Vitamin D Status and Behavioral Impulsivity in Anorexia Nervosa: Insights from a Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Instruments
2.2.1. Self-Report Questionnaires
2.2.2. Neuropsychological Evaluation
2.2.3. Serum Vitamin D
2.3. Statistical Analysis
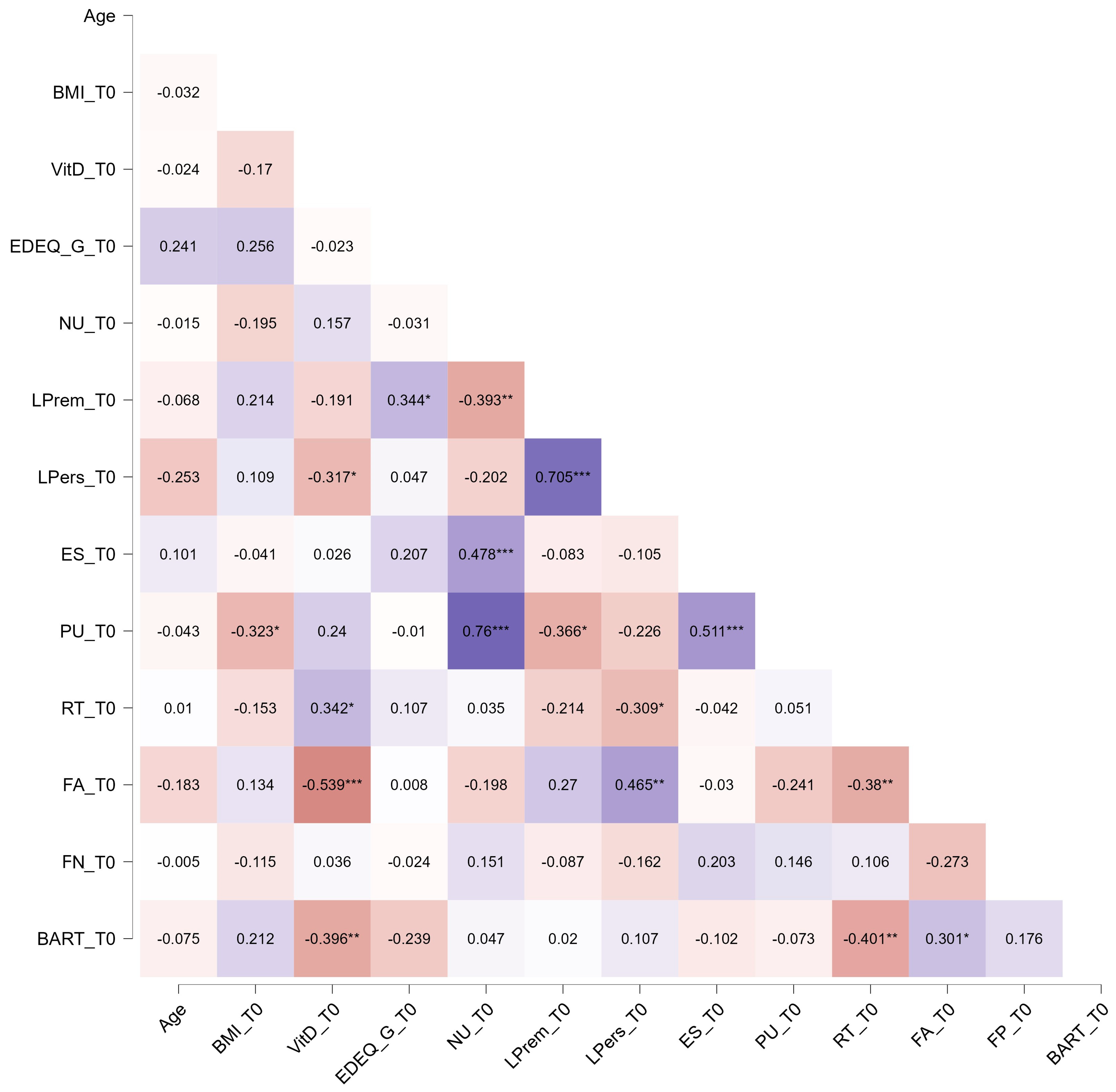
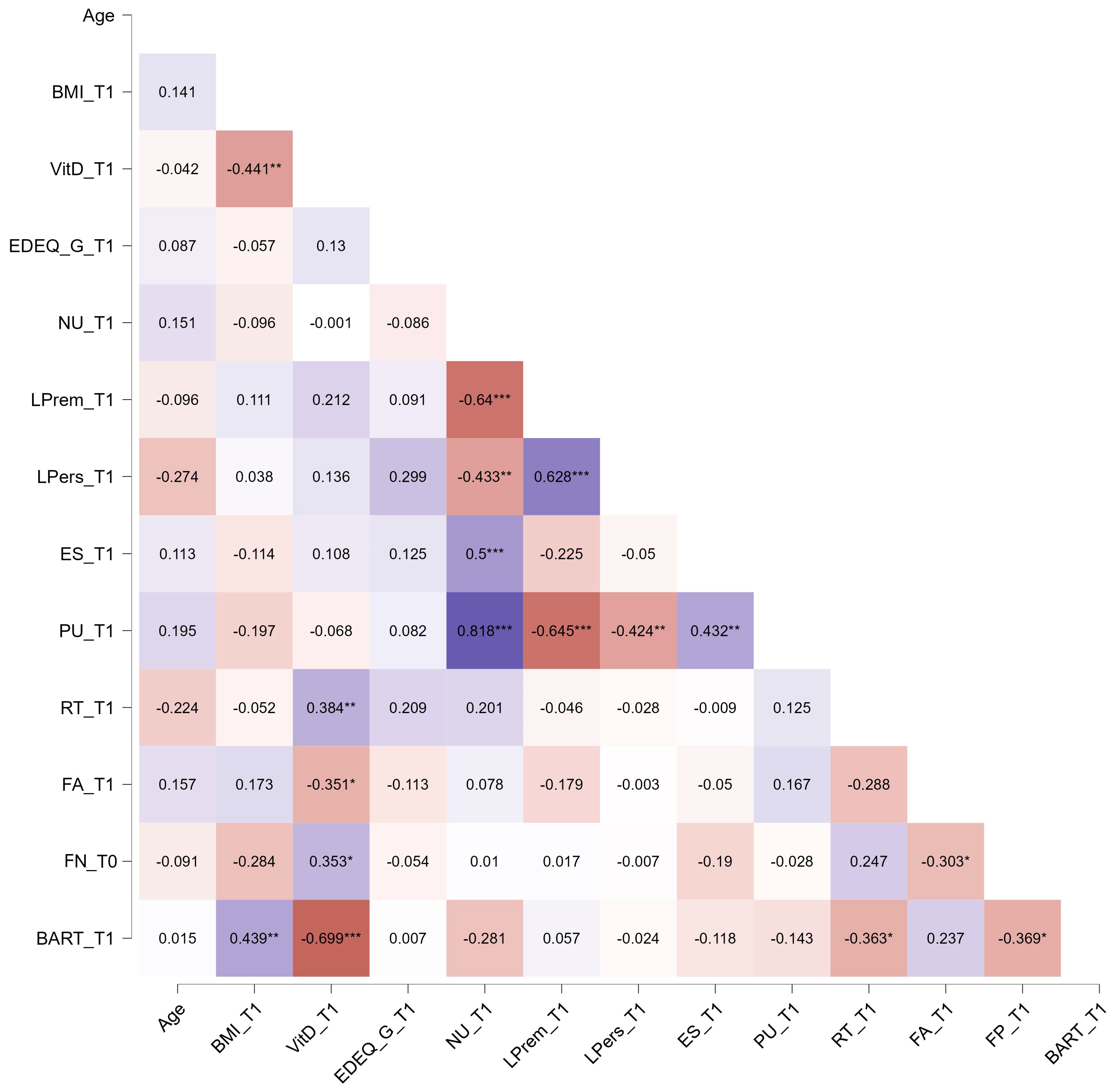
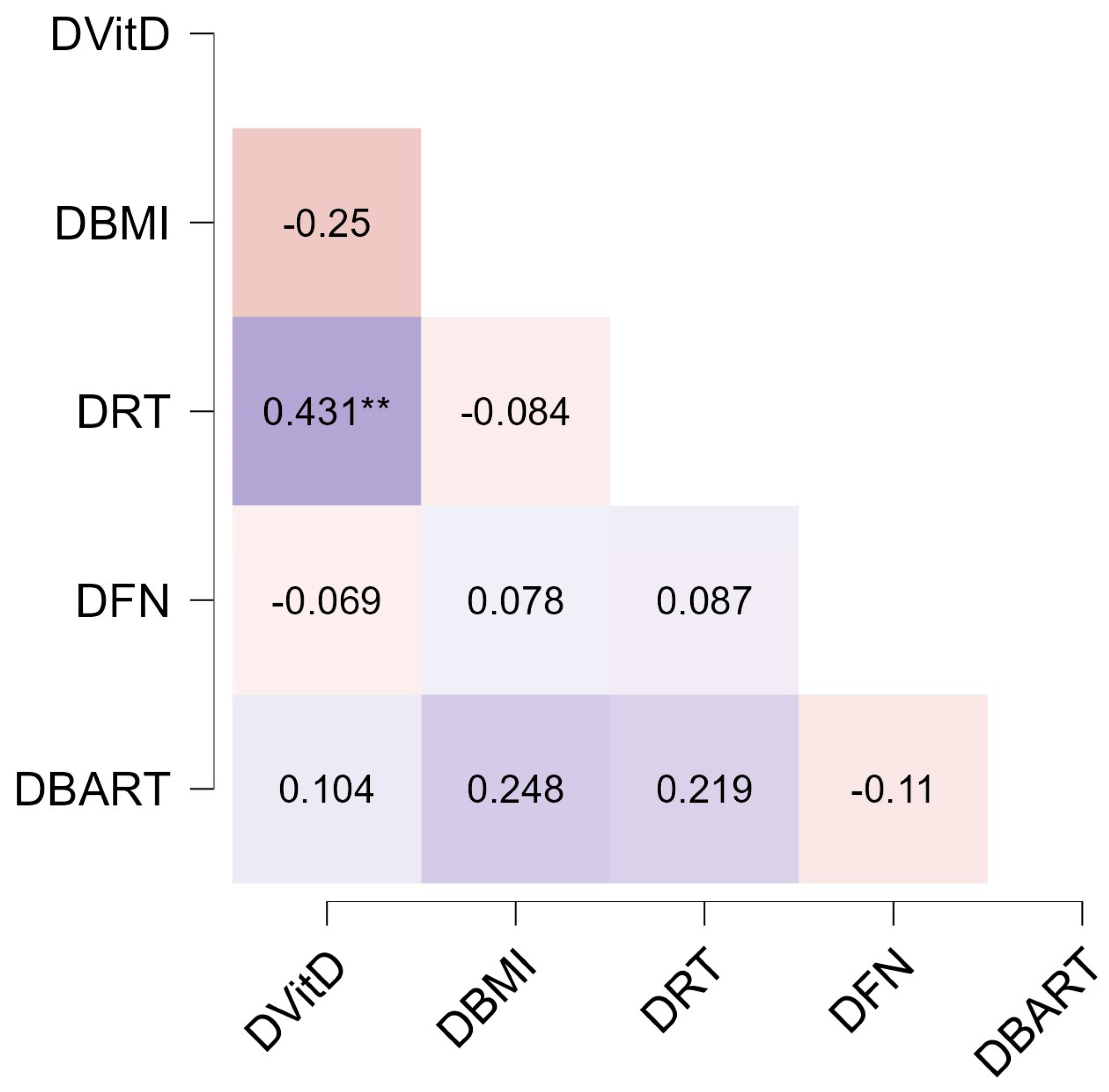
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013.
- Meneguzzo, P.; Todisco, P.; Calonaci, S.; Mancini, C.; Dal Brun, D.; Collantoni, E.; Donini, L.M.; Tenconi, E.; Favaro, A. Health-Related Quality of Life Assessment in Eating Disorders: Adjustment and Validation of a Specific Scale with the Inclusion of an Interpersonal Domain. Eat. Weight Disord. 2020, 26, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia Nervosa: Aetiology, Assessment, and Treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.; Łukaszkiewicz, J.; Wrzosek, M.; Jakubczyk, A.; Matsumoto, H.; Piątkiewicz, P.; Radziwoń-Zaleska, M.; Wojnar, M.; Nowicka, G. Vitamin D and the Central Nervous System. Pharmacol. Rep. 2013, 65, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Modan-Moses, D.; Levy-Shraga, Y.; Pinhas-Hamiel, O.; Kochavi, B.; Enoch-Levy, A.; Vered, I.; Stein, D. High Prevalence of Vitamin D Deficiency and Insufficiency in Adolescent Inpatients Diagnosed with Eating Disorders. Int. J. Eat. Disord. 2015, 48, 607–614. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not Just the Bone. Evidence for Beneficial Pleiotropic Extraskeletal Effects. Eat. Weight. Disord.-Stud. Anorex. Bulim. Obes. 2017, 22, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Murhadi, L.L.; Kurpad, A.V.; Chan She Ping-Delfos, W.L.; Piers, L.S. Mechanistic Roles for Calcium and Vitamin D in the Regulation of Body Weight. Obes. Rev. 2012, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Trinko, J.R.; Land, B.B.; Solecki, W.B.; Wickham, R.J.; Tellez, L.A.; Maldonado-Aviles, J.; de Araujo, I.E.; Addy, N.A.; DiLeone, R.J. Vitamin D3: A Role in Dopamine Circuit Regulation, Diet-Induced Obesity, and Drug Consumption. eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Gambino, C.M.; Iacolino, G.; Ciaccio, M. Vitamin D and the Nervous System. Neurol. Res. 2019, 41, 827–835. [Google Scholar] [CrossRef]
- Lavender, J.M.; Mitchell, J.E. Eating Disorders and Their Relationship to Impulsivity. Curr. Treat. Options Psychiatry 2015, 2, 394–401. [Google Scholar] [CrossRef]
- Bénard, M.; Bellisle, F.; Kesse-Guyot, E.; Julia, C.; Andreeva, V.A.; Etilé, F.; Reach, G.; Dechelotte, P.; Tavolacci, M.P.; Hercberg, S.; et al. Impulsivity Is Associated with Food Intake, Snacking, and Eating Disorders in a General Population. Am. J. Clin. Nutr. 2019, 109, 117–126. [Google Scholar] [CrossRef]
- Waxman, S.E. A Systematic Review of Impulsivity in Eating Disorders. Eur. Eat. Disord. Rev. 2009, 17, 408–425. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Schiöth, H. Impulsivity and Compulsivity in Anorexia Nervosa: Cognitive Systems Underlying Variation in Appetite Restraint from an RDoC Perspective. In Anorexia and Bulimia Nervosa; IntechOpen: London, UK, 2019; p. 3. [Google Scholar]
- Howard, M.; Gregertsen, E.C.; Hindocha, C.; Serpell, L. Impulsivity and Compulsivity in Anorexia and Bulimia Nervosa: A Systematic Review. Psychiatry Res. 2020, 293, 113354. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, P.; Todisco, P.; Collantoni, E.; Meregalli, V.; Dal Brun, D.; Tenconi, E.; Favaro, A. A Multi-Faceted Evaluation of Impulsivity Traits and Early Maladaptive Schemas in Patients with Anorexia Nervosa. J. Clin. Med. 2021, 10, 5895. [Google Scholar] [CrossRef] [PubMed]
- Lavender, J.M.; Goodman, E.L.; Culbert, K.M.; Wonderlich, S.A.; Crosby, R.D.; Engel, S.G.; Mitchell, J.E.; Le Grange, D.; Crow, S.J.; Peterson, C.B. Facets of Impulsivity and Compulsivity in Women with Anorexia Nervosa. Eur. Eat. Disord. Rev. 2017, 25, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Bevione, F.; Martini, M.; Toppino, F.; Longo, P.; Abbate-Daga, G.; Brustolin, A.; Panero, M. Cognitive Impulsivity in Anorexia Nervosa in Correlation with Eating and Obsessive Symptoms: A Comparison with Healthy Controls. Nutrients 2024, 16, 1156. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Granero, R.; Misiolek, A.; Vintró-Alcaraz, C.; Mallorqui-Bagué, N.; Lozano-Madrid, M.; Heras, M.V.D.L.; Sánchez, I.; Jiménez-Murcia, S.; Fernández-Aranda, F. Impact of impulsivity and therapy response in eating disorders from a neurophysiological, personality and cognitive perspective. Nutrients 2022, 14, 5011. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.K.; Hopko, D.R.; Bare, R.; Lejuez, C.W.; Robinson, E.V. Construct Validity of the Balloon Analog Risk Task (BART): Associations with Psychopathy and Impulsivity. Assessment 2005, 12, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Wonderlich, S.A.; Connolly, K.M.; Stice, E. Impulsivity as a Risk Factor for Eating Disorder Behavior: Assessment Implications with Adolescents. Int. J. Eat. Disord. 2004, 36, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Vandereycken, W.; Vertommen, H. Impulsivity-Related Traits in Eating Disorder Patients. Personal. Individ. Differ. 2005, 39, 739–749. [Google Scholar] [CrossRef]
- Wrzosek, M.; Sawicka, A.; Tałałaj, M.; Wojnar, M.; Nowicka, G. Impulsivity and Vitamin D in Bariatric Surgery Candidates. Pharmacol. Rep. 2018, 70, 688–693. [Google Scholar] [CrossRef]
- Velickovic, K.M.C.; Makovey, J.; Abraham, S.F. Vitamin D, Bone Mineral Density and Body Mass Index in Eating Disorder Patients. Eat. Behav. 2013, 14, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Todisco, P.; Meneguzzo, P.; Vogazianos, P.; Garolla, A.; Antoniades, A.; Tozzi, F. Relation between Vitamin D and Impulse Behaviours in Patients with Eating Disorder: A Pilot Observational Study. Eur. Eat. Disord. Rev. 2020, 28, 587–593. [Google Scholar] [CrossRef]
- Meneguzzo, P.; Mancini, C.; Ormitti, A.; Garolla, A.; Bonello, E.; Donini, L.M.; Todisco, P. Impulsivity and Eating Disorders: The Relationship between Serum 25-Hydroxyvitamin D and Different Impulsivity Facets in a Transdiagnostic Sample. World J. Biol. Psychiatry 2022, 23, 401–409. [Google Scholar] [CrossRef]
- Fairburn, C.G.; Beglin, S.J. Assessment of Eating Disorder: Interview or Self-Report Questionnaire? Int. J. Eat. Disord. 1994, 16, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Calugi, S.; Milanese, C.; Sartirana, M.; El Ghoch, M.; Sartori, F.; Geccherle, E.; Coppini, A.; Franchini, C.; Dalle Grave, R. The Eating Disorder Examination Questionnaire: Reliability and Validity of the Italian Version. Eat. Weight. Disord.-Stud. Anorex. Bulim. Obes. 2017, 22, 509–514. [Google Scholar] [CrossRef]
- Argyriou, E.; Um, M.; Wu, W.; Cyders, M.A. Measurement Invariance of the UPPS-P Impulsive Behavior Scale Across Age and Sex Across the Adult Life Span. Assessment 2020, 27, 432–453. [Google Scholar] [CrossRef]
- D’Orta, I.; Burnay, J.; Aiello, D.; Niolu, C.; Siracusano, A.; Timpanaro, L.; Khazaal, Y.; Billieux, J. Development and Validation of a Short Italian UPPS-P Impulsive Behavior Scale. Addict. Behav. Rep. 2015, 2, 19–22. [Google Scholar] [CrossRef]
- Gomez, P.; Ratcliff, R.; Perea, M. A Model of the Go/No-Go Task. J. Exp. Psychol. Gen. 2007, 136, 389. [Google Scholar] [CrossRef] [PubMed]
- Lauriola, M.; Panno, A.; Levin, I.P.; Lejuez, C.W. Individual Differences in Risky Decision Making: A Meta-Analysis of Sensation Seeking and Impulsivity with the Balloon Analogue Risk Task. J. Behav. Decis. Mak. 2014, 27, 20–36. [Google Scholar] [CrossRef]
- Pettersen, J.A. Does High Dose Vitamin D Supplementation Enhance Cognition?: A Randomized Trial in Healthy Adults. Exp. Gerontol. 2017, 90, 90–97. [Google Scholar] [CrossRef]
- Overeem, K.; Eyles, D.W.; McGrath, J.J.; Burne, T.H.J. The Impact of Vitamin D Deficiency on Behaviour and Brain Function in Rodents. Curr. Opin. Behav. Sci. 2016, 7, 47–52. [Google Scholar] [CrossRef]
- Bond, M.; Moll, N.; Rosello, A.; Bond, R.; Schnell, J.; Burger, B.; Hoekstra, P.J.; Dietrich, A.; Schrag, A.; Kocovska, E.; et al. Vitamin D Levels in Children and Adolescents with Chronic Tic Disorders: A Multicentre Study. Eur. Child Adolesc. Psychiatry 2022, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.G.; Kerlan, V.; Desailloud, R. Non-Classical Effects of Vitamin D: Non-Bone Effects of Vitamin D. Ann. Endocrinol. 2021, 82, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chakkera, M.; Ravi, N.; Ramaraju, R.; Vats, A.; Nair, A.R.; Bandhu, A.K.; Koirala, D.; Pallapothu, M.R.; Mariñez, M.G.Q.; Khan, S. The Efficacy of Vitamin D Supplementation in Patients With Alzheimer’s Disease in Preventing Cognitive Decline: A Systematic Review. Cureus 2022, 14, e31710. [Google Scholar] [CrossRef] [PubMed]
- Dehbokri, N.; Noorazar, G.; Ghaffari, A.; Mehdizadeh, G.; Sarbakhsh, P.; Ghaffary, S. Effect of Vitamin D Treatment in Children with Attention-Deficit Hyperactivity Disorder. World J. Pediatr. 2019, 15, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Naeini, A.A.; Fasihi, F.; Najafi, M.; Ghazvini, M.R.; Hasanzadeh, A. The Effects of Vitamin D Supplementation on ADHD (Attention Deficit Hyperactivity Disorder) in 6–13 Year-Old Students: A Randomized, Double-Blind, Placebo-Controlled Study. Eur. J. Integr. Med. 2019, 25, 28–33. [Google Scholar] [CrossRef]
- Elshorbagy, H.H.; Barseem, N.F.; Abdelghani, W.E.; Suliman, H.A.I.; Al-shokary, A.H.; Abdulsamea, S.E.; Elsadek, A.E.; Abdel Maksoud, Y.H.; Nour El Din, D.M.A.E.H. Impact of Vitamin D Supplementation on Attention-Deficit Hyperactivity Disorder in Children. Ann. Pharmacother. 2018, 52, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Kotsi, E.; Kotsi, E.; Perrea, D.N. Vitamin D Levels in Children and Adolescents with Attention-Deficit Hyperactivity Disorder (ADHD): A Meta-Analysis. ADHD Atten. Deficit Hyperact. Disord. 2019, 11, 221–232. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D and the Omega-3 Fatty Acids Control Serotonin Synthesis and Action, Part 2: Relevance for ADHD, Bipolar Disorder, Schizophrenia, and Impulsive Behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef]
| T0 | T1 | Z | p (δ) | |
|---|---|---|---|---|
| BMI, kg/m2 | 15.70 1.93 | 18.39 1.99 | −5.842 | <0.001 1.37 |
| Vit-D, ng/mL | 28.57 11.90 | 44.35 15.31 | −4.868 | <0.001 1.15 |
| EDE-Q total score | 4.31 1.18 | 2.77 1.17 | −4.509 | <0.001 1.31 |
| UPPS | ||||
| NU | 9.51 3.73 | 9.53 3.97 | −1.276 | 0.202 |
| LPrem | 7.80 3.48 | 7.82 3.40 | −0.030 | 0.976 |
| LPers | 8.02 3.60 | 7.44 3.32 | −2.416 | 0.016 0.17 |
| ES | 10.20 3.79 | 10.89 3.56 | −2.343 | 0.019 0.19 |
| PU | 10.58 3.30 | 11.16 2.98 | −1.637 | 0.102 |
| Go-No go | ||||
| RT | 326.10 51.54 | 340.76 43.29 | −2.594 | 0.009 0.31 |
| False alarm | 16.52 14.47 | 7.65 6.39 | −3.455 | 0.001 0.79 |
| False negative | 0.65 2.87 | 0.50 1.68 | −0.141 | 0.888 |
| BART | 80.48 17.59 | 78.07 12.49 | −0.699 | 0.484 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todisco, P.; De Mico, A.; Meneguzzo, P. Vitamin D Status and Behavioral Impulsivity in Anorexia Nervosa: Insights from a Longitudinal Study. Nutrients 2024, 16, 2523. https://doi.org/10.3390/nu16152523
Todisco P, De Mico A, Meneguzzo P. Vitamin D Status and Behavioral Impulsivity in Anorexia Nervosa: Insights from a Longitudinal Study. Nutrients. 2024; 16(15):2523. https://doi.org/10.3390/nu16152523
Chicago/Turabian StyleTodisco, Patrizia, Alberto De Mico, and Paolo Meneguzzo. 2024. "Vitamin D Status and Behavioral Impulsivity in Anorexia Nervosa: Insights from a Longitudinal Study" Nutrients 16, no. 15: 2523. https://doi.org/10.3390/nu16152523





