A Systematic Review of the Gene–Lifestyle Interactions on Metabolic Disease-Related Outcomes in Arab Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria and Search Strategy
2.2. Data Collection, Extraction, and Synthesis Methods
2.3. Quality and Risk of Bias Assessments
3. Results and Discussion
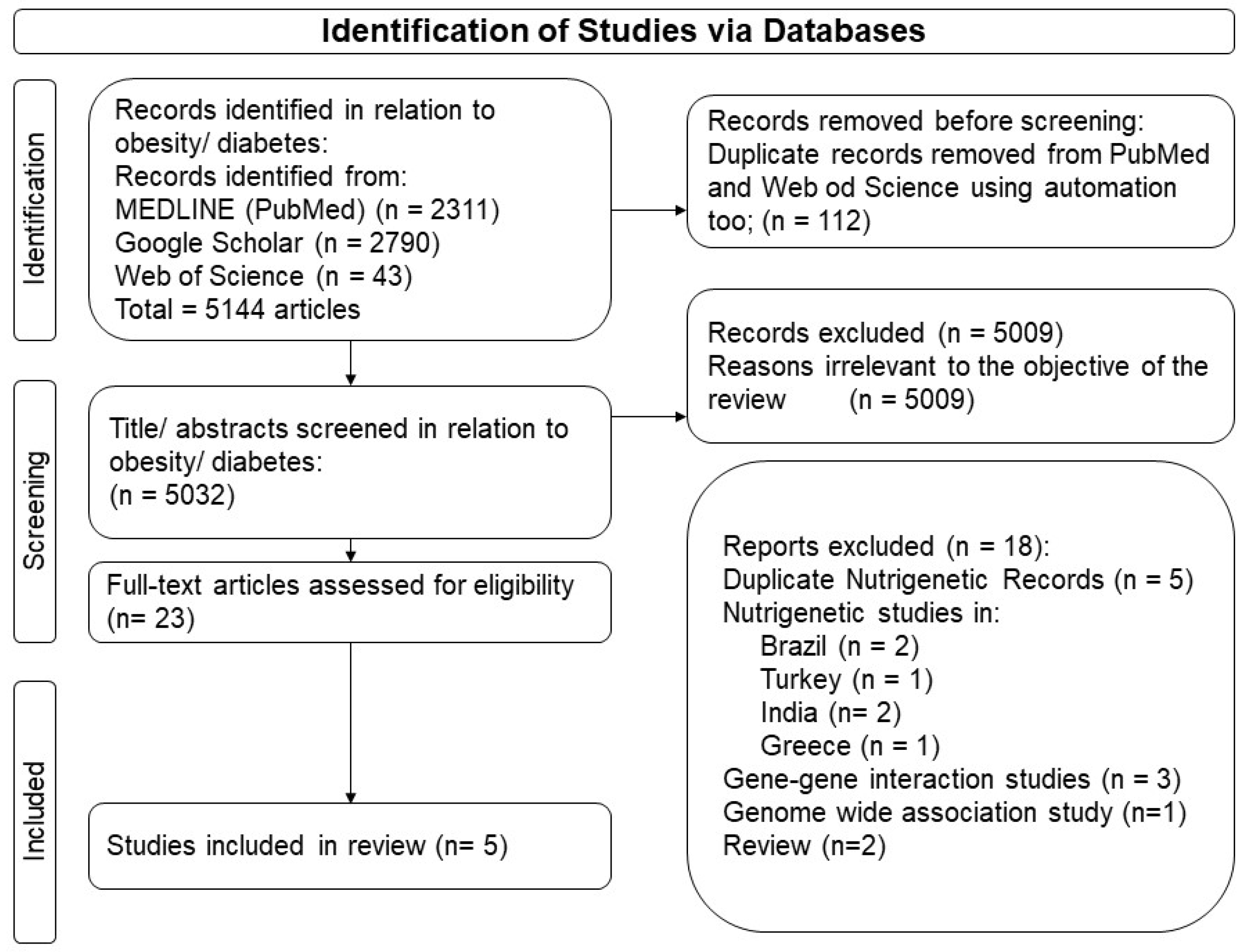
3.1. Outputs from the Search Strategy
3.2. Gene–Lifestyle Interactions on Obesity and Diabetes Traits
3.2.1. Gene–Diet and Gene–Physical Activity Interactions on Obesity Traits
Interaction between SNP TCF7L2 rs7903146 and Diet on Obesity in the Lebanese Population
Interaction between MC4R SNP rs17782313 and Diet or Physical Activity on Obesity in the Lebanese Population
Interaction between MTHFR SNP rs1801133 and Diet on Obesity in the Lebanese Population
Interaction between FTO rs9939609 and rs1558902 and Diet or Physical Activity on Obesity in the Lebanese and Emirati Populations
Interaction between VDR rs1544410 (BsmI) and Physical Activity on Obesity in the Emirati Population
3.2.2. Gene–Diet Interactions on Diabetes Traits
Interaction between TCF7L2 SNPs rs7903146 and Diet on T2D in the Algerian Population
The Interaction between CYP1A2 SNPs rs2470890, rs2069526, rs762551, rs2472304, and rs4646427 and Diet on T2D in the Lebanese Population
3.3. Risk of Bias Assessment
Quality and Risk of Bias Assessments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.W.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Loos, R.J.; Marshall, S.M.; Zierath, J.R. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell 2021, 184, 1530–1544. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Loos, R.J. Progress in the genetics of common obesity and type 2 diabetes. Expert Rev. Mol. Med. 2010, 12, e7. [Google Scholar] [CrossRef]
- Temelkova-Kurktschiev, T.; Stefanov, T. Lifestyle and genetics in obesity and type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2012, 120, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hamamy, H.A.; Al-Allawi, N.A. Epidemiological profile of common haemoglobinopathies in Arab countries. J. Community Genet. 2013, 4, 147–167. [Google Scholar] [CrossRef]
- Lewis, B. Arabs in History; OUP Oxford: Oxford, UK, 2002. [Google Scholar]
- Population, Total-Arab World; The World Bank Group: Washington, DC, USA, 2002; Available online: https://data.worldbank.org/region/arab-world (accessed on 19 July 2024).
- Mokdad, A.H.; Jaber, S.; Aziz, M.I.A.; AlBuhairan, F.; AlGhaithi, A.; AlHamad, N.M.; Al-Hooti, S.N.; Al-Jasari, A.; AlMazroa, M.A.; AlQasmi, A.M. The state of health in the Arab world, 1990–2010: An analysis of the burden of diseases, injuries, and risk factors. Lancet 2014, 383, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Alzaman, N.; Ali, A. Obesity and diabetes mellitus in the Arab world. J. Taibah Univ. Med. Sci. 2016, 11, 301–309. [Google Scholar] [CrossRef]
- Younes, S.; Ibrahim, A.; Al-Jurf, R.; Zayed, H. Genetic polymorphisms associated with obesity in the Arab world: A systematic review. Int. J. Obes. 2021, 45, 1899–1913. [Google Scholar] [CrossRef]
- Teebi, A.S. Introduction: Genetic diversity among arabs. Genet. Disord. Among Arab Popul. 2005, 3–34. [Google Scholar] [CrossRef]
- Mirkin, B. Population Levels, Trends and Policies in the Arab Region: Challenges and Opportunities; United Nations Development Programme, Regional Bureau for Arab States USA: New York, NY, USA, 2010. [Google Scholar]
- Rahim, H.F.A.; Sibai, A.; Khader, Y.; Hwalla, N.; Fadhil, I.; Alsiyabi, H.; Mataria, A.; Mendis, S.; Mokdad, A.H.; Husseini, A. Non-communicable diseases in the Arab world. Lancet 2014, 383, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Sharara, E.; Akik, C.; Ghattas, H.; Makhlouf Obermeyer, C. Physical inactivity, gender and culture in Arab countries: A systematic assessment of the literature. BMC Public Health 2018, 18, 639. [Google Scholar] [CrossRef] [PubMed]
- Abuhendi, N.; Qush, A.; Naji, F.; Abunada, H.; Al Buainain, R.; Shi, Z.; Zayed, H. Genetic polymorphisms associated with type 2 diabetes in the Arab world: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2019, 151, 198–208. [Google Scholar] [CrossRef]
- Abuyassin, B.; Laher, I. Obesity-linked diabetes in the Arab world: A review. East Mediterr. Health J. 2015, 21, 420–439. [Google Scholar] [CrossRef]
- Osman, W.; Tay, G.K.; Alsafar, H. Multiple genetic variations confer risks for obesity and type 2 diabetes mellitus in arab descendants from UAE. Int. J. Obes. 2018, 42, 1345–1353. [Google Scholar] [CrossRef]
- O’Beirne, S.L.; Salit, J.; Rodriguez-Flores, J.L.; Staudt, M.R.; Abi Khalil, C.; Fakhro, K.A.; Robay, A.; Ramstetter, M.D.; Al-Azwani, I.K.; Malek, J.A. Type 2 diabetes risk allele loci in the Qatari population. PLoS ONE 2016, 11, e0156834. [Google Scholar] [CrossRef]
- Serván, P.R. Obesity and diabetes. Nutr. Hosp. 2013, 28, 138–143. [Google Scholar]
- Vimaleswaran, K.S. Gene–Nutrient Interactions on Metabolic Diseases: Findings from the GeNuIne Collaboration; Wiley Online Library: Hoboken, NJ, USA, 2017. [Google Scholar]
- Hosseini-Esfahani, F.; Koochakpoor, G.; Daneshpour, M.S.; Sedaghati-Khayat, B.; Mirmiran, P.; Azizi, F. Mediterranean dietary pattern adherence modify the association between FTO genetic variations and obesity phenotypes. Nutrients 2017, 9, 1064. [Google Scholar] [CrossRef]
- Khan, S.M.; El Hajj Chehadeh, S.; Abdulrahman, M.; Osman, W.; Al Safar, H. Establishing a genetic link between FTO and VDR gene polymorphisms and obesity in the Emirati population. BMC Med. Genet. 2018, 19, 11. [Google Scholar] [CrossRef]
- Nasreddine, L.; Akika, R.; Mailhac, A.; Tamim, H.; Zgheib, N.K. The interaction between genetic polymorphisms in FTO and TCF7L2 genes and dietary intake with regard to body mass and composition: An exploratory study. J. Pers. Med. 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Aoun, C.; Hajj, A.; Hajj, F.; Papazian, T.; Khabbaz, L.R. The interaction between genetic polymorphisms in FTO, MC4R and MTHFR genes and adherence to the Mediterranean Diet in relation to obesity. Gene 2022, 809, 146037. [Google Scholar] [CrossRef]
- Ouhaibi-Djellouli, H.; Mediene-Benchekor, S.; Lardjam-Hetraf, S.A.; Hamani-Medjaoui, I.; Meroufel, D.N.; Boulenouar, H.; Hermant, X.; Saidi-Mehtar, N.; Amouyel, P.; Houti, L. The TCF7L2rs7903146 polymorphism, dietary intakes and type 2 diabetes risk in an Algerian population. BMC Genet. 2014, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Platt, D.E.; Ghassibe-Sabbagh, M.; Salameh, P.; Salloum, A.K.; Haber, M.; Mouzaya, F.; Gauguier, D.; Al-Sarraj, Y.; El-Shanti, H.; Zalloua, P.A. Caffeine impact on metabolic syndrome components is modulated by a CYP1A2 variant. Ann. Nutr. Metab. 2016, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Alathari, B.E.; Bodhini, D.; Jayashri, R.; Lakshmipriya, N.; Shanthi Rani, C.S.; Sudha, V.; Lovegrove, J.A.; Anjana, R.M.; Mohan, V.; Radha, V.; et al. A Nutrigenetic Approach to Investigate the Relationship between Metabolic Traits and Vitamin D Status in an Asian Indian Population. Nutrients 2020, 12, 1357. [Google Scholar] [CrossRef]
- Roswall, N.; Ängquist, L.; Ahluwalia, T.S.; Romaguera, D.; Larsen, S.C.; Østergaard, J.N.; Halkjær, J.; Vimaleswaran, K.S.; Wareham, N.J.; Bendinelli, B. Association between Mediterranean and Nordic diet scores and changes in weight and waist circumference: Influence of FTO and TCF7L2 loci. Am. J. Clin. Nutr. 2014, 100, 1188–1197. [Google Scholar] [CrossRef]
- Bodhini, D.; Gaal, S.; Shatwan, I.; Ramya, K.; Ellahi, B.; Surendran, S.; Sudha, V.; Anjana, M.R.; Mohan, V.; Lovegrove, J.A. Interaction between TCF7L2 polymorphism and dietary fat intake on high density lipoprotein cholesterol. PLoS ONE 2017, 12, e0188382. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Coltell, O.; Sorli, J.V.; Estruch, R.; Quiles, L.; Martínez-González, M.Á.; Salas-Salvado, J.; Castañer, O.; Arós, F.; Ortega-Calvo, M. Polymorphism of the transcription factor 7-like 2 gene (TCF7L2) interacts with obesity on type-2 diabetes in the PREDIMED study emphasizing the heterogeneity of genetic variants in type-2 diabetes risk prediction: Time for obesity-specific genetic risk scores. Nutrients 2016, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, I. The public health rationale for reducing saturated fat intakes: Is a maximum of 10% energy intake a good recommendation? Nutr. Bull. 2020, 45, 271–280. [Google Scholar] [CrossRef]
- Council, N.R. Recommended Dietary Allowances; National Academies Press: Washington, DC, USA, 1989. [Google Scholar] [CrossRef]
- Hwalla, N.; Jomaa, L.; Hachem, F.; Kharroubi, S.; Hamadeh, R.; Nasreddine, L.; Naja, F. Promoting sustainable and healthy diets to mitigate food insecurity amidst economic and health crises in lebanon. Front. Nutr. 2021, 8, 697225. [Google Scholar] [CrossRef] [PubMed]
- Fat: The Facts. Available online: https://www.nhs.uk/live-well/eat-well/food-types/different-fats-nutrition/#:~:text=Saturated%20fat%20guidelines,of%20saturated%20fat%20a%20day (accessed on 6 February 2024).
- Grau, K.; Cauchi, S.; Holst, C.; Astrup, A.; Martinez, J.A.; Saris, W.H.; Blaak, E.E.; Oppert, J.-M.; Arner, P.; Rössner, S. TCF7L2 rs7903146–macronutrient interaction in obese individuals’ responses to a 10-wk randomized hypoenergetic diet. Am. J. Clin. Nutr. 2010, 91, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Mattei, J.; Qi, Q.; Hu, F.B.; Sacks, F.M.; Qi, L. TCF7L2 genetic variants modulate the effect of dietary fat intake on changes in body composition during a weight-loss intervention. Am. J. Clin. Nutr. 2012, 96, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Haupt, A.; Thamer, C.; Heni, M.; Ketterer, C.; Machann, J.; Schick, F.; Machicao, F.; Stefan, N.; Claussen, C.D.; Häring, H.-U. Gene variants of TCF7L2 influence weight loss and body composition during lifestyle intervention in a population at risk for type 2 diabetes. Diabetes 2010, 59, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Kraft, P.; Hunter, D.J.; Hu, F.B. The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum. Mol. Genet. 2008, 17, 3502–3508. [Google Scholar] [CrossRef]
- Xi, B.; Chandak, G.R.; Shen, Y.; Wang, Q.; Zhou, D. Association between common polymorphism near the MC4R gene and obesity risk: A systematic review and meta-analysis. PLoS ONE 2012, 7, e45731. [Google Scholar] [CrossRef]
- Yu, K.; Li, L.; Zhang, L.; Guo, L.; Wang, C. Association between MC4R rs17782313 genotype and obesity: A meta-analysis. Gene 2020, 733, 144372. [Google Scholar] [CrossRef]
- Helou, K.; El Helou, N.; Mahfouz, M.; Mahfouz, Y.; Salameh, P.; Harmouche-Karaki, M. Validity and reliability of an adapted arabic version of the long international physical activity questionnaire. BMC Public Health 2018, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, E.; Shalash, A.; Martin, R.; Rmeileh, N.A. Measurement and prevalence of adult physical activity levels in Arab countries. Public Health 2021, 198, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Cherfan, M.; Blacher, J.; Asmar, R.; Chahine, M.N.; Zeidan, R.K.; Farah, R.; Salameh, P. Prevalence and risk factors of hypertension: A nationwide cross-sectional study in Lebanon. J. Clin. Hypertens. 2018, 20, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Koochakpoor, G.; Hosseini-Esfahani, F.; Daneshpour, M.S.; Hosseini, S.A.; Mirmiran, P. Effect of interactions of polymorphisms in the Melanocortin-4 receptor gene with dietary factors on the risk of obesity and Type 2 diabetes: A systematic review. Diabet. Med. 2016, 33, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, M.; Jafarabadi, M.A.; Farhangi, M.A. Melanocortin-4 receptor (MC4R) rs17782313 polymorphism interacts with Dietary Approach to Stop Hypertension (DASH) and Mediterranean Dietary Score (MDS) to affect hypothalamic hormones and cardio-metabolic risk factors among obese individuals. Genes Nutr. 2020, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, M.; Kahroba, H.; Jafarabadi, M.A.; Mesgari-Abbasi, M.; Farhangi, M.A. Dietary quality indices modifies the effects of melanocortin-4 receptor (MC4R) rs17782313 polymorphism on cardio-metabolic risk factors and hypothalamic hormones in obese adults. BMC Cardiovasc. Disord. 2020, 20, 57. [Google Scholar] [CrossRef]
- Moehlecke, M.; Canani, L.H.; Trindade, M.R.M.; Friedman, R.; Leitão, C.B. Determinants of body weight regulation in humans. Arch. Endocrinol. Metab. 2016, 60, 152–162. [Google Scholar] [CrossRef]
- Yarizadeh, H.; Bahiraee, A.; Asadi, S.; Maddahi, N.S.; Setayesh, L.; Casazza, K.; Mirzaei, K. The interaction between dietary approaches to stop hypertension and MC4R gene variant in predicting cardiovascular risk factors. Int. J. Vitam. Nutr. Res. 2020, 92, 376–384. [Google Scholar] [CrossRef]
- Mousavizadeh, Z.; Hosseini-Esfahani, F.; Javadi, A.; Daneshpour, M.S.; Akbarzadeh, M.; Javadi, M.; Mirmrian, P.; Azizi, F. The interaction between dietary patterns and melanocortin-4 receptor polymorphisms in relation to obesity phenotypes. Obes. Res. Clin. Pract. 2020, 14, 249–256. [Google Scholar] [CrossRef]
- Vesnina, A.; Prosekov, A.; Kozlova, O.; Atuchin, V. Genes and eating preferences, their roles in personalized nutrition. Genes 2020, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Bordoni, L.; Muscogiuri, G.; Colao, A.; Savastano, S.; Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group. Nutrigenetics—Personalized nutrition in obesity and cardiovascular diseases. Int. J. Obes. Suppl. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; You, D.; Wang, H.; Yang, Y.; Zhang, D.; Lv, J.; Luo, S.; Liao, R.; Ma, L. Association between homocysteine and obesity: A meta-analysis. J. Evid. Based Med. 2021, 14, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Bodhini, D.; Lakshmipriya, N.; Ramya, K.; Anjana, R.M.; Sudha, V.; Lovegrove, J.A.; Kinra, S.; Mohan, V.; Radha, V. Interaction between FTO gene variants and lifestyle factors on metabolic traits in an Asian Indian population. Nutr. Metab. 2016, 13, 39. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Sun, B.-F.; Zhao, Y.-L.; Yang, Y.-G. FTO and obesity: Mechanisms of association. Curr. Diab. Rep. 2014, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.L.; Yeo, G.S. From GWAS to biology: Lessons from FTO. Ann. N. Y. Acad. Sci. 2011, 1220, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Shimaoka, I.; Kamide, K.; Ohishi, M.; Katsuya, T.; Akasaka, H.; Saitoh, S.; Sugimoto, K.; Oguro, R.; Congrains, A.; Fujisawa, T. Association of gene polymorphism of the fat-mass and obesity-associated gene with insulin resistance in Japanese. Hypertens. Res. 2010, 33, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, S.; Xu, H.; Qian, J.; Yang, J. Genetic variants in FTO associated with metabolic syndrome: A meta-and gene-based analysis. Mol. Biol. Rep. 2012, 39, 5691–5698. [Google Scholar] [CrossRef]
- Bhurosy, T.; Jeewon, R. Pitfalls of using body mass index (BMI) in assessment of obesity risk. Curr. Res. Nutr. Food Sci. J. 2013, 1, 71–76. [Google Scholar] [CrossRef]
- Haddad, C.; Lahoud, N.; Akel, M.; Sacre, H.; Hajj, A.; Hallit, S.; Salameh, P. Knowledge, attitudes, harm perception, and practice related to waterpipe smoking in Lebanon. Environ. Sci. Pollut. Res. 2020, 27, 17854–17863. [Google Scholar] [CrossRef]
- Baalbaki, R.; Itani, L.; El Kebbi, L.; Dehni, R.; Abbas, N.; Farsakouri, R.; Awad, D.; Tannir, H.; Kreidieh, D.; El Masri, D. Association between smoking hookahs (shishas) and higher risk of obesity: A systematic review of population-based studies. J. Cardiovasc. Dev. Dis. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Bonanni, A.E.; Di Castelnuovo, A.; De Lucia, F.; Donati, M.B.; De Gaetano, G.; Iacoviello, L.; Investigators, M.-s.P. Low income is associated with poor adherence to a Mediterranean diet and a higher prevalence of obesity: Cross-sectional results from the Moli-sani study. BMJ Open 2012, 2, e001685. [Google Scholar] [CrossRef] [PubMed]
- Musaiger, A.O. Overweight and obesity in eastern mediterranean region: Prevalence and possible causes. J. Obes. 2011, 2011, 407237. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019, 34, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S. GeNuIne (gene–nutrient interactions) Collaboration: Towards implementing multi-ethnic population-based nutrigenetic studies of vitamin B12 and D deficiencies and metabolic diseases. Proc. Nutr. Soc. 2021, 80, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Leońska-Duniec, A.; Jastrzębski, Z.; Zarębska, A.; Maciejewska, A.; Ficek, K.; Cięszczyk, P. Assessing effect of interaction between the FTO A/T polymorphism (rs9939609) and physical activity on obesity-related traits. J. Sport Health Sci. 2018, 7, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Isgin-Atici, K.; Alsulami, S.; Turan-Demirci, B.; Surendran, S.; Sendur, S.N.; Lay, I.; Karabulut, E.; Ellahi, B.; Lovegrove, J.A.; Alikasifoglu, M.; et al. FTO gene-lifestyle interactions on serum adiponectin concentrations and central obesity in a Turkish population. Int. J. Food Sci. Nutr. 2021, 72, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Tachmazidou, I.; Zhao, J.H.; Hirschhorn, J.N.; Dudbridge, F.; Loos, R.J. Candidate genes for obesity-susceptibility show enriched association within a large genome-wide association study for BMI. Hum. Mol. Genet. 2012, 21, 4537–4542. [Google Scholar] [CrossRef]
- Molag, M.L.; de Vries, J.H.; Ocké, M.C.; Dagnelie, P.C.; van den Brandt, P.A.; Jansen, M.C.; van Staveren, W.A.; van’t Veer, P. Design characteristics of food frequency questionnaires in relation to their validity. Am. J. Epidemiol. 2007, 166, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. A meta-analysis of the reproducibility of food frequency questionnaires in nutritional epidemiological studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 12. [Google Scholar] [CrossRef]
- Gordon, D.C. The Republic of Lebanon: Nation in Jeopardy; Routledge: London, UK, 2016. [Google Scholar] [CrossRef]
- Makhoul, N.; Wells, R.; Kaspar, H.; Shbaklo, H.; Taher, A.; Chakar, N.; Zalloua, P. Genetic heterogeneity of Beta thalassemia in Lebanon reflects historic and recent population migration. Ann. Hum. Genet. 2005, 69, 55–66. [Google Scholar] [CrossRef]
- Haddad, S. Lebanon: From consociationalism to conciliation. Natl. Ethn. Politics 2009, 15, 398–416. [Google Scholar] [CrossRef]
- Maktabi, R. The Lebanese census of 1932 revisited. Who are the Lebanese? Br. J. Middle East. Stud. 1999, 26, 219–241. [Google Scholar] [CrossRef]
- Alghamdi, J.; Padmanabhan, S. Fundamentals of complex trait genetics and association studies. Handb. Pharmacogenomics Stratif. Med. 2014, 1, 235–257. [Google Scholar]
- Kilpeläinen, T.O.; Qi, L.; Brage, S.; Sharp, S.J.; Sonestedt, E.; Demerath, E.; Ahmad, T.; Mora, S.; Kaakinen, M.; Sandholt, C.H. Physical activity attenuates the influence of FTO variants on obesity risk: A meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011, 8, e1001116. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alkharfy, K.M.; Khan, N.; Mohammed, A.K.; Vinodson, B.; Ansari, M.G.; Alenad, A.; Alokail, M.S. Association of VDR-gene variants with factors related to the metabolic syndrome, type 2 diabetes and vitamin D deficiency. Gene 2014, 542, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Jin, H.S.; Eom, Y.B. The interaction between FTO rs9939609 and physical activity is associated with a 2-fold reduction in the risk of obesity in Korean population. Am. J. Hum. Biol. 2021, 33, e23489. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Ortega-Azorin, C.; Sorli, J.V.; Covas, M.I.; Carrasco, P.; Salas-Salvado, J.; Martínez-González, M.Á.; Aros, F.; Lapetra, J.; Serra-Majem, L. Statistical and biological gene-lifestyle interactions of MC4R and FTO with diet and physical activity on obesity: New effects on alcohol consumption. PLoS ONE 2012, 7, e52344. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Ängquist, L.; Hansen, R.D.; Van Der A, D.L.; Bouatia-Naji, N.; Holst, C.; Tjønneland, A.; Overvad, K.; Jakobsen, M.U.; Boeing, H. Association between FTO variant and change in body weight and its interaction with dietary factors: The DiOGenes study. Obesity 2012, 20, 1669–1674. [Google Scholar] [CrossRef]
- Sonestedt, E.; Roos, C.; Gullberg, B.; Ericson, U.; Wirfält, E.; Orho-Melander, M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am. J. Clin. Nutr. 2009, 90, 1418–1425. [Google Scholar] [CrossRef]
- Petkeviciene, J.; Smalinskiene, A.; Klumbiene, J.; Petkevicius, V.; Kriaucioniene, V.; Lesauskaite, V. Physical activity, but not dietary intake, attenuates the effect of the FTO rs9939609 polymorphism on obesity and metabolic syndrome in Lithuanian adult population. Public Health 2016, 135, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.; Power, C.; Hyppönen, E. Interaction between vitamin D receptor gene polymorphisms and 25-hydroxyvitamin D concentrations on metabolic and cardiovascular disease outcomes. Diabetes Metab. 2014, 40, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.; Pols, H.A.; Van Leeuwen, J.P. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Alathari, B.E.; Sabta, A.A.; Kalpana, C.A.; Vimaleswaran, K.S. Vitamin D pathway-related gene polymorphisms and their association with metabolic diseases: A literature review. J. Diabetes Metab. Disord. 2020, 19, 1701–1729. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.A.; AbuOdeh, R.O.; Muda, W.; Mohamed, H.; Samsudin, A.R. Association of Vitamin D receptor gene polymorphisms with metabolic syndrome and its components among adult Arabs from the United Arab Emirates. Diabetes Metab. Syndr. 2017, 11 (Suppl. S2), S531–S537. [Google Scholar] [CrossRef] [PubMed]
- Hemkens, L.G.; Ewald, H.; Naudet, F.; Ladanie, A.; Shaw, J.G.; Sajeev, G.; Ioannidis, J.P. Interpretation of epidemiologic studies very often lacked adequate consideration of confounding. J. Clin. Epidemiol. 2018, 93, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, N.; Drinkwater, E.J.; Rachele, J.N. Improving the accuracy of self-reported height and weight in surveys: An experimental study. BMC Med. Res. Methodol. 2022, 22, 241. [Google Scholar] [CrossRef] [PubMed]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary assessment methods in epidemiological research: Current state of the art and future prospects. F1000Research 2017, 6, 926. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L. Assessment of usual dietary intake in population studies of gene–diet interaction. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 74–81. [Google Scholar] [CrossRef]
- Culliford, A.E.; Bradbury, J.; Medici, E.B. Improving Communication of the UK Sustainable Healthy Dietary Guidelines the Eatwell Guide: A Rapid Review. Sustainability 2023, 15, 6149. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S. A nutrigenetics approach to study the impact of genetic and lifestyle factors on cardiometabolic traits in various ethnic groups: Findings from the GeNuIne Collaboration. Proc. Nutr. Soc. 2020, 79, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Byrne, E.; Johnson, J.; Chenevix-Trench, G.; Walter, S.; Nolte, I.; Vink, J.; Rawal, R.; Mangino, M.; Teumer, A. Genome-wide association analysis of coffee drinking suggests association with CYP1A1/CYP1A2 and NRCAM. Mol. Psychiatry 2012, 17, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Tornio, A.; Backman, J.T. Cytochrome P450 in pharmacogenetics: An update. Adv. Pharmacol. 2018, 83, 3–32. [Google Scholar] [PubMed]
- Liu, J.; Sridhar, J.; Foroozesh, M. Cytochrome P450 family 1 inhibitors and structure-activity relationships. Molecules 2013, 18, 14470–14495. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Dalton, T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960. [Google Scholar] [CrossRef]
- Wang, C. Revealing obesity through diet-gene interactions. Health Sci. Inq. 2020, 11, 158–161. [Google Scholar] [CrossRef]
- Palatini, P.; Benetti, E.; Mos, L.; Garavelli, G.; Mazzer, A.; Cozzio, S.; Fania, C.; Casiglia, E. Association of coffee consumption and CYP1A2 polymorphism with risk of impaired fasting glucose in hypertensive patients. Eur. J. Epidemiol. 2015, 30, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.M.; Clifford, M.N.; Penson, S.; Williams, P.; Robertson, M.D. Postprandial glycaemic and lipaemic responses to chronic coffee consumption may be modulated by CYP1A2 polymorphisms. Br. J. Nutr. 2018, 119, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Banks, N.; Tomko, P.; Colquhoun, R.; Muddle, T.; Emerson, S.; Jenkins, N. Genetic polymorphisms in ADORA2A and CYP1A2 influence caffeine’s effect on postprandial glycaemia. Sci. Rep. 2019, 9, 10532. [Google Scholar] [CrossRef]
- Hüls, A.; Krämer, U.; Carlsten, C.; Schikowski, T.; Ickstadt, K.; Schwender, H. Comparison of weighting approaches for genetic risk scores in gene-environment interaction studies. BMC Genet. 2017, 18, 115. [Google Scholar] [CrossRef]

| Gene Symbol and Name | SNP and Nucleotide Change | Minor Allele | Study Design | Ethnicity/Sample Size | BMI Status | Dietary Intake/Physical Activity Assessment Tool | Outcome Measure | Pinteraction | References |
|---|---|---|---|---|---|---|---|---|---|
| FTO Fat Mass and Obesity-Associated gene | rs9939609 A/T | A | Case-control study | Emiratis 414 | Normal/ overweight/obese | Lifestyle questionnaire | BMI | 0.027 SNP * PA | [24] |
| FTO | rs9939609 T/A | A | Cross-sectional study | Lebanese 308 | Normal/overweight/obese | Culture-specific, 80-item semi-quantitative FFQ | Body fat/BMI/Muscle Mass | - | [25] |
| FTO | rs9939609 T/A | A | Cross-sectional study | Lebanese 392 | Normal/overweight | IPAQ * and 157-item, semi-quantitative FFQ | BMI | 0.02 SNP * PA | [26] |
| WHR | 0.023 SNP * MD | ||||||||
| FTO | rs1558902 T/A | A | Cross-sectional study | Lebanese 308 | Normal/overweight/obese | Culture-specific, 80-item semi-quantitative FFQ | Body fat/BMI/Muscle Mass | - | [25] |
| TCF7L2 Transcription Factor 7 Like 2 gene | rs7903146 C/T | T | Cross-sectional study | Lebanese 308 | Normal/overweight/obese | Culture-specific, 80-item semi-quantitative FFQ | Body fat | 0.013 SNP * dietary fat | [25] |
| BMI | 0.016 SNP * dietary fat | ||||||||
| Muscle mass | 0.032 SNP * dietary fat | ||||||||
| TCF7L2 | rs7903146 C/T | T | Cross-sectional study | Algerian 737 | Normal/overweight/obese | Weekly FFQ | T2D risks | 0.05 SNP * dessert | [27] |
| T2D risks | 0.01 SNP * milk intake | ||||||||
| FBG | 0.02 SNP * desserts | ||||||||
| MC4R Melanocortin-4 receptor gene | rs17782313 T/C | C | Cross-sectional study | Lebanese 392 | Normal/overweight | IPAQ and 157-item, semi-quantitative FFQ | WC | 0.039 SNP * MD | [26] |
| WHR | 0.037 SNP * MD | ||||||||
| WHR | 0.006 SNP * PA | ||||||||
| MTHFR Methylenetetrahydrofolate reductase gene | rs1801133 C/T | T | Cross-sectional study | Lebanese 392 | Normal/overweight | IPAQ and 157-item, semi-quantitative FFQ | WC | 0.012 SNP * dietary energy | [26] |
| WC | 0.039 SNP * MD | ||||||||
| CYP1A2 Cytochrome P450 proteins gene | rs2470890 T/C | C | Cross-sectional study | Lebanese 7607 | Normal/overweight/obese | Self-reported caffeine consumption | T2D | 0.0512 SNP * Caffeine intake | [28] |
| rs2069526 T/G | G | ||||||||
| rs762551 A/C | C | ||||||||
| rs2572304 A/G | G | ||||||||
| rs4646427 C/T | C | ||||||||
| VDR Vitamin D Receptor gene | rs1544410 C/T | C | Case- control study | Emiratis 414 | Normal/overweight/obese | Lifestyle questionnaire | BMI | - | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlAnazi, M.M.; Ventura, E.F.; Lovegrove, J.A.; Vimaleswaran, K.S. A Systematic Review of the Gene–Lifestyle Interactions on Metabolic Disease-Related Outcomes in Arab Populations. Nutrients 2024, 16, 2519. https://doi.org/10.3390/nu16152519
AlAnazi MM, Ventura EF, Lovegrove JA, Vimaleswaran KS. A Systematic Review of the Gene–Lifestyle Interactions on Metabolic Disease-Related Outcomes in Arab Populations. Nutrients. 2024; 16(15):2519. https://doi.org/10.3390/nu16152519
Chicago/Turabian StyleAlAnazi, Maria M., Eduard Flores Ventura, Julie A. Lovegrove, and Karani Santhanakrishnan Vimaleswaran. 2024. "A Systematic Review of the Gene–Lifestyle Interactions on Metabolic Disease-Related Outcomes in Arab Populations" Nutrients 16, no. 15: 2519. https://doi.org/10.3390/nu16152519







