Nutritional Management and Physical Activity in the Treatment of Sarcopenic Obesity: A Review of the Literature
Abstract
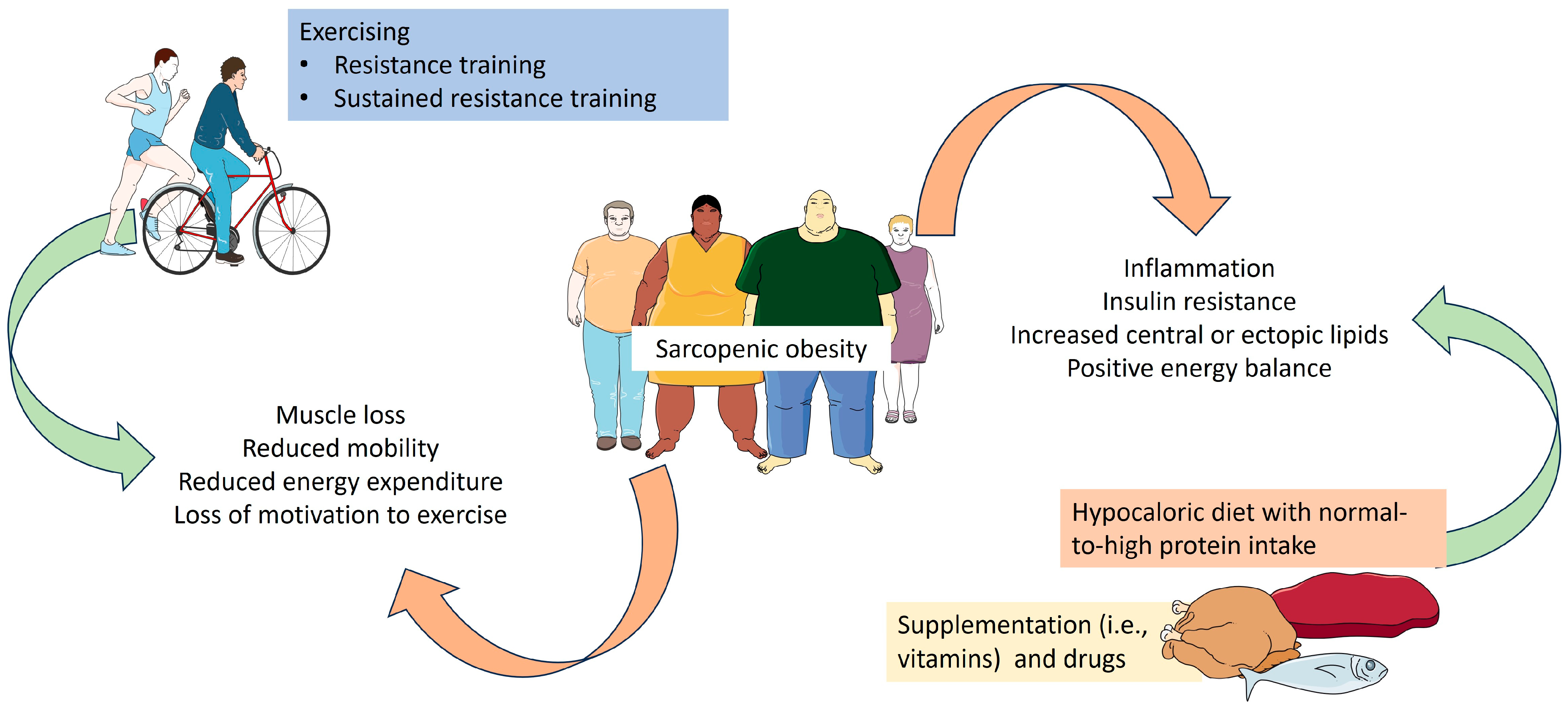
:1. Introduction
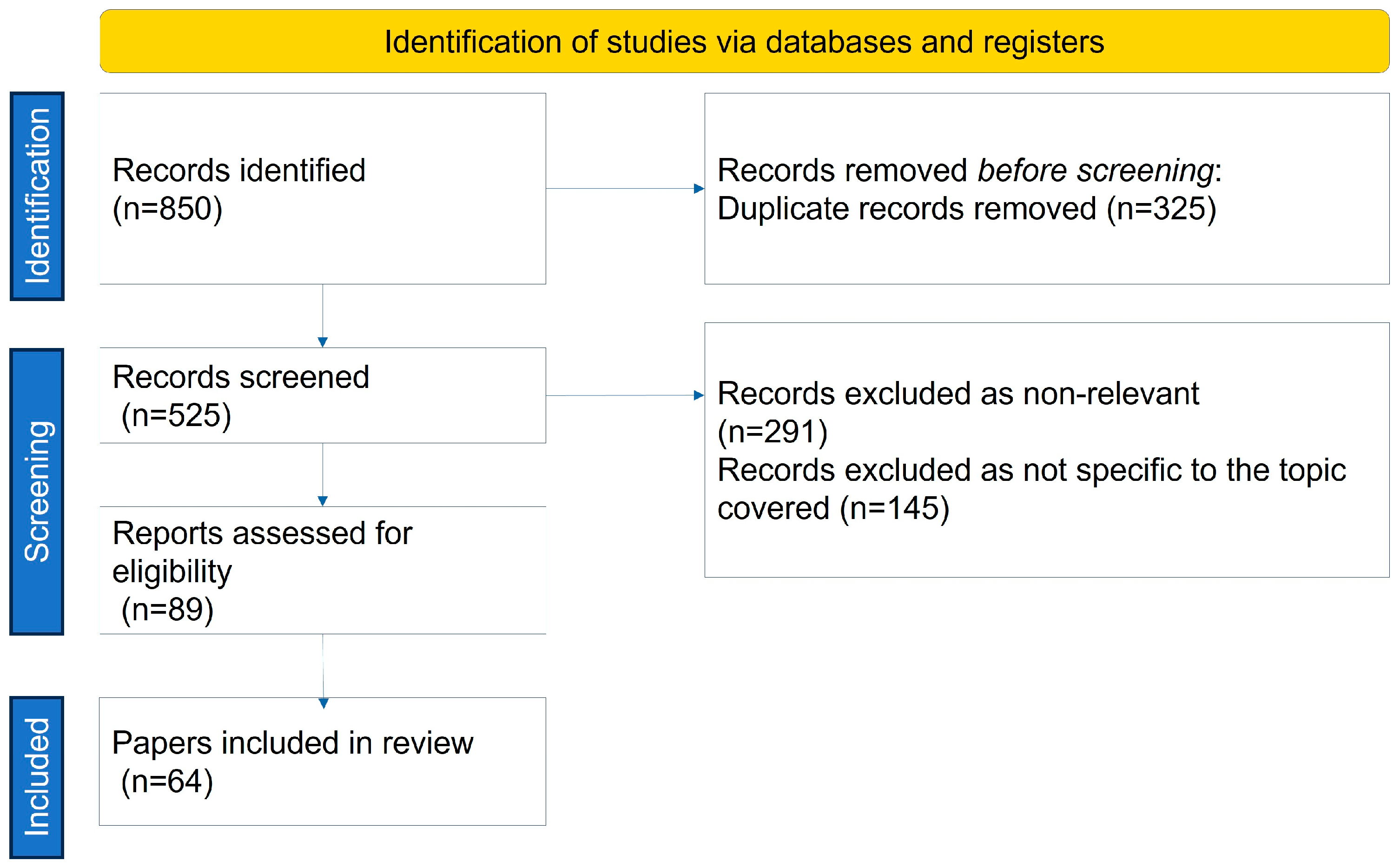
2. Methods
3. Nutritional Management of Sarcopenic Obesity
4. Physical Activity and Sarcopenic Obesity
4.1. Resistance Training
4.2. Aerobic Training
4.3. Combination Training
5. Combined Nutritional and Physical Intervention
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010, 21, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Anker, S.D.; von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology-update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ellison-Barnes, A.; Johnson, S.; Gudzune, K. Trends in Obesity Prevalence Among Adults Aged 18 Through 25 Years, 1976–2018. JAMA 2021, 326, 2073. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Freiberger, E.; Goisser, S.; Porzel, S.; Volkert, D.; Kemmler, W.; Sieber, C.; Bollheimer, C. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons—A narrative review. Clin. Interv. Aging 2015, 10, 1267–1282. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic Obesity: Time to Meet the Challenge. Obes. Facts 2018, 11, 294–305. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef]
- Gross, D.C.; Cheever, C.R.; Batsis, J.A. Understanding the development of sarcopenic obesity. Expert Rev. Endocrinol. Metab. 2023, 18, 469–488. [Google Scholar]
- Trouwborst, I.; Verreijen, A.; Memelink, R.; Massanet, P.; Boirie, Y.; Weijs, P.; Tieland, M. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients 2018, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Petroni, M.L.; Caletti, M.T.; Dalle Grave, R.; Bazzocchi, A.; Aparisi Gómez, M.P.; Marchesini, G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients 2019, 11, 1302. [Google Scholar] [CrossRef] [PubMed]
- Aubertin-Leheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Six months of isoflavone supplement increases fat-free mass in obese–sarcopenic postmenopausal women: A randomized double-blind controlled trial. Eur. J. Clin. Nutr. 2007, 61, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Aleman-Mateo, H.; Macias, L.; Esparza-Romero, J.; Astiazaran-Garcia, H.; Blancas, A.L. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: Evidence from a randomized clinical trial using a protein-rich food. Clin. Interv. Aging 2012, 7, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Coker, R.H.; Miller, S.; Schutzler, S.; Deutz, N.; Wolfe, R.R. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr. J. 2012, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.J.; Marcotte, G.R.; Churchward-Venne, T.A.; Murphy, C.H.; Breen, L.; von Allmen, M.; Baker, S.K.; Phillips, S.M. Whey Protein Supplementation Preserves Postprandial Myofibrillar Protein Synthesis during Short-Term Energy Restriction in Overweight and Obese Adults. J. Nutr. 2015, 145, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Hamer, H.M.; De Lange, A.; Kiskini, A.; Groen, B.B.L.; Senden, J.M.G.; Gijsen, A.P.; Verdijk, L.B.; Van Loon, L.J.C. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin. Nutr. 2013, 32, 412–419. [Google Scholar]
- Muscariello, E.; Nasti, G.; Siervo, M.; Di Maro, M.; Lapi, D.; D’Addio, G.; Colantuoni, A. Dietary protein intake in sarcopenic obese older women. Clin. Interv. Aging 2016, 11, 133–140. [Google Scholar] [CrossRef]
- Sammarco, R.; Marra, M.; Di Guglielmo, M.L.; Naccarato, M.; Contaldo, F.; Poggiogalle, E.; Donini, L.M.; Pasanisi, F. Evaluation of Hypocaloric Diet With Protein Supplementation in Middle-Aged Sarcopenic Obese Women: A Pilot Study. Obes. Facts 2017, 10, 160–167. [Google Scholar] [CrossRef]
- Bouchonville, M.F.; Villareal, D.T. Sarcopenic obesity: How do we treat it? Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 412–419. [Google Scholar] [CrossRef]
- Porter Starr, K.N.; Pieper, C.F.; Orenduff, M.C.; McDonald, S.R.; McClure, L.B.; Zhou, R.; Payne, M.E.; Bales, C.W. Improved Function With Enhanced Protein Intake per Meal: A Pilot Study of Weight Reduction in Frail, Obese Older Adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 1369–1375. [Google Scholar] [CrossRef]
- Weinheimer, E.M.; Sands, L.P.; Campbell, W.W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 2010, 68, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Damms-Machado, A.; Weser, G.; Bischoff, S.C. Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr. J. 2012, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T. Bone Mineral Density Response to Caloric Restriction–Induced Weight Loss or Exercise-Induced Weight Loss: A Randomized Controlled Trial. Arch. Intern. Med. 2006, 166, 2502. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Weissenfels, A.; Teschler, M.; Willert, S.; Bebenek, M.; Shojaa, M.; Kohl, M.; Freiberger, E.; Sieber, C.; von Stengel, S. Whole-body electromyostimulation and protein supplementation favorably affect sarcopenic obesity in community-dwelling older men at risk: The randomized controlled FranSO study. Clin. Interv. Aging 2017, 12, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.J.; Liao, C.D.; Tsai, M.W.; Chen, C.N. Effects of Exercise and Nutritional Intervention on Body Composition, Metabolic Health, and Physical Performance in Adults with Sarcopenic Obesity: A Meta-Analysis. Nutrients 2019, 11, 2163. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the “anabolic resistance” of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef]
- Deer, R.R.; Volpi, E. Protein intake and muscle function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 248–253. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Hita-Contreras, F.; Bueno-Notivol, J.; Martínez-Amat, A.; Cruz-Díaz, D.; Hernandez, A.V.; Pérez-López, F.R. Effect of exercise alone or combined with dietary supplements on anthropometric and physical performance measures in community-dwelling elderly people with sarcopenic obesity: A meta-analysis of randomized controlled trials. Maturitas 2018, 116, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, T.; Yin, Z.; Espinoza, S.; Xie, Y.; Gentry, C.; Tian, Q.; Zhao, L.-J.; Shen, H.; Luo, Z.; et al. Associations of physical activity with sarcopenia and sarcopenic obesity in middle-aged and older adults: The Louisiana osteoporosis study. BMC Public Health 2022, 22, 896. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.D.; Rhea, M.R.; Sen, A.; Gordon, P.M. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Res. Rev. 2010, 9, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.T.; de Oliveira, G.S.; Gonçalves, V.S.S.; Neri, S.G.R.; de Carvalho, K.M.B.; Dutra, E.S. Effect of physical exercise on muscle strength in adults following bariatric surgery: A systematic review and meta-analysis of different muscle strength assessment tests. PLoS ONE 2022, 17, e0269699. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Kenyon, A.J.; Eberle, P.; Skye, A.; Kraus, W.E. Preventing sarcopenic obesity among breast cancer patients who receive adjuvant chemotherapy: Results of a feasibility study. Clin. Exerc. Physiol. 2002, 4, 44–49. [Google Scholar] [PubMed]
- Huang, S.W.; Ku, J.W.; Lin, L.F.; Liao, C.D.; Chou, L.C.; Liou, T.H. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: A pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 556–563. Available online: https://www.minervamedica.it/index2.php?show=R33Y2017N04A0556 (accessed on 2 September 2022). [CrossRef] [PubMed]
- Liao, C.D.; Tsauo, J.Y.; Lin, L.F.; Huang, S.W.; Ku, J.W.; Chou, L.C.; Liou, T.H. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: A CONSORT-compliant prospective randomized controlled trial. Medicine 2017, 96, e7115. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Tsauo, J.Y.; Huang, S.W.; Ku, J.W.; Hsiao, D.J.; Liou, T.H. Effects of elastic band exercise on lean mass and physical capacity in older women with sarcopenic obesity: A randomized controlled trial. Sci. Rep. 2018, 8, 2317. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, P.H.; Lin, L.F.; Liao, C.D.; Liou, T.H.; Huang, S.W. Effects of progressive elastic band resistance exercise for aged osteosarcopenic adiposity women. Exp. Gerontol. 2021, 147, 111272. [Google Scholar] [CrossRef]
- Park, J.; Kwon, Y.; Park, H. Effects of 24-Week Aerobic and Resistance Training on Carotid Artery Intima-Media Thickness and Flow Velocity in Elderly Women with Sarcopenic Obesity. J. Atheroscler. Thromb. 2017, 24, 1117–1124. [Google Scholar] [CrossRef]
- Fry, A.C. The Role of Resistance Exercise Intensity on Muscle Fibre Adaptations. Sports Med. 2004, 34, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.B.; Paiva, F.M.L.; Gauche, R.; de Oliveira, R.J.; Lima, R.M. Effects of resistance training on sarcopenic obesity index in older women: A randomized controlled trial. Arch. Gerontol. Geriatr. 2016, 65, 168–173. [Google Scholar] [CrossRef]
- Chen, H.T.; Chung, Y.C.; Chen, Y.J.; Ho, S.Y.; Wu, H.J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef]
- Chiu, S.C.; Yang, R.S.; Yang, R.J.; Chang, S.F. Effects of resistance training on body composition and functional capacity among sarcopenic obese residents in long-term care facilities: A preliminary study. BMC Geriatr. 2018, 18, 21. [Google Scholar] [CrossRef]
- Vasconcelos, K.S.S.; Dias, J.M.D.; Araújo, M.C.; Pinheiro, A.C.; Moreira, B.S.; Dias, R.C. Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: A randomized controlled trial. Braz. J. Phys. Ther. 2016, 20, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Pincivero, D.M.; Lephart, S.M.; Karunakara, R.G. Effects of rest interval on isokinetic strength and functional performance after short-term high intensity training. Br. J. Sports Med. 1997, 31, 229–234. [Google Scholar] [CrossRef]
- Willardson, J.M.; Burkett, L.N. The Effect of Different Rest Intervals Between Sets on Volume Components and Strength Gains. J. Strength Cond. Res. 2008, 22, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, A.; Krawczyk, S.N.; Potiaumpai, M.; Signorile, J.F. High-speed circuit training vs hypertrophy training to improve physical function in sarcopenic obese adults: A randomized controlled trial. Exp. Gerontol. 2014, 60, 64–71. [Google Scholar] [CrossRef]
- de Oliveira Silva, A.; Dutra, M.T.; de Moraes, W.M.A.M.; Funghetto, S.S.; Lopes de Farias, D.; Dos Santos, P.H.F.; Vieira, D.C.L.; Nascimento, D.D.C.; Orsano, V.S.M.; Schoenfeld, B.J.; et al. Resistance training-induced gains in muscle strength, body composition, and functional capacity are attenuated in elderly women with sarcopenic obesity. Clin. Interv. Aging 2018, 13, 411–417. [Google Scholar] [CrossRef]
- Stoever, K.; Heber, A.; Eichberg, S.; Brixius, K. Influences of Resistance Training on Physical Function in Older, Obese Men and Women With Sarcopenia. J. Geriatr. Phys. Ther. 2018, 41, 20–27. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H. Exercise Therapy for People With Sarcopenic Obesity: Myokines and Adipokines as Effective Actors. Front. Endocrinol. 2022, 13, 811751. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Nair, K.S. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol.-Endocrinol. Metab. 2004, 286, E92–E101. [Google Scholar] [CrossRef] [PubMed]
- Bocalini, D.S.; Lima, L.S.; De Andrade, S.; Madureira, A.; Rica, R.L.; Dos Santos, R.N.; Serra, A.J.; Silva, J.A.; Rodriguez, D.; Figueira, A.; et al. Effects of circuit-based exercise programs on the body composition of elderly obese women. Clin. Interv. Aging 2012, 7, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef]
- Gutiérrez-López, L.; Olivares-Corichi, I.M.; Martínez-Arellanes, L.Y.; Mejía-Muñoz, E.; Polanco-Fierro, J.A.; García-Sánchez, J.R. A moderate intensity exercise program improves physical function and oxidative damage in older women with and without sarcopenic obesity. Exp. Gerontol. 2021, 150, 111360. [Google Scholar] [CrossRef]
- Maltais, M.L.; Perreault, K.; Courchesne-Loyer, A.; Lagacé, J.C.; Barsalani, R.; Dionne, I.J. Effect of Resistance Training and Various Sources of Protein Supplementation on Body Fat Mass and Metabolic Profile in Sarcopenic Overweight Older Adult Men: A Pilot Study. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 71–77. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef]
- Nabuco, H.C.; Tomeleri, C.M.; Fernandes, R.R.; Junior, P.S.; Cavalcante, E.F.; Cunha, P.M.; Antunes, M.; Nunes, J.P.; Venturini, D.; Barbosa, D.S.; et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2019, 32, 88–95. [Google Scholar]
- Verreijen, A.M.; Verlaan, S.; Engberink, M.F.; Swinkels, S.; de Vogel-van den Bosch, J.; Weijs, P.J. A high whey protein–, leucine-, and vitamin D–enriched supplement preserves muscle mass during intentional weight loss in obese older adults: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 279–286. [Google Scholar] [CrossRef]
- Mojtahedi, M.C.; Thorpe, M.P.; Karampinos, D.C.; Johnson, C.L.; Layman, D.K.; Georgiadis, J.G.; Evans, E.M. The Effects of a Higher Protein Intake During Energy Restriction on Changes in Body Composition and Physical Function in Older Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Eglseer, D.; Traxler, M.; Schoufour, J.D.; Weijs, P.J.M.; Voortman, T.; Boirie, Y.; Cruz-Jentoft, A.J.; Reiter, L.; Bauer, S.; Ben Allouch, S.; et al. Nutritional and exercise interventions in individuals with sarcopenic obesity around retirement age: A systematic review and meta-analysis. Nutr. Rev. 2023, 81, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Reiter, L.; Bauer, S.; Traxler, M.; Schoufour, J.D.; Weijs, P.J.M.; Cruz-Jentoft, A.; Topinková, E.; Eglseer, D. Effects of Nutrition and Exercise Interventions on Persons with Sarcopenic Obesity: An Umbrella Review of Meta-Analyses of Randomised Controlled Trials. Curr. Obes. Rep. 2023, 12, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Gortan Cappellari, G.; Guillet, C.; Poggiogalle, E.; Ballesteros Pomar, M.D.; Batsis, J.A.; Boirie, Y.; Breton, I.; Frara, S.; Genton, L.; Gepner, Y.; et al. Sarcopenic obesity research perspectives outlined by the sarcopenic obesity global leadership initiative (SOGLI)—Proceedings from the SOGLI consortium meeting in rome November 2022. Clin. Nutr. 2023, 42, 687–699. [Google Scholar] [CrossRef] [PubMed]
| Publication Title | Authors | Length of Intervention | No. of Subjects | Intervention | Clinical Outcome |
|---|---|---|---|---|---|
| Six months of isoflavone supplement increases fat-free mass in obese–sarcopenic postmenopausal women: a randomized double-blind controlled trial | Aubertin-Leheudre et al. [14] | 6 months | 18 postmenopausal women with SO | isoflavone supplementation vs. placebo |
|
| Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: evidence from a randomized clinical trial using a protein-rich food | Aleman-Mateo et al. [15] | 3 months | 40 elderly men and women with sarcopenia over 60 years | Addition of protein-rich food to the diet—210 g/day of ricotta cheese plus the habitual diet vs. habitual diet alone |
|
| Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals | Coker et al. [16] | 8 weeks | 12 elderly individuals | Caloric restriction-based with meal replacements (EAAMR) vs. competitive meal replacement (CMR) with 400 kcal of solid food |
|
| Whey Protein Supplementation Preserves Postprandial Myofibrillar Protein Synthesis during Short-Term Energy Restriction in Overweight and Obese Adults | Hector et al. [17] | 14 days | 19 men and 21 women with BMI 28–50 kg/m2 | Controlled hypocaloric diet (−750 kcal/d)—isolated whey (27 g/supplement) or soy (26 g/supplement) vs. isoenergetic carbohydrate (25 g maltodextrin/supplement) |
|
| Whey protein stimulates postprandial muscle protein accretion more effectively than casein and casein hydrolysate in older men | Pennings et al. [18] | N/A | 48 older men aged 74 years | Ingestion of meal-like amount (20 g) of whey, casein, or casein hydrolysate |
|
| Leucine co-ingestion improves postprandial muscle protein accretion in elderly men | Wall et al. [19] | N/A | 24 elderly men at 74 years | Ingestion of 20 g intrinsically casein protein with (PRO + LEU) or without (PRO) 2.5 g crystalline leucine |
|
| Dietary protein intake in sarcopenic obese older women | Muscariello et al. [20] | 3 months | 1030 females over 65 years and BMI > 30 kg/m2, 104 with sarcopenia | hypocaloric diet (0.8 g/kg desirable body weight/day of proteins) (n = 50), vs. hypocaloric diet with high protein intake (n = 54) |
|
| Evaluation of Hypocaloric Diet With Protein Supplementation in Middle-Aged Sarcopenic Obese Women: A Pilot Study | Sammarco et al. [21] | 4 months | 18 women with obesity aged 41–74 years | Hypocaloric diet plus placebo vs. hypocaloric high-protein diet |
|
| Function With Enhanced Protein Intake per Meal: A Pilot Study of Weight Reduction in Frail, Obese Older Adults | Porter Starr et al. [23] | 6 months | 67 (body mass index ≥30 kg/m2) older (≥60 years) adults with obesity and a Short Physical Performance Battery score of 4–10 | traditional weight loss regimen vs. higher protein intake (>30 g) |
|
| Micronutrient deficiency in obese subjects undergoing low-calorie diet | Damms-Machado et al. [25] | 3 months | 104 subjects | Dietetic intervention with formula diet |
|
| Whole-body electromyostimulation and protein supplementation favorably affect sarcopenic obesity in community-dwelling older men at risk: the randomized controlled FranSO study | Kemmler et al. [27] | 16 weeks | 100 community-dwelling northern Bavarian men aged ≥70 years with sarcopenia and obesity | Whole-body electromyostimulation with protein supplementation vs. Isolated protein supplementation vs. non-intervention control group |
|
| Publication | Authors | Length | No. of Sub. | Intervention | Clinical Outcome |
|---|---|---|---|---|---|
| Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity | Liao et al. [38] | 12 weeks | 46 | RT (EB) |
|
| Effects of elastic band exercise on lean mass and physical capacity in older women with sarcopenic obesity: A randomized controlled trial | Liao et al. [39] | 12 weeks | 56 | RT (EB) |
|
| Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: a pilot randomized controlled trial | Huang et al. [37] | 12 weeks | 35 | RT (EB) |
|
| Effects of progressive elastic band resistance exercise for aged osteosarcopenic adiposity women | Lee et al. [40] | 12 weeks | 27 | RT (EB) |
|
| Effects of 24-Week Aerobic and Resistance Training on Carotid Artery Intima-Media Thickness and Flow Velocity in Elderly Women with Sarcopenic Obesity | Park et al. [41] | 24 weeks | 50 | CT |
|
| Effects of resistance training on sarcopenic obesity index in older women: A randomized controlled trial | Gadelha et al. [43] | 24 weeks | 113 | RT |
|
| Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity | Chen et al. [44] | 8 weeks | 60 | RT, AT, CT |
|
| Effects of resistance training on body composition and functional capacity among sarcopenic obese residents in long-term care facilities: a preliminary study | Chiu et al. [45] | 13 weeks | 64 | RT |
|
| Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: a randomized controlled trial | Vasconcelos et al. [46] | 10 weeks | 28 | RT (with high-speed component) |
|
| High-speed circuit training vs. hypertrophy training to improve physical function in sarcopenic obese adults: a randomized controlled trial | Balachandran et al. [49] | 15 weeks | 21 | RT, AT |
|
| Resistance training-induced gains in muscle strength, body composition, and functional capacity are attenuated in elderly women with sarcopenic obesity | Silva et al. [50] | 16 weeks | 49 totalSO (8)Non-SO (41) | RT |
|
| Influences of Resistance Training on Physical Function in Older, Obese Men and Women With Sarcopenia | Stoever et al. [51] | 16 weeks | SAR (28)NSAR (20) | RT |
|
| Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial | Dieli-Conwright et al. [55] | 16 weeks | 100 | CT |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assyov, Y.; Nedeva, I.; Spassov, B.; Gerganova, A.; Velikov, T.; Kamenov, Z.; Velikova, T. Nutritional Management and Physical Activity in the Treatment of Sarcopenic Obesity: A Review of the Literature. Nutrients 2024, 16, 2560. https://doi.org/10.3390/nu16152560
Assyov Y, Nedeva I, Spassov B, Gerganova A, Velikov T, Kamenov Z, Velikova T. Nutritional Management and Physical Activity in the Treatment of Sarcopenic Obesity: A Review of the Literature. Nutrients. 2024; 16(15):2560. https://doi.org/10.3390/nu16152560
Chicago/Turabian StyleAssyov, Yavor, Iveta Nedeva, Borian Spassov, Antonina Gerganova, Toni Velikov, Zdravko Kamenov, and Tsvetelina Velikova. 2024. "Nutritional Management and Physical Activity in the Treatment of Sarcopenic Obesity: A Review of the Literature" Nutrients 16, no. 15: 2560. https://doi.org/10.3390/nu16152560






