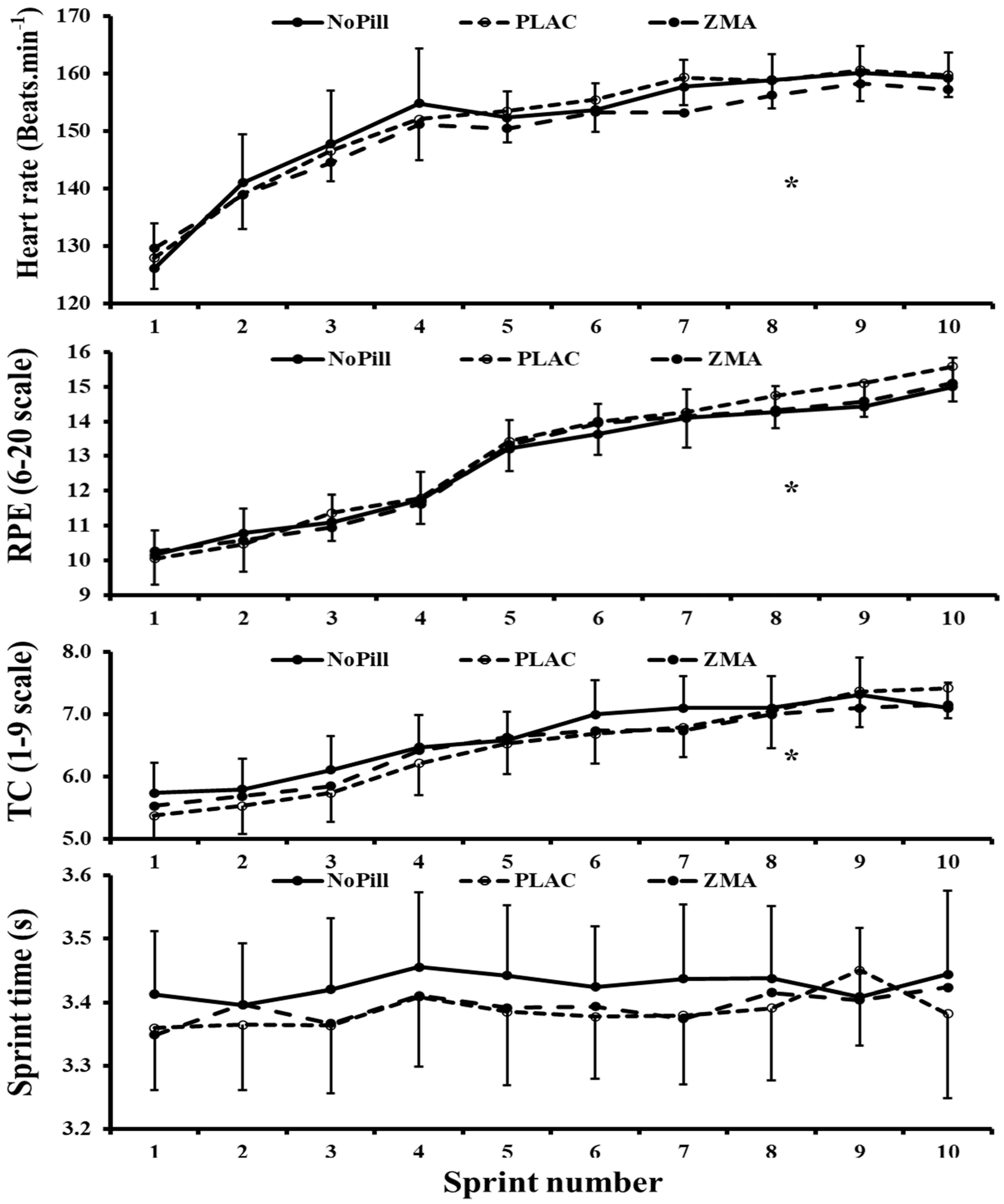
3.2.2. Repeated Sprint Performance: Split Times, HR, RPE and TC Levels
3.3. Blood Lactate
3.4. Resting POMS Questionnaire Variables
3.5. Stroop (Color–Word, Word–Color Interference Test)
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lun, V.; Erdman, K.A.; Fung, T.S.; Reimer, R.A. Dietary supplementation practices in Canadian high-performance athletes. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C. Micronutrients (magnesium, zinc, and copper): Are mineral supplements needed for athletes? Int. J. Sport Nutr. 1995, 5, S74–S83. [Google Scholar] [CrossRef] [PubMed]
- Cinar, V.; Polat, Y.; Baltaci, A.K.; Mogulkoc, R. Effects of magnesium supplementation on testosterone levels of athletes and sedentary subjects at rest and after exhaustion. Biol. Trace Elem. Res. 2011, 140, 18–23. [Google Scholar] [CrossRef]
- Ikonte, C.J.; Mun, J.G.; Reider, C.A.; Grant, R.W.; Mitmesser, S.H. Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005–2016. Nutrients 2019, 11, 2335. [Google Scholar] [CrossRef] [PubMed]
- Halson, S. Nutrition, sleep and recovery. Eur. J. Sports Sci. 2008, 8, 119–126. [Google Scholar] [CrossRef]
- Romyn, G.; Robey, E.; Dimmock, J.A.; Halson, S.L.; Peeling, P. Sleep, anxiety and electronic device use by athletes in the training and competition environments. Eur. J. Sport Sci. 2015, 16, 301–308. [Google Scholar] [CrossRef]
- Venter, R.E. Perceptions of team athletes on the importance of recovery modalities. Eur. J. Sport Sci. 2014, 14, S69. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. Br. J. Sports Med. 2021, 55, 356–368. [Google Scholar] [CrossRef]
- Edwards, B.J.; Gallagher, C.; Pullinger, S.A.; de Mello, T.M.; Walsh, N.P. Athletic Performance; Effects of Sleep Loss. In The Encyclopedia of Sleep and Circadian Rhythms, 2nd ed.; Kushida, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 434–443. [Google Scholar]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643. [Google Scholar] [CrossRef]
- Rugby Renegde: The 6 Best Supplements for Rugby Players. Available online: https://rugbyrenegade.com/the-6-best-supplements-for-rugby-players/ (accessed on 24 June 2024).
- Gleeson, M.; Nieman, D.C.; Pederson, B.K. Exercise, nutrition and immune function. J. Sports Sci. 2004, 22, 115–125. [Google Scholar] [CrossRef]
- Wilborn, C.D.; Kerksick, C.M.; Campbell, B.I.; Taylor, L.W.; Marcello, B.M.; Rasmussen, C.J.; Greenwood, M.C.; Almada, A.; Kreider, R.B. Effects of zinc magnesium aspartate (ZMA) supplementation on training adaptations and markers of anabolism and catabolism. J. Int. Soc. Sports Nutr. 2004, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and its role in cell metabolism and physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef]
- Solomon, T.M.; Leech, J.; Murphy, C.; Debros, G.; Budson, A.; Solomon, P. A randomized, double-blind, placebo controlled, parallel group, efficacy study of alpha BRAIN® administered orally. J. Int. Soc. Sports Nutr. 2015, 12, 54. [Google Scholar] [CrossRef]
- Adventure-Heart, D.J.; Madden, N.A.; Delfabbro, P. Effects of vitamin B6 (pyridoxine) and a B complex preparation on dreaming and sleep. Percept. Mot. Ski. 2018, 125, 451–462. [Google Scholar]
- Deuster, P.A.; Hodgson, A.B.; Steer, S.J.; Burke, L.M.; Castell, L.M. A–Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance: Part 46. Br. J. Sports Med. 2013, 47, 809–810. [Google Scholar] [CrossRef]
- Ji, X.; Grandner, M.A.; Liu, J. The relationship between micronutrient status and sleep patterns: A systematic review. Public Health Nutr. 2017, 20, 687–701. [Google Scholar] [CrossRef]
- Gallagher, C.; Austin, V.; Dunlop, K.A.; Dally, J.; Taylor, K.; Pullinger, S.A.; Edwards, B.J. Effects of Supplementing Zinc Magnesium Aspartate on Sleep Quality and Submaximal Weightlifting Performance, following Two Consecutive Nights of Partial Sleep Deprivation. Nutrients 2024, 16, 251. [Google Scholar] [CrossRef]
- Pullinger, S.A.; Cocking, S.; Robertson, C.M.; Tod, D.; Doran, D.A.; Burniston, J.G.; Varamenti, E.; Edwards, B.J. Time-of-day variation on performance measures in repeated-sprint tests: A systematic review. Chronobiol. Int. 2019, 37, 451–468. [Google Scholar] [CrossRef]
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Chisholm, D.M.; Collis, M.L.; Kulak, L.L.; Davenport, W.; Gruber, N. Physical activity readiness. BC Med. J. 1975, 17, 375–378. [Google Scholar]
- Smith, C.S.; Reilly, C.; Midkiff, K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J. Appl. Psychol. 1989, 74, 728. [Google Scholar] [CrossRef] [PubMed]
- Nutrition Science Team. Government Dietary Recommendations: Government Recommendations for Energy and Nutrients for Males and Females Aged 1–18 Years and 19+ Years; Public Health England: London, UK, 2016. [Google Scholar]
- Terry, P.C.; Lane, A.M.; Fogarty, G.J. Construct validity of the POMS-A for use with adults. Psychol. Sport Exerc. 2003, 4, 125–139. [Google Scholar] [CrossRef]
- Hoddes, E.; Zarcone, V.; Smythe, H.; Phillips, R.; Dement, W.C. Quantification of sleepiness: A new approach. Psychophysiology 1973, 10, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, J.; Nevill, A.; Finnegan, J.; Williams, P.; Edwards, B.; Kao, S.Y.; Reilly, T. Further assessments of the relationship between jet lag and some of its symptoms. Chronobiol. Int. 2005, 22, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.J.; Harris, C.; Jackman, Z.; Linares-Alcolea, R.; Matta, R.; Giacomoni, M.; Mahon, E.; Pullinger, S.A.; Burniston, J.G.; Doran, D.A. Is there a diurnal variation in 4-km cycling time-trial performance, conducted in an environmental chamber where a standardised approach has been employed? Biol. Rhythm. Res. 2024, 55, 184–209. [Google Scholar] [CrossRef]
- Vanrenterghem, J.; De Clercq, D.; Cleven, P.V. Necessary precautions in measuring correct vertical jumping height by means of force plate measurements. Ergonomics 2001, 44, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Physical training. 3. Perceived exertion in physical work. Lakartidningen 1970, 67, 4548–4557. [Google Scholar] [PubMed]
- Bakkevig, M.K.; Nielsen, R. Impact of wet underwear on thermoregulatory responses and thermal comfort in the cold. Ergonomics 1994, 37, 1375–1389. [Google Scholar] [CrossRef]
- Batterham, A.M.; Hopkins, W.G. Making meaningful inferences about magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef]
- Hornyak, M.; Voderholzer, U.; Hohagen, F.; Berger, M.; Riemann, D. Magnesium therapy for periodic leg movements-related Insomnia and Restless Legs syndrome: An open pilot study. Pharmacol. Sleep 1998, 21, 501–505. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Monteferrario, F.; Antoniello, N.; Manni, R.; Klersy, C. The effect of melatonin, magnesium, and zinc on primary insomnia in long-term care facility residents in Italy: A double-blind, placebo-controlled clinical trial. J. Am. Geriatr. Soc. 2011, 59, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Laires, M.J.; Monteiro, C.P.; Bicho, M. Role of cellular magnesium in health and human disease. Front. Biosci. 2004, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Frey, S.; Anders, D.; Späti, J.; Bues, M.; Pross, A.; Mager, R.; Wirz-Justice, A.; Stefani, O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. 2011, 110, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.F.; Land, J.M.; Heales, S.J. A new perspective on the treatment of aromatic L-amino acid decarboxylase deficiency. Mol. Genet. Metab. 2009, 97, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Luboshitzky, R.; Ophir, U.; Nave, R.; Epstein, R.; Shen-Orr, Z.; Herer, P. The effect of pyridoxine administration on melatonin secretion in normal men. Neuroendocrinol. Lett. 2002, 23, 213–218. [Google Scholar]
- Lichstein, K.L.; Payne, K.L.; Soeffing, J.P.; Durrence, H.H.; Taylor, J.; Riedel, B.W.; Bush, A.J. Vitamins and sleep: An exploratory study. Sleep Med. 2007, 9, 27–32. [Google Scholar] [CrossRef]
- Leeder, J.; Glaister, M.; Pizzoferro, K.; Dawson, J.; Pedlar, C. Sleep duration and quality in elite athletes measured using wristwatch actigraphy. J. Sports Sci. 2012, 30, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.; Edwards, B. Altered sleep–wake cycles and physical performance in athletes. Physiol. Behav. 2007, 90, 274–784. [Google Scholar] [CrossRef]
- Beedie, C.J.; Foad, A.J. The placebo effect in sports performance: A brief review. Sports Med. 2009, 39, 313–329. [Google Scholar] [CrossRef]
- Moëzzi, N.; Peeri, M.; Matin, H. Effects of zinc, magnesium and vitamin B6 supplementation on hormones and performance in weightlifters. Ann. Biol. Res. 2013, 4, 163–168. [Google Scholar]
- Brilla, L.R.; Conte, V. Effects of a novel zinc-magnesium formulation on hormones and strength. J. Exerc. Physiol. Online 2000, 3, 26–36. [Google Scholar]
- Di Salvo, V.; Baron, R.; González-Haro, C.; Gormasz, C.; Pigozzi, F.; Bachl, N. Sprinting analysis of elite soccer players during European Champions League and UEFA Cup matches. J. Sports Sci. 2010, 28, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Jeacocke, N.A.; Burke, L.M. Methods to standardize dietary intake before performance testing. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Favier, A.E. The role of zinc in reproduction: Hormonal mechanisms. Biol. Trace Elem. Res. 1992, 32, 363–382. [Google Scholar] [CrossRef]
- Prasad, A.S.; Mantzoros, C.S.; Beck, F.W.; Hess, J.W.; Brewer, G.J. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996, 12, 344–348. [Google Scholar] [CrossRef]
| Participant Variables | ||
|---|---|---|
| Physical characteristics | ||
| Age (year) | 24.6 ± 5 | |
| Height (cm) | 175.9 ± 7.5 | |
| Mass (kg) | 77.9 ± 9.7 | |
| Baseline actimetry | ||
| Fragmentation (Au) | 29.0 ± 13.0 | |
| Sleep efficiency (%) | 82.0 ± 7.0 | |
| Actual sleep time (h:mm) | 6:44 ± 00:45 | |
| Habitual retiring time (h:mm) | 23:10 ± 00:31 | |
| Habitual wake time (h:mm) | 7:13 ± 00:38 | |
| Sleep onset latency (h:mm) | 0:12 ± 00:12 | |
| Time in bed (h:mm) | 22:30 ± 00:32 | |
| Wake time (h:min) | 07:10 ± 00:32 | |
| Baseline food intake | Participants intake | RDA * |
| Daily calories (kcal) | 2688 ± 632 | 2500 (p = 0.210) |
| Fats (g) | 153 ± 85 | 97 (p = 0.010) |
| Protein (g) | 140 ± 45 | 55.5 (p < 0.001) |
| Carbohydrates (g) | 188 ± 100 | 330 (p < 0.001) |
| Zinc (mg) | 11.9 ± 3.4 | 11 (p = 0.257) |
| Magnesium (mg) | 395 ± 103 | 400 (p = 0.832) |
| Vitamin B6 (mg) | 2.7 ± 0.9 | 1.4 (p < 0.001) |
| Variable | NoPill | PLAC | ZMA | Main Effect for | Main Effect Sprint/Pre-Post, d and Observed Power | Interaction | ||
|---|---|---|---|---|---|---|---|---|
| Condition, d and Observed Power | ||||||||
| Actigraphy | ||||||||
| Sleep onset latency (h:mm) | 0:20 ± 0:20 | 0:21 ± 0:27 | 0:15 ± 0:12 | p = 0.532 | 0.35, 13.5 | |||
| Sleep efficiency (%) | 80.0 ± 7.0 | 81.0 ± 8.0 | 79.0 ± 7.0 | p = 0.408 | 0.30, 18.7 | |||
| Actual sleep time (h:mm) | 6:09 ± 0:31 | 6:08 ± 0:31 | 6:00 ± 0:32 | p = 0.558 | 0.24, 14.1 | |||
| Fragmentation index (%) | 30.9 ± 11.5 | 28.1 ± 10.1 | 29.2 ± 13.8 | p = 0.702 | 0.22, 97.0 | |||
| Subjective Sleep QTN | ||||||||
| Waterhouse QTN | ||||||||
| Ease to sleep | −0.7 ± 1.8 | −0.7 ± 2.3 | −1.1 ± 2.6 | p = 0.812 | 0.17, 80.0 | |||
| Time to sleep | −0.6 ± 1.9 | −0.2 ± 2.5 | −0.5 ± 2.1 | p = 0.808 | 0.14, 81.0 | |||
| How well did you sleep | −0.9 ± 1.9 | −0.4 ± 2.2 | −0.9 ± 2.0 | p = 0.679 | 0.23, 10.8 | |||
| What was your waking time | −1.3 ± 2.3 | −0.8 ± 1.9 | −0.8 ± 1.6 | p = 0.592 | 0.27, 100.0 | |||
| Alertness 30-min after waking | −0.3 ± 2.3 | 0.1 ± 1.5 | 0.2 ± 1.9 | p = 0.672 | 0.06, 94.0 | |||
| Stanford Sleep QTN | ||||||||
| Degree of sleepiness | 3.2 ± 0.7 | 2.9 ± 0.7 | 2.9 ± 1.1 | p = 0.613 | 0.05, 11.6 | |||
| Cognitive Function | ||||||||
| Stroop (no. of mistakes) | 4.2 ± 3.1 | 6.5 ± 1.2 | 6.5 ± 1.2 | p = 0.609 | 0.28, 12.5 | |||
| Stroop (no. completed) | 149.8 ± 24.1 | 150.1 ± 25.1 | 153.2 ± 30.5 | p = 0.795 | 0.13, 83.0 | |||
| Jump (CMJ) | ||||||||
| Height (cm) | 32.5 ± 6.2 | 30.7 ± 5.6 * | 32.3 ± 5.7 | p < 0.001 | 0.354, 97.5 | |||
| Repeated Sprint Ability (RS) | ||||||||
| Split time (s) | 3.43 ± 0.23 | 3.39 ± 0.25 | 3.39 ± 0.24 | p = 0.052 | 0.147, 57.0 | p = 0.217 | 0.176, 0.439 | p = 0.162 |
| RPE (6–20) | 12.8 ± 2.3 | 13.1 ± 2.6 | 12.9 ± 2.6 | p = 0.640 | 0.017, 11.7 | p < 0.001 | 2.797, 100.0 | p = 0.354 |
| TC (1–9) | 6.6 ± 1.2 | 4.0 ± 2.1 | 3.8 ± 2.2 | p = 0.454 | 0.122, 17.7 | p < 0.001 | 1.707, 100.0 | p = 0.218 |
| HR (beats.min−1) | 151 ± 18 | 151 ± 17 | 149 ± 14 | p = 0.543 | 0.108, 14.6 | p < 0.001 | 2.560, 100.0 | p = 0.752 |
| Blood | ||||||||
| Lactate Pre (mmol L−1) | 0.9 ± 0.2 | 1.1 ± 0.4 | 1.0 ± 0.3 | p = 0.075 | 0.199, 51.3 | p < 0.005 | 3.998, 100.0 | p = 0.081 |
| Lactate Post (mmol L−1) | 5.7 ± 2.2 | 5.7 ± 1.9 | 6.2 ± 2.2 | |||||
| Variable | NoPill | PLAC | ZMA | CON | Time-of-Day | Interaction | |||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Sleep (30-min) | After Wake (30-min) | Pre-Sleep (30-min) | After Wake (30-min) | Pre-Sleep (30-min) | After Wake (30-min) | ||||
| POMS | |||||||||
| Anger | 0.9 ± 1.7 | 0.7 ± 1.7 | 1.2 ± 2.8 | 0.6 ± 1.5 | 1.3 ± 2.1 | 1.2 ± 2.3 | p = 0.682 | p = 0.111 | p = 0.786 |
| Depression | 1.1 ± 2.1 | 0.7 ± 1.7 | 1.1 ± 2.1 | 0.5 ± 1.1 | 0.8 ± 1.5 | 0.8 ± 1.5 | p = 0.900 | p = 0.052 | p = 0.484 |
| Vigour | 4.2 ± 3.3 | 5.0 ± 3.1 | 5.4 ± 3.5 | 5.8 ± 3.3 | 4.9 ± 3.1 | 5.3 ± 2.6 | p = 0.234 | p = 0.371 | p = 0.878 |
| Calm | 8.6 ± 3.9 | 7.5 ± 3.6 | 8.4 ± 2.9 | 6.3 ± 3.1 | 7.5 ± 3.9 | 6.7 ± 2.8 | p = 0.465 | p = 0.005 | p = 0.295 |
| Tension | 0.3 ± 0.9 | 0.3 ± 0.8 | 0.5 ± 1.2 | 0.4 ± 0.8 | 0.7 ± 1.4 | 0.1 ± 0.2 | p = 0.610 | p = 0065 | p = 0.036 |
| Confusion | 1.3 ± 1.9 | 0.9± 2.0 | 1.5 ± 2.6 | 1.0 ± 1.6 | 1.2 ± 1.9 | 0.7 ± 1.6 | p = 0.713 | p = 0.002 | p = 0.934 |
| Fatigue | 7.3 ± 2.2 | 5.9 ± 1.8 | 6.8 ± 2.2 | 4.4 ± 2.2 | 5.9 ± 2.7 | 5.1 ± 2.5 | p = 0.022 | p = 0.001 | p = 0.016 |
| Happy | 8.1 ± 3.7 | 5.6 ± 2.8 | 7.4 ± 3.4 | 6.2 ± 2.9 | 6.6 ± 3.4 | 5.3 ± 3.0 | p = 0.223 | p = 0.001 | p = 0.347 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, B.J.; Adam, R.L.; Drummond, D.; Gallagher, C.; Pullinger, S.A.; Hulton, A.T.; Richardson, L.D.; Donovan, T.F. Effects of an Acute Dose of Zinc Monomethionine Asparate and Magnesium Asparate (ZMA) on Subsequent Sleep and Next-Day Morning Performance (Countermovement Jumps, Repeated Sprints and Stroop Test). Nutrients 2024, 16, 2466. https://doi.org/10.3390/nu16152466
Edwards BJ, Adam RL, Drummond D, Gallagher C, Pullinger SA, Hulton AT, Richardson LD, Donovan TF. Effects of an Acute Dose of Zinc Monomethionine Asparate and Magnesium Asparate (ZMA) on Subsequent Sleep and Next-Day Morning Performance (Countermovement Jumps, Repeated Sprints and Stroop Test). Nutrients. 2024; 16(15):2466. https://doi.org/10.3390/nu16152466
Chicago/Turabian StyleEdwards, Ben J., Ryan L. Adam, Dan Drummond, Chloe Gallagher, Samuel A. Pullinger, Andrew T. Hulton, Lucinda D. Richardson, and Timothy F. Donovan. 2024. "Effects of an Acute Dose of Zinc Monomethionine Asparate and Magnesium Asparate (ZMA) on Subsequent Sleep and Next-Day Morning Performance (Countermovement Jumps, Repeated Sprints and Stroop Test)" Nutrients 16, no. 15: 2466. https://doi.org/10.3390/nu16152466
APA StyleEdwards, B. J., Adam, R. L., Drummond, D., Gallagher, C., Pullinger, S. A., Hulton, A. T., Richardson, L. D., & Donovan, T. F. (2024). Effects of an Acute Dose of Zinc Monomethionine Asparate and Magnesium Asparate (ZMA) on Subsequent Sleep and Next-Day Morning Performance (Countermovement Jumps, Repeated Sprints and Stroop Test). Nutrients, 16(15), 2466. https://doi.org/10.3390/nu16152466







