The Relationship between Healthy Vascular Aging with the Mediterranean Diet and Other Lifestyles in the Spanish Population: The EVA Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
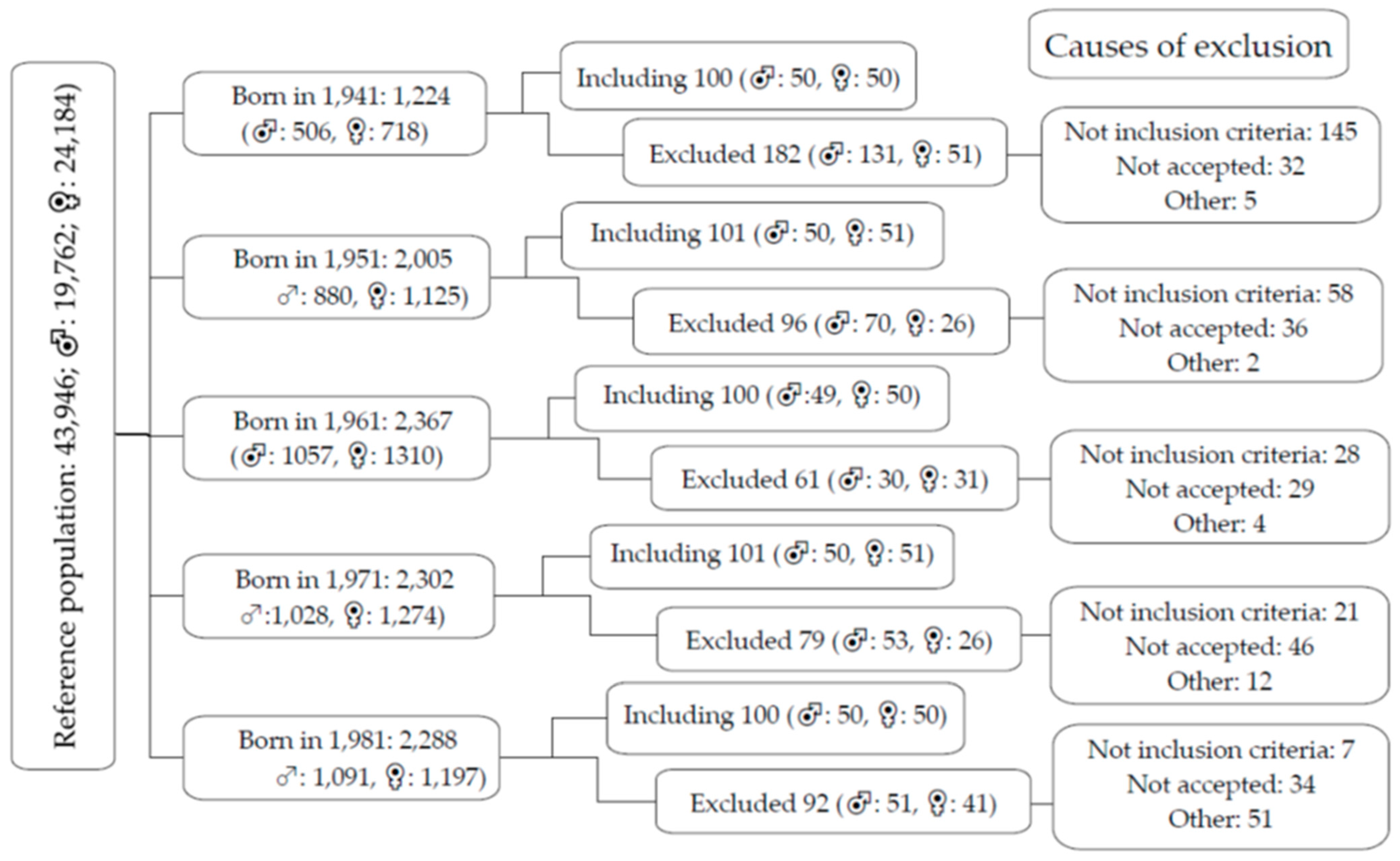
2.2. Study Population
2.3. Variables and Measuring Instruments
2.3.1. Healthy Lifestyles
Mediterranean Diet
Alcohol and Smoking
Physical Activity and Sedentary Time
2.3.2. Assessment of Vascular Structure, Function and Vascular Aging
Assessment of Intima–Media Thickness
The Ankle–Brachial Index
Assessment of Carotid–Femoral Pulse Wave Velocity
Vascular Aging Index (VAI)
2.3.3. Definition of Healthy Vascular Aging
2.3.4. Anthropometric Measurements and Cardiovascular Risk Factors
2.3.5. Analytical Tests
2.4. Statistical Analysis
2.5. Ethical Principles
3. Results
3.1. Lifestyles, Risk Factors, and Vascular Structure and Function of the Subjects Included, Overall and by Sex
3.2. Lifestyles, Risk Factors, and Vascular Structure and Function According to Vascular Aging
3.3. Correlation of Vascular Aging with Mediterranean Diet and Other Lifestyles
3.4. Association between Vascular Aging Index and Healthy Lifestyles: Multiple Regression Analysis
3.5. Association between Vascular Aging Index and Healthy Lifestyles: Logistic Regression Analysis
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, E.; Walker, R. Global ageing: Successes, challenges and opportunities. Br. J. Hosp. Med. 2020, 81, 1–9. [Google Scholar] [CrossRef]
- Moreau, K.L.; Clayton, Z.S.; DuBose, L.E.; Rosenberry, R.; Seals, D.R. Effects of regular exercise on vascular function with aging: Does sex matter? Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H123–H137. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Cunha, P.G.; Lacolley, P.; Nilsson, P.M. Concept of Extremes in Vascular Aging. Hypertension 2019, 74, 218–228. [Google Scholar] [CrossRef]
- Nowak, K.L.; Rossman, M.J.; Chonchol, M.; Seals, D.R. Strategies for Achieving Healthy Vascular Aging. Hypertension 2018, 71, 389–402. [Google Scholar] [CrossRef]
- Clayton, Z.S.; Craighead, D.H.; Darvish, S.; Coppock, M.; Ludwig, K.R.; Brunt, V.E.; Seals, D.R.; Rossman, M.J. Promoting healthy cardiovascular aging: Emerging topics. J. Cardiovasc. Aging 2022, 2, 43. [Google Scholar] [CrossRef]
- Laurent, S. Defining vascular aging and cardiovascular risk. J. Hypertens. 2012, 30, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.M.; Nilsson, P.M.; Engström, G.; Wadström, B.N.; Empana, J.P.; Boutouyrie, P.; Laurent, S. Early and Supernormal Vascular Aging: Clinical Characteristics and Association With Incident Cardiovascular Events. Hypertension 2020, 76, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Nilsson Wadström, B.; Fatehali, A.H.; Engström, G.; Nilsson, P.M. A Vascular Aging Index as Independent Predictor of Cardiovascular Events and Total Mortality in an Elderly Urban Population. Angiology 2019, 70, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, M.; Gomez-Sanchez, L.; Patino-Alonso, M.C.; Cunha, P.G.; Recio-Rodriguez, J.I.; Alonso-Dominguez, R.; Sanchez-Aguadero, N.; Rodriguez-Sanchez, E.; Maderuelo-Fernandez, J.A.; Garcia-Ortiz, L.; et al. Vascular aging and its relationship with lifestyles and other risk factors in the general Spanish population: Early Vascular Ageing Study. J. Hypertens. 2020, 38, 1110–1122. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, L.; Xu, L.; Sun, X.; Liu, W.; Zhou, S.; van de Vosse, F.; Greenwald, S.E. Effects of exercise modalities on central hemodynamics, arterial stiffness and cardiac function in cardiovascular disease: Systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0200829. [Google Scholar] [CrossRef]
- Murray, K.O.; Mahoney, S.A.; Venkatasubramanian, R.; Seals, D.R.; Clayton, Z.S. Aging, aerobic exercise, and cardiovascular health: Barriers, alternative strategies and future directions. Exp. Gerontol. 2023, 173, 112105. [Google Scholar] [CrossRef]
- Kucharska-Newton, A.M.; Stoner, L.; Meyer, M.L. Determinants of Vascular Age: An Epidemiological Perspective. Clin. Chem. 2019, 65, 108–118. [Google Scholar] [CrossRef]
- Appiah, D.; Capistrant, B.D. Cardiovascular Disease Risk Assessment in the United States and Low- and Middle-Income Countries Using Predicted Heart/Vascular Age. Sci. Rep. 2017, 7, 16673. [Google Scholar] [CrossRef]
- Niiranen, T.J.; Lyass, A.; Larson, M.G.; Hamburg, N.M.; Benjamin, E.J.; Mitchell, G.F.; Vasan, R.S. Prevalence, Correlates, and Prognosis of Healthy Vascular Aging in a Western Community-Dwelling Cohort: The Framingham Heart Study. Hypertension 2017, 70, 267–274. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Reis, J.; Román, A.N.; Toledo, J.B.; Toledo, E. Extra-virgin olive oil for potential prevention of Alzheimer disease. Rev. Neurol. 2019, 175, 705–723. [Google Scholar] [CrossRef]
- Martini, D. Health Benefits of Mediterranean Diet. Nutrients 2019, 11, 1802. [Google Scholar] [CrossRef] [PubMed]
- Kaddoumi, A.; Denney, T.S., Jr.; Deshpande, G.; Robinson, J.L.; Beyers, R.J.; Redden, D.T.; Praticò, D.; Kyriakides, T.C.; Lu, B.; Kirby, A.N.; et al. Extra-Virgin Olive Oil Enhances the Blood-Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial. Nutrients 2022, 14, 5102. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-Style Diet for the Primary and Secondary Prevention of Cardiovascular Disease: A Cochrane Review. Glob. Heart 2020, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean diet in the management and prevention of obesity. Exp. Gerontol. 2023, 174, 112121. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 3, CD009825. [Google Scholar] [CrossRef] [PubMed]
- Andreo-López, M.C.; Contreras-Bolívar, V.; Muñoz-Torres, M.; García-Fontana, B.; García-Fontana, C. Influence of the Mediterranean Diet on Healthy Aging. Int. J. Mol. Sci. 2023, 24, 4491. [Google Scholar] [CrossRef] [PubMed]
- Mazza, E.; Ferro, Y.; Pujia, R.; Mare, R.; Maurotti, S.; Montalcini, T.; Pujia, A. Mediterranean Diet in Healthy Aging. J. Nutr. Health Aging 2021, 25, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Román-Viñas, B.; Sanchez-Villegas, A.; Guasch-Ferré, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Aspects Med. 2019, 67, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, Y.; Teixido-Tura, G.; Ambale-Venkatesh, B.; Noda, C.; Chugh, A.R.; Liu, C.Y.; Redheuil, A.; Stacey, R.B.; Dietz, H.; Gomes, A.S.; et al. Ten-year longitudinal change in aortic stiffness assessed by cardiac MRI in the second half of the human lifespan: The multi-ethnic study of atherosclerosis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1044–1053. [Google Scholar] [CrossRef]
- Karimi, L.; Mattace-Raso, F.U.; van Rosmalen, J.; van Rooij, F.; Hofman, A.; Franco, O.H. Effects of combined healthy lifestyle factors on functional vascular aging: The Rotterdam Study. J. Hypertens. 2016, 34, 853–859. [Google Scholar] [CrossRef]
- Weber, T.; Wassertheurer, S.; Hametner, B.; Moebus, S.; Pundt, N.; Mahabadi, A.A.; Roggenbuck, U.; Lehmann, N.; Jockel, K.H.; Erbel, R. Cross-sectional analysis of pulsatile hemodynamics across the adult life span: Reference values, healthy and early vascular aging: The Heinz Nixdorf Recall and the MultiGeneration Study. J. Hypertens. 2019, 37, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Del Giorno, R.; Maddalena, A.; Bassetti, S.; Gabutti, L. Association between Alcohol Intake and Arterial Stiffness in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 1207. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.; Britton, A.; Brunner, E.J.; Bell, S. Twenty-Five-Year Alcohol Consumption Trajectories and Their Association with Arterial Aging: A Prospective Cohort Study. J. Am. Heart Assoc. 2017, 6, e005288. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Marcos, M.A.; Martinez-Salgado, C.; Gonzalez-Sarmiento, R.; Hernandez-Rivas, J.M.; Sanchez-Fernandez, P.L.; Recio-Rodriguez, J.I.; Rodriguez-Sanchez, E.; García-Ortiz, L. Association between different risk factors and vascular accelerated ageing (EVA study): Study protocol for a cross-sectional, descriptive observational study. BMJ Open 2016, 6, e011031. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Zomeño, M.D.; Martínez-González, M.A.; Salas-Salvadó, J.; Corella, D.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Tinahones, F.J.; Miranda, J.L.; et al. Validity of the energy-restricted Mediterranean Diet Adherence Screener. Clin. Nutr. 2021, 40, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Sanidad, M.D. Límites de Consumo de Bajo Riesgo de Alcohol. In Actualización del Riesgo Relacionado con los Niveles de Consumo de Alcohol, el Patrón de Consumo y el Tipo de Bebida; Ministerio de Sanidad: Madrid, Spain, 2020. [Google Scholar]
- WHO MONICA Project Principal Investigators. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): A major international collaboration. J. Clin. Epidemiol. 1988, 41, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Melanson, E.L., Jr.; Freedson, P.S. Validity of the Computer Science and Applications, Inc. (CSA) activity monitor. Med. Sci. Sports Exerc. 1995, 27, 934–940. [Google Scholar] [CrossRef]
- Gómez-Marcos, M.A.; Recio-Rodríguez, J.I.; Patino-Alonso, M.C.; Agudo-Conde, C.; Gómez-Sanchez, L.; Gómez-Sanchez, M.; Rodríguez-Sánchez, E.; García-Ortiz, L. Protocol for measuring carotid intima-media thickness that best correlates with cardiovascular risk and target organ damage. Am. J. Hypertens. 2012, 25, 955–961. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Navarro Cáceres, A.; Navarro-Matías, E.; Gómez-Sánchez, M.; Tamayo-Morales, O.; Lugones-Sánchez, C.; González-Sánchez, S.; Rodríguez-Sánchez, E.; García-Ortiz, L.; Gómez-Sánchez, L.; Gómez-Marcos, M.A.; et al. Increase in Vascular Function Parameters According to Lifestyles in a Spanish Population without Previous Cardiovascular Disease-EVA Follow-Up Study. Nutrients 2023, 15, 4614. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Abhari, S.; Sabia, S.; Shipley, M.J.; Kivimäki, M.; Singh-Manoux, A.; Tabak, A.; McEniery, C.; Wilkinson, I.B.; Brunner, E.J. Physical Activity, Sedentary Behavior, and Long-Term Changes in Aortic Stiffness: The Whitehall II Study. J. Am. Heart Assoc. 2017, 6, e005974. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.Y.; Grunseit, A.; Midthjell, K.; Holmen, J.; Holmen, T.L.; Bauman, A.E.; van der Ploeg, H.P. Cross-sectional associations of total sitting and leisure screen time with cardiometabolic risk in adults. J. Sci. Med. Sport 2014, 17, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wilkens, L.R.; Park, S.Y.; Goodman, M.T.; Monroe, K.R.; Kolonel, L.N. Association between various sedentary behaviours and all-cause, cardiovascular disease and cancer mortality: The Multiethnic Cohort Study. Int. J. Epidemiol. 2013, 42, 1040–1056. [Google Scholar] [CrossRef] [PubMed]
- Wennman, H.; Vasankari, T.; Borodulin, K. Where to Sit? Type of Sitting Matters for the Framingham Cardiovascular Risk Score. AIMS Public Health 2016, 3, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Park, H.Y.; Lim, K.; Park, J. The role of habitual physical activity on arterial stiffness in elderly Individuals: A systematic review and meta-analysis. J. Exerc. Nutr. Biochem. 2017, 21, 16–21. [Google Scholar] [CrossRef]
- Leiva, A.M.; Martínez, M.A.; Cristi-Montero, C.; Salas, C.; Ramírez-Campillo, R.; Díaz Martínez, X.; Aguilar-Farías, N.; Celis-Morales, C. Sedentary lifestyle is associated with metabolic and cardiovascular risk factors independent of physical activity. Rev. Med. Chil. 2017, 145, 458–467. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 2020, 41, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Tigbe, W.W.; Granat, M.H.; Sattar, N.; Lean, M.E.J. Time spent in sedentary posture is associated with waist circumference and cardiovascular risk. Int. J. Obes. 2017, 45, 689–696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caparello, G.; Galluccio, A.; Giordano, C.; Lofaro, D.; Barone, I.; Morelli, C.; Sisci, D.; Catalano, S.; Andò, S.; Bonofiglio, D. Adherence to the Mediterranean diet pattern among university staff: A cross-sectional web-based epidemiological study in Southern Italy. Int. J. Food Sci. Nutr. 2020, 71, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Perez-Caballero, A.I.; Perez-Jimenez, F.; Lopez-Miranda, J. Mediterranean Diet and Cardiovascular Risk: Beyond Traditional Risk Factors. Crit. Rev. Food Sci. Nutr. 2016, 56, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Mangano, K.M.; McLean, R.R.; Hannan, M.T.; Kiel, D.P. Dietary Approaches for Bone Health: Lessons from the Framingham Osteoporosis Study. Curr. Osteoporos. Rep. 2015, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rey, J.; Roncero-Martín, R.; Rico-Martín, S.; Rey-Sánchez, P.; Pedrera-Zamorano, J.D.; Pedrera-Canal, M.; López-Espuela, F.; Lavado García, J.M. Adherence to a Mediterranean Diet and Bone Mineral Density in Spanish Premenopausal Women. Nutrients 2019, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Daviglus, M.L.; Bell, C.C.; Berrettini, W.; Bowen, P.E.; Connolly, E.S., Jr.; Cox, N.J.; Dunbar-Jacob, J.M.; Granieri, E.C.; Hunt, G.; McGarry, K.; et al. National Institutes of Health State-of-the-Science Conference statement: Preventing alzheimer disease and cognitive decline. Ann. Intern. Med. 2010, 153, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Remie, C.M.E.; Roumans, K.H.M.; Moonen, M.P.B.; Connell, N.J.; Havekes, B.; Mevenkamp, J.; Lindeboom, L.; de Wit, V.H.W.; van de Weijer, T.; Aarts, S.; et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am. J. Clin. Nutr. 2020, 112, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Casso, A.G.; VanDongen, N.S.; Ziemba, B.P.; Sapinsley, Z.J.; Richey, J.J.; Zigler, M.C.; Neilson, A.P.; Davy, K.P.; et al. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension 2020, 76, 101–112. [Google Scholar] [CrossRef]
- Ashor, A.W.; Shannon, O.M.; Werner, A.D.; Scialo, F.; Gilliard, C.N.; Cassel, K.S.; Seal, C.J.; Zheng, D.; Mathers, J.C.; Siervo, M. Effects of inorganic nitrate and vitamin C co-supplementation on blood pressure and vascular function in younger and older healthy adults: A randomised double-blind crossover trial. Clin. Nutr. 2020, 39, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Denman, B.A.; Mazzo, M.R.; Armstrong, M.L.; Reisdorph, N.; McQueen, M.B.; Chonchol, M.; Seals, D.R. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat. Commun. 2018, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat. Rev. Cardiol. 2018, 15, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.L.; Piano, M.R.; Thur, L.A.; Peters, T.A.; da Silva, A.L.G.; Phillips, S.A. The effects of repeated binge drinking on arterial stiffness and urinary norepinephrine levels in young adults. J. Hypertens. 2020, 38, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, J.; Garcia-Ortiz, L.; Rodriguez-Sanchez, E.; Maderuelo-Fernandez, J.A.; Tamayo-Morales, O.; Lugones-Sanchez, C.; Recio-Rodriguez, J.I.; Gomez-Marcos, M.A. The Relationship Between Alcohol Consumption with Vascular Structure and Arterial Stiffness in the Spanish Population: EVA Study. Alcohol. Clin. Exp. Res. 2020, 44, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Isath, A.; Rosenson, R.S.; Khawaja, M.; Wang, Z.; Fogg, S.E.; Virani, S.S.; Qi, L.; Cao, Y.; Long, M.T.; et al. Alcohol Consumption and Cardiovascular Health. Am. J. Med. 2022, 135, 1213–1230. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.F.J. Alcohol and Human Health: What Is the Evidence? Annu. Rev. Food Sci. Technol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, E.; Chahal, H.S.; Mukamal, K.J.; Rimm, E.B.; Mittleman, M.A. Alcohol and Immediate Risk of Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis. Circulation 2016, 133, 979–987. [Google Scholar] [CrossRef]
- Hahad, O.; Schmitt, V.H.; Arnold, N.; Keller, K.; Prochaska, J.H.; Wild, P.S.; Schulz, A.; Lackner, K.J.; Pfeiffer, N.; Schmidtmann, I.; et al. Chronic cigarette smoking is associated with increased arterial stiffness in men and women: Evidence from a large population-based cohort. Clin. Res. Cardiol. 2023, 112, 270–284. [Google Scholar] [CrossRef]
- Münzel, T.; Hahad, O.; Kuntic, M.; Keaney, J.F.; Deanfield, J.E.; Daiber, A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur. Heart J. 2020, 41, 4057–4070. [Google Scholar] [CrossRef]
- Claas, S.A.; Arnett, D.K. The Role of Healthy Lifestyle in the Primordial Prevention of Cardiovascular Disease. Curr. Cardiol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Abrignani, M.G.; Lucà, F.; Favilli, S.; Benvenuto, M.; Rao, C.M.; Di Fusco, S.A.; Gabrielli, D.; Gulizia, M.M. Lifestyles and Cardiovascular Prevention in Childhood and Adolescence. Pediatr. Cardiol. 2019, 40, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Howlett, S.E. Differences in Cardiovascular Aging in Men and Women. Adv. Exp. Med. Biol. 2018, 1065, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.M.; Howlett, S.E. Sex Differences in the Biology and Pathology of the Aging Heart. Can. J. Cardiol. 2016, 32, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
| Lifestyles | Overall (501) | Men (249) | Women (252) | p Value |
|---|---|---|---|---|
| MD score | 7.15 ± 2.07 | 6.68 ± 1.97 | 7.60 ± 2.08 | <0.001 |
| Adherence to MD, n (%) | 214, 42.7% | 89, 35.7% | 125, 49.6% | 0.001 |
| Alcohol consumption (g/W) | 40.47 ± 63.15 | 61.54 ± 74.65 | 19.64 ± 39.54 | <0.001 |
| Adequate alcohol consumption, n (%) | 251, 50.1% | 94, 37.8% | 157, 62.3% | <0.001 |
| Years of smoking | 12.98 ± 17.41 | 14.43 ± 18.91 | 11.55 ± 15.69 | 0.064 |
| Never smoked, n (%) | 367, 73.3% | 183, 73.5% | 184, 73.0% | 0.920 |
| Total physical activity (h/W) | 27.09 ± 9.52 | 26.01 ± 9.51 | 28.17 ± 9.43 | 0.011 |
| More than 26 h/W, n (%) | 246, 49.7% | 107, 43.1% | 139, 56.3% | 0.004 |
| Sedentary time (h/W) | 140.75 ± 9.56 | 141.78 ± 9.57 | 139.72 ± 9.45 | 0.017 |
| Less than 142 h/W, n (%) | 253, 51.1% | 113, 45.6% | 140, 56.7% | 0.015 |
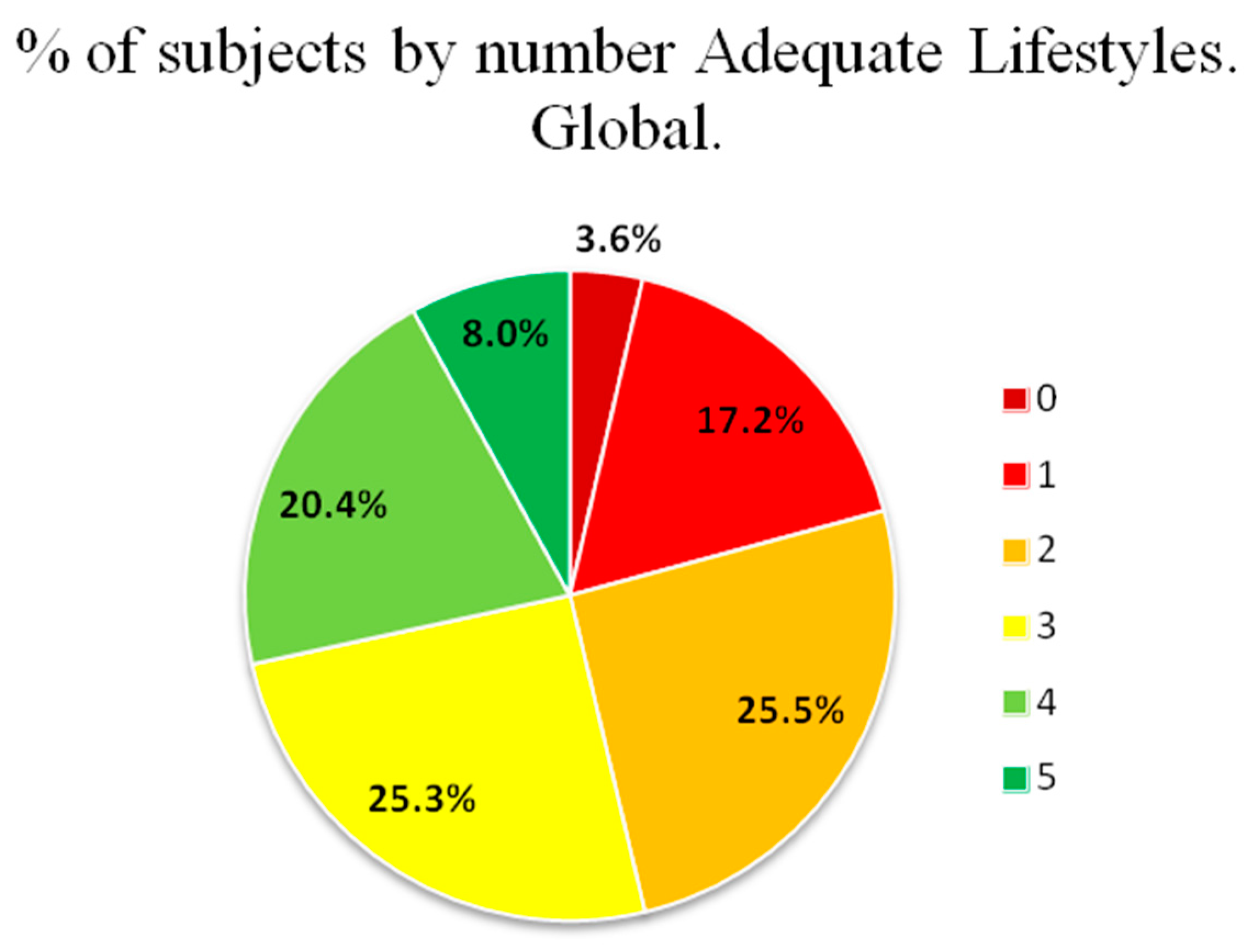
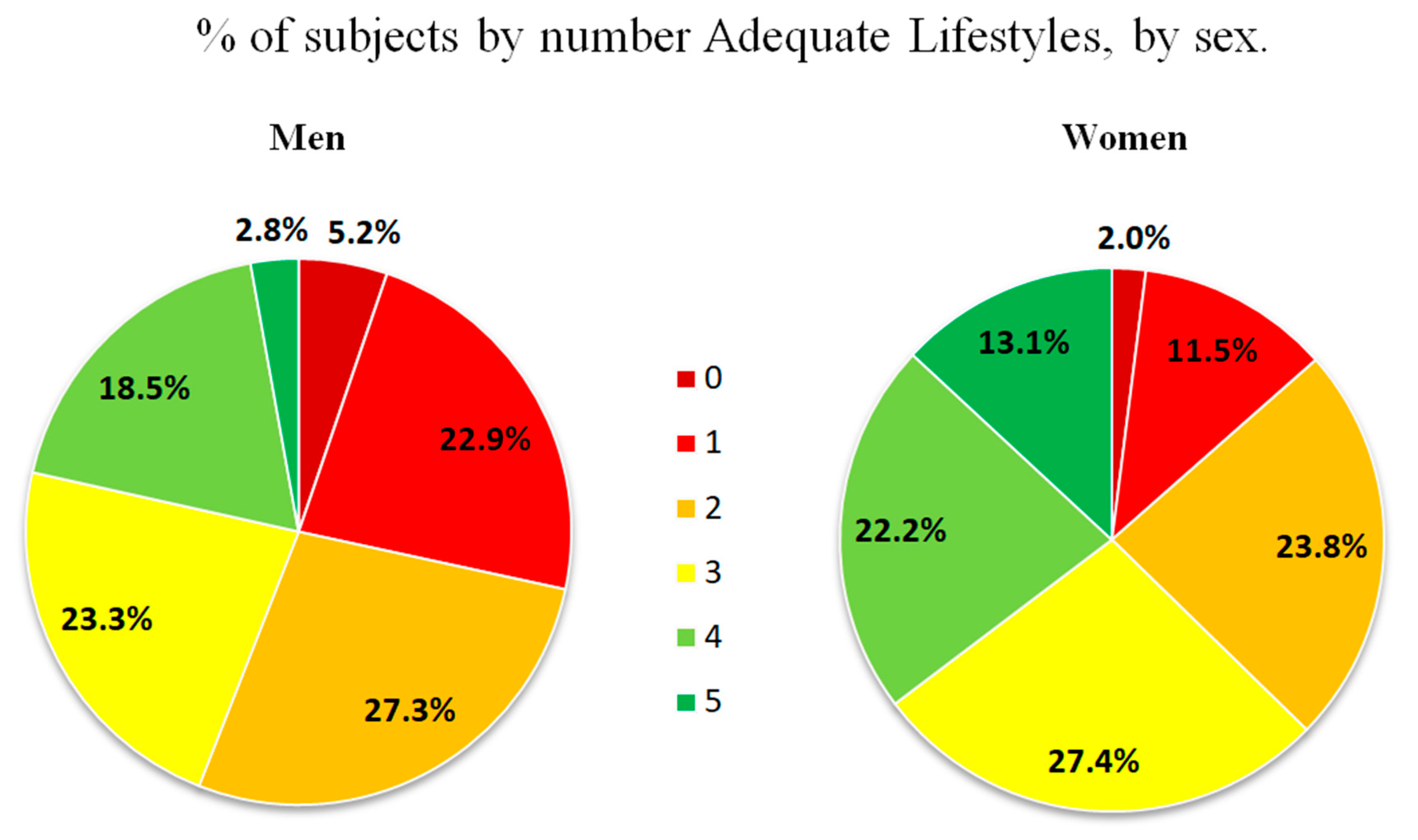
| Number of healthy lifestyles | 2.66 ± 1.29 | 2.35 ± 1.24 | 2.96 ± 1.28 | <0.001 |
| More than 2 healthy lifestyles, n (%) | 253, 50.5% | 102, 41.0% | 151, 59.9% | <0.001 |
| Conventional risk factors | ||||
| Age (years) | 55.90 ± 14.24 | 55.95 ± 14.31 | 55.85 ± 14.19 | 0.935 |
| Systolic blood pressure (mmHg) | 120.69 ± 23.13 | 126.47 ± 19.52 | 114.99 ± 24.96 | <0.001 |
| Diastolic blood pressure (mmHg) | 75.53 ± 10.10 | 77.40 ± 9.38 | 73.67, ± 10.46 | <0.001 |
| Mean arterial pressure (mmHg) | 90.58 ± 12.61 | 93.76 ± 11.13 | 87.44 ± 13.21 | <0.001 |
| Pulse pressure (mmHg) | 45.17 ± 19.81 | 49.06 ± 16.68 | 41.31 ± 21.83 | <0.001 |
| Hypertensive, n (%) | 147, 29.3% | 82, 32.9% | 65, 25.8% | 0.095 |
| Antihypertensive, n (%) | 96, 19.2% | 50, 20.1% | 46, 18.3% | 0.650 |
| Total cholesterol (mg/dL) | 194.76 ± 32.49 | 192.61 ± 32.26 | 196.88 ± 32.65 | 0.142 |
| LDL cholesterol (mg/dL) | 115.51 ± 29.37 | 117.43 ± 30.12 | 113.61 ± 28.54 | 0.148 |
| HDL cholesterol (mg/dL) | 58.75 ± 16.16 | 53.19 ± 14.12 | 64.22 ± 16.20 | <0.001 |
| Triglycerides (mg/dL) | 103.06 ± 53.20 | 112.28 ± 54.40 | 93.95 ± 50.46 | <0.001 |
| Atherogenic index | 3.53 ± 1.07 | 3.84 ± 1.12 | 3.24 ± 0.93 | <0.001 |
| Dyslipidemic | 191, 38.1% | 95 ± 38.2% | 96, 38.1% | 98.9% |
| Lipid-lowering, n (%) | 102, 20.4% | 49, 19.7% | 53, 21.0% | 0.740 |
| Plasma glucose (mg/dL) | 88.21 ± 17.37 | 90.14 ± 18.71 | 86.30 ± 15.73 | 0.013 |
| HbA1c (%) | 5.49 ± 0.56 | 5.54 ± 0.63 | 5.44 ± 0.47 | 0.043 |
| Diabetes mellitus type 2, n (%) | 38, 7.6% | 26, 10.4% | 12, 4.8% | 0.018 |
| Hypoglycemic, n (%) | 35, 7.0% | 23, 9.2% | 12, 4.8% | 0.055 |
| Body mass index (kg/m2) | 26.52 ± 4.23 | 26.90 ± 3.54 | 26.14 ± 4.79 | 0.044 |
| Waist circumference (cm) | 93.33 ± 12.01 | 98.76 ± 9.65 | 87.93 ± 11.70 | <0.001 |
| Obesity, n (%) | 94, 18.8% | 42, 16.9% | 52, 20.6% | 0.304 |
| Abdominal obesity, n (%) | 193, 38.6% | 78, 31.3% | 115, 45.8% | 0.001 |
| Structure—vascular function and aging | ||||
| Intima–media thickness (mm) | 0.682 ± 0.109 | 0.699 ± 0.116 | 0.665 ± 0.100 | 0.001 |
| cfPWV (m/s) | 6.53 ± 2.03 | 6.86 ± 2.20 | 6.21 ± 1.79 | <0.001 |
| Vascular aging index | 61.23 ± 12.86 | 63.47 ± 13.75 | 59.04 ± 11.54 | <0.001 |
| Lifestyles | HVA (94, 18.9%) | Non HVA (407, 81.1%) | p |
|---|---|---|---|
| MD score | 6.91 ± 2.20 | 7.20 ± 2.05 | 0.226 |
| Adherence to MD, n (%) | 33, 35.1% | 180, 44.6% | 0.106 |
| Alcohol consumption (g/W) | 34.89 ± 57.99 | 41.94 ± 64.46 | 0.331 |
| Adequate alcohol consumption, n (%) | 47, 50.0% | 202, 50.0% | 1.000 |
| Years of smoking | 12.45 ± 15.41 | 12.90 ± 17.70 | 0.820 |
| Never smoked, n (%) | 65, 69.1% | 299, 67.0% | 0.037 |
| Total physical activity (h/W) | 29.19 ± 9.79 | 26.58 ± 9.41 | 0.017 |
| More than 26 h/W, n (%) | 56, 60.2% | 188, 47.1% | 0.028 |
| Sedentary time (h/W) | 138.57 ± 9.81 | 141.28 ± 9.44 | 0.014 |
| Less than 142 h/W, n (%) | 57, 61.3% | 194, 48.6% | 0.029 |
| Number of healthy lifestyles | 2.74 ± 1.20 | 2.63 ± 1.32 | 0.444 |
| More than 2 healthy lifestyles, n (%) | 57, 60.6% | 195, 48.3% | 0.039 |
| Conventional risk factors | |||
| Age (years) | 52.64 ± 13.29 | 56.66 ± 14.34 | 0.010 |
| Systolic blood pressure (mmHg) | 109.59 ± 12.61 | 123.32 ± 24.28 | <0.001 |
| Diastolic blood pressure (mmHg) | 71.10 ± 8.28 | 76.56 ± 10.24 | <0.001 |
| Mean arterial pressure (mmHg) | 83.93 ± 9.00 | 92.14 ± 12.87 | <0.001 |
| Pulse pressure (mmHg) | 38.48 ± 8.90 | 46.76, 21.30 | <0.001 |
| Hypertensive, n (%) | 0, 0.0% | 147, 36.4% | <0.001 |
| Antihypertensive, n (%) | 0, 0.0% | 96, 19.3% | <0.001 |
| Total cholesterol (mg/dL) | 193.27 ± 32.39 | 194.95 ± 32.50 | 0.651 |
| LDL cholesterol (mg/dL) | 113.42 ± 28.02 | 115.87 ± 29.72 | 0.469 |
| HDL cholesterol (mg/dL) | 62.42 ± 18.00 | 57.85 ± 15.62 | 0.014 |
| Triglycerides (mg/dL) | 83.33 ± 32.99 | 107.66 ± 55.97 | <0.001 |
| Atherogenic index | 3.32 ± 1.09 | 3.59 ± 1.06 | 0.027 |
| Dyslipidemic | 29, 30.9% | 160, 39.6% | 0.126 |
| Lipid-lowering, n (%) | 13, 13.8% | 88, 21.8% | 0.086 |
| Plasma glucose (mg/dL) | 83.41 ± 10.27 | 89.41 ± 18.49 | <0.001 |
| HbA1c (%) | 5.30 ± 0.29 | 5.53 ± 0.59 | <0.001 |
| Diabetes mellitus type 2, n (%) | 0, 0.0% | 38, 9.4% | 0.002 |
| Hypoglycemic, n (%) | 0, 0.0% | 35, 7.0% | 0.003 |
| Body mass index (kg/m2) | 24.43 ± 3.26 | 27.00 ± 4.29 | <0.001 |
| Waist circumference (cm) | 88.48 ± 9.41 | 94.45 ± 12.28 | <0.001 |
| Obesity, n (%) | 6, 6.4% | 88, 21.8% | 0.001 |
| Abdominal obesidad, n (%) | 21, 22.3% | 171, 42.4% | <0.001 |
| Structure—vascular function and aging | |||
| Intima–media thickness (mm) | 0.62 ± 0.08 | 0.70 ± 0.11 | <0.001 |
| cfPWV (m/s) | 4.83 ± 0.75 | 6.93 ± 2.02 | <0.001 |
| Vascular aging index | 50.51 ± 5.67 | 63.74 ± 12.78 | <0.001 |
| VAI | Overall (501) | Men (249) | Women (251) |
|---|---|---|---|
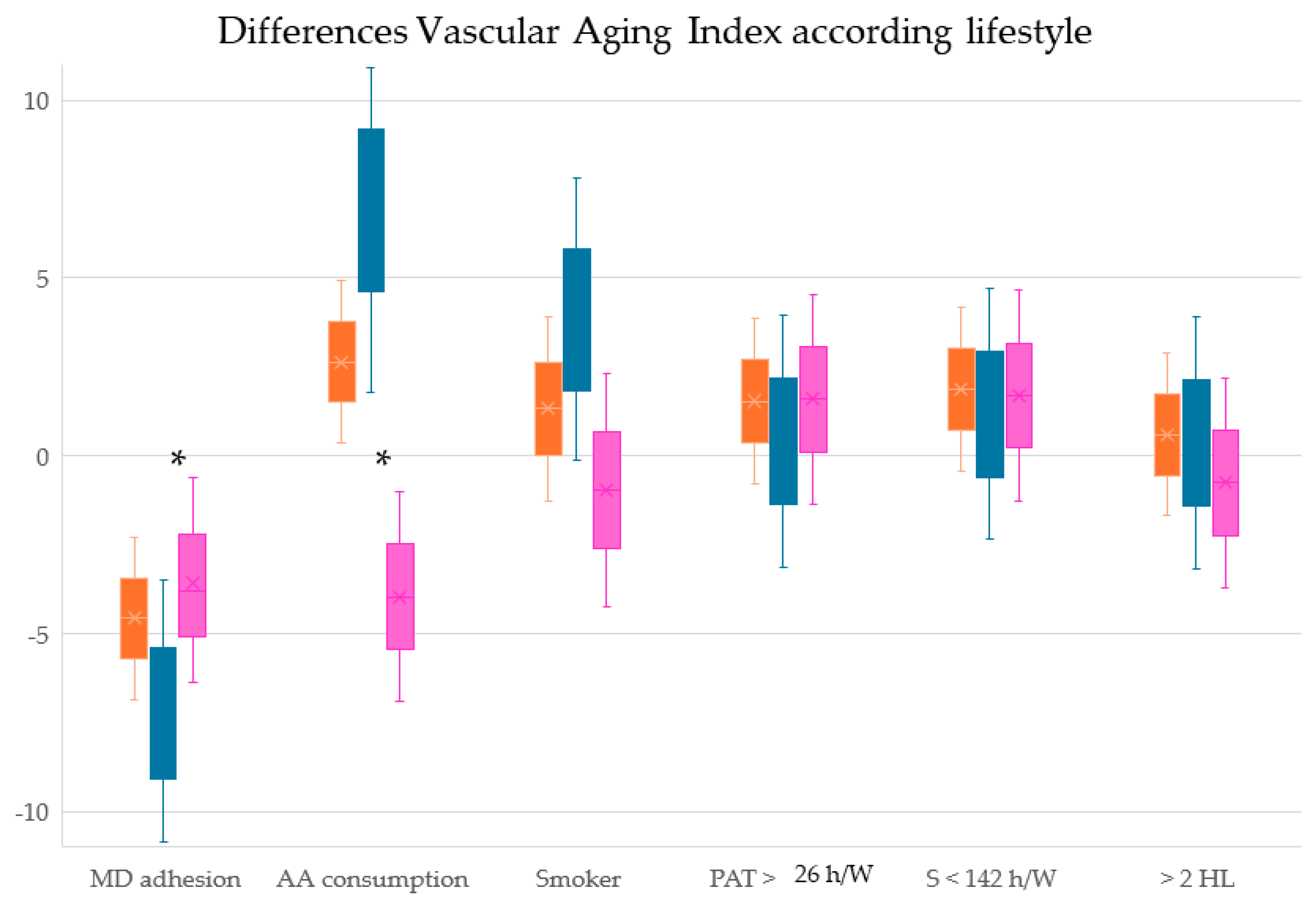
| MD score | −0.102 * | −0.082 * | 0.058 |
| Alcohol consumption, g/W | 0.228 ** | 0.221 ** | −0.040 |
| Year smoking | 0.092 * | 0.105 | 0.010 |
| Total physical activity (h/W) | −0.158 * | −0.120 | −0.161 * |
| Sedentary time (h/W) | 0.165 ** | 0.135 * | −0.162 * |
| Number of healthy lifestyles | −0.199 ** | −0.197 ** | −0.063 |
| cfPWV (m/s) | |||
| MD score | −0.082 | −0.048 | 0.075 |
| Alcohol consumption, g/W | 0.145 ** | 0.187 * | −0.031 |
| Year smoking | 0.082 | 0.099 | 0.026 |
| Total physical activity (h/W) | −0.174 ** | −0.155 * | −0.159 * |
| Sedentary time (h/W) | 0.181 ** | 0.170 * | −0.160 * |
| Number of healthy lifestyles | −0.178 ** | −0.173 * | −0.076 |
| Intima–media thickness (mm) | |||
| MD score | −0.082 | −0.047 | −0.058 |
| Alcohol consumption, g/W | 0.209 ** | 0.159 * | 0.120 * |
| Year smoking | 0.074 | 0.042 | 0.088 |
| Total physical activity (h/W) | 0.013 | 0.082 | 0.054 |
| Sedentary time (h/W) | 0.016 | 0.076 | 0.057 |
| Number of healthy lifestyles | 0.080 | −0.012 | −0.046 |
| VAI | β | (95% CI) | p |
|---|---|---|---|
| Overall | |||
| MD score | −0.056 | −0.418–0.307 | 0.763 |
| Alcohol consumption, g/W | 0.020 | 0.008–0.032 | 0.001 |
| Year smoking | 0.028 | −0.013–0.069 | 0.185 |
| Total physical activity (h/W) | −0.102 | −0.176–−0.028 | 0.007 |
| Sedentary time (h/W) | 0.109 | 0.036–0.183 | 0.004 |
| Number of healthy lifestyles | −0.640 | −1.195–−0.086 | 0.024 |
| Men | |||
| MD score | −0.044 | −0.598–0.509 | 0.875 |
| Alcohol consumption, g/W | 0.020 | 0.005–0.034 | 0.008 |
| Year smoking | 0.045 | −0.014–0.103 | 0.133 |
| Total physical activity (h/W) | −0.096 | −0.203–0.011 | 0.080 |
| Sedentary time (h/W) | 0.108 | 0.001–0.214 | 0.048 |
| Number of healthy lifestyles | −1.054 | −1.741–−0.367 | 0.003 |
| Women | |||
| MD score | −0.010 | −0.459–0.439 | 0.965 |
| Alcohol consumption, g/W | −0.005 | −0.027–0.018 | 0.690 |
| Year smoking | −0.023 | −0.080–0.033 | 0.416 |
| Total physical activity (h/W) | −0.099 | −0.195–−0.002 | 0.044 |
| Sedentary time (h/W) | 0.099 | 0.195–0.003 | 0.043 |
| Number of healthy lifestyles | −0.256 | −0.820–0.309 | 0.373 |
| VAI | OR | (95% CI) | p |
|---|---|---|---|
| Overall | |||
| MD adherence | 0.571 | 0.333–0.981 | 0.042 |
| AA consumption | 0.993 | 0.606–1.627 | 0.977 |
| Smoker | 0.648 | 0.375–1.119 | 0.119 |
| PAT > 26 h/W | 1.735 | 1.048–2.871 | 0.032 |
| ST—142 h/W | 1.696 | 1.025–2.805 | 0.040 |
| >2 HL | 1.877 | 1.123–3.136 | 0.016 |
| Men | |||
| MD adherence | 0.413 | 0.170–1.004 | 0.051 |
| AA consumption | 0.852 | 0.425–1.708 | 0.653 |
| Smoker | 0.382 | 0.162–0.902 | 0.028 |
| PAT > 26 h/W | 1.825 | 0.878–3.793 | 0.107 |
| ST—142 h/W | 1.738 | 0.838–3.604 | 0.136 |
| >2 HL | 1.998 | 0.956–4.175 | 0.066 |
| Women | |||
| MD adherence | 0.766 | 0.377–1.558 | 0.462 |
| AA consumption | 0.968 | 0.465–2.014 | 0.931 |
| Smoker | 0.812 | 0.381–1.731 | 0.589 |
| PAT > 26 h/W | 1.668 | 0.817–3.404 | 0.160 |
| ST—142 h/W | 1.655 | 0.810–3.379 | 0.167 |
| >2 HL | 1.769 | 0.848–3.692 | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Sánchez, L.; González-Falcon, D.; Llamas-Ramos, R.; Rodríguez, M.C.; Rodríguez-Sánchez, E.; García-Ortiz, L.; Llamas-Ramos, I.; Gómez-Sánchez, M.; Gómez-Marcos, M.A. The Relationship between Healthy Vascular Aging with the Mediterranean Diet and Other Lifestyles in the Spanish Population: The EVA Study. Nutrients 2024, 16, 2565. https://doi.org/10.3390/nu16152565
Gómez-Sánchez L, González-Falcon D, Llamas-Ramos R, Rodríguez MC, Rodríguez-Sánchez E, García-Ortiz L, Llamas-Ramos I, Gómez-Sánchez M, Gómez-Marcos MA. The Relationship between Healthy Vascular Aging with the Mediterranean Diet and Other Lifestyles in the Spanish Population: The EVA Study. Nutrients. 2024; 16(15):2565. https://doi.org/10.3390/nu16152565
Chicago/Turabian StyleGómez-Sánchez, Leticia, David González-Falcon, Rocío Llamas-Ramos, María Cortés Rodríguez, Emiliano Rodríguez-Sánchez, Luis García-Ortiz, Inés Llamas-Ramos, Marta Gómez-Sánchez, and Manuel A. Gómez-Marcos. 2024. "The Relationship between Healthy Vascular Aging with the Mediterranean Diet and Other Lifestyles in the Spanish Population: The EVA Study" Nutrients 16, no. 15: 2565. https://doi.org/10.3390/nu16152565
APA StyleGómez-Sánchez, L., González-Falcon, D., Llamas-Ramos, R., Rodríguez, M. C., Rodríguez-Sánchez, E., García-Ortiz, L., Llamas-Ramos, I., Gómez-Sánchez, M., & Gómez-Marcos, M. A. (2024). The Relationship between Healthy Vascular Aging with the Mediterranean Diet and Other Lifestyles in the Spanish Population: The EVA Study. Nutrients, 16(15), 2565. https://doi.org/10.3390/nu16152565







