A Mixture of Lactobacillus HY7601 and KY1032 Regulates Energy Metabolism in Adipose Tissue and Improves Cholesterol Disposal in High-Fat-Diet-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Bacteria and Preparation
2.2. Animal Study Design
2.3. Measurement of Body Temperature
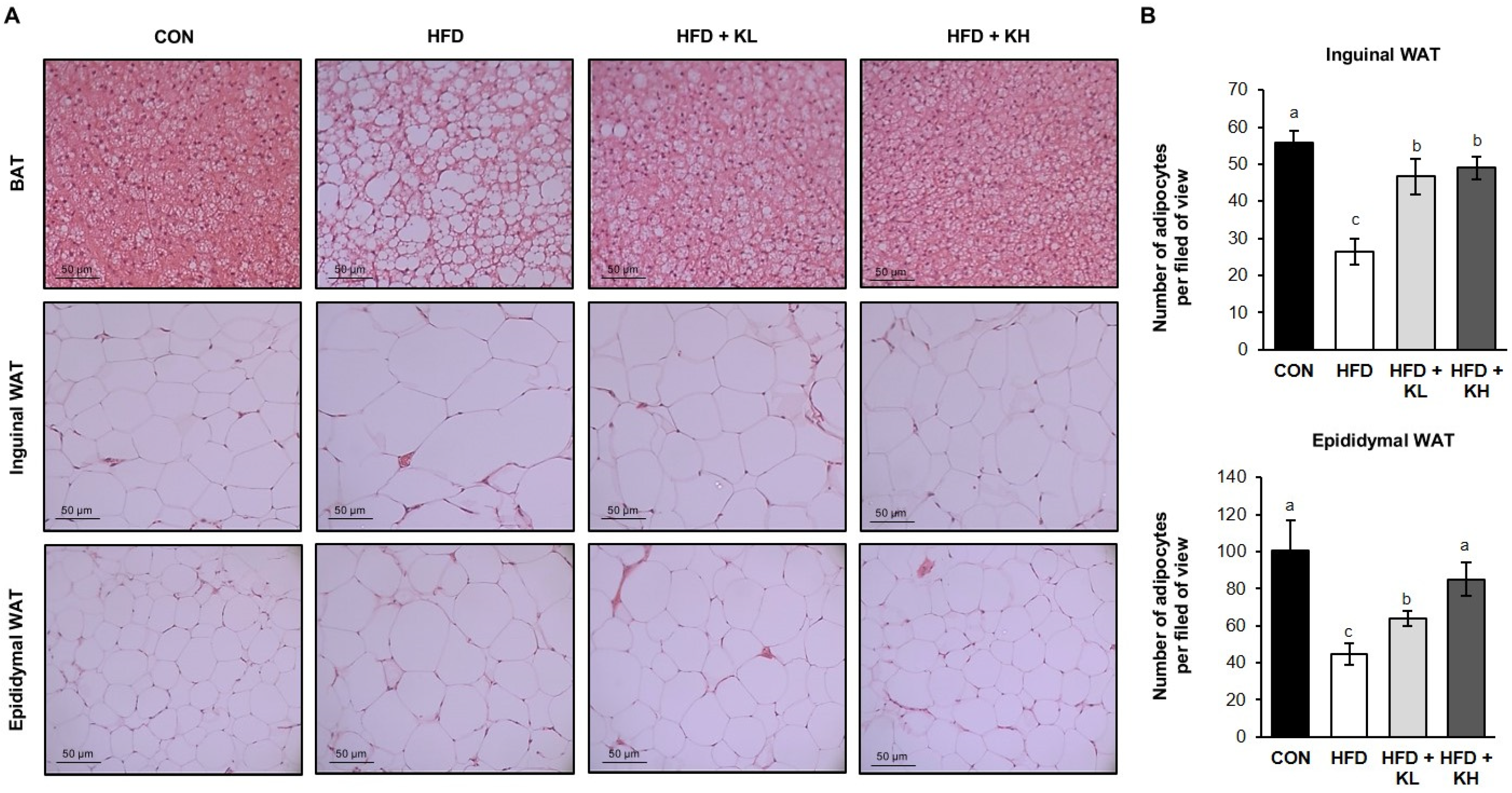
2.4. Histologic Analysis
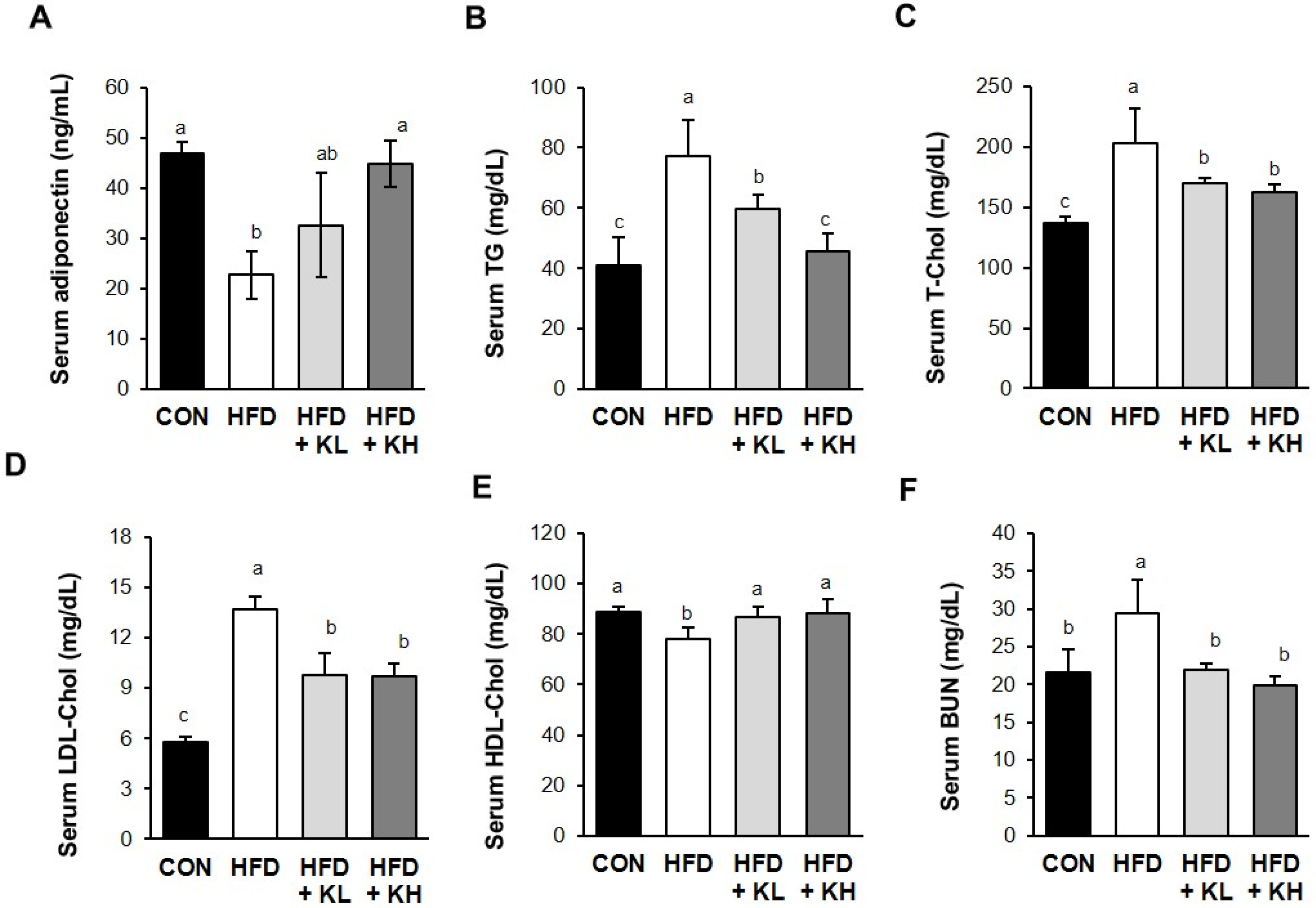
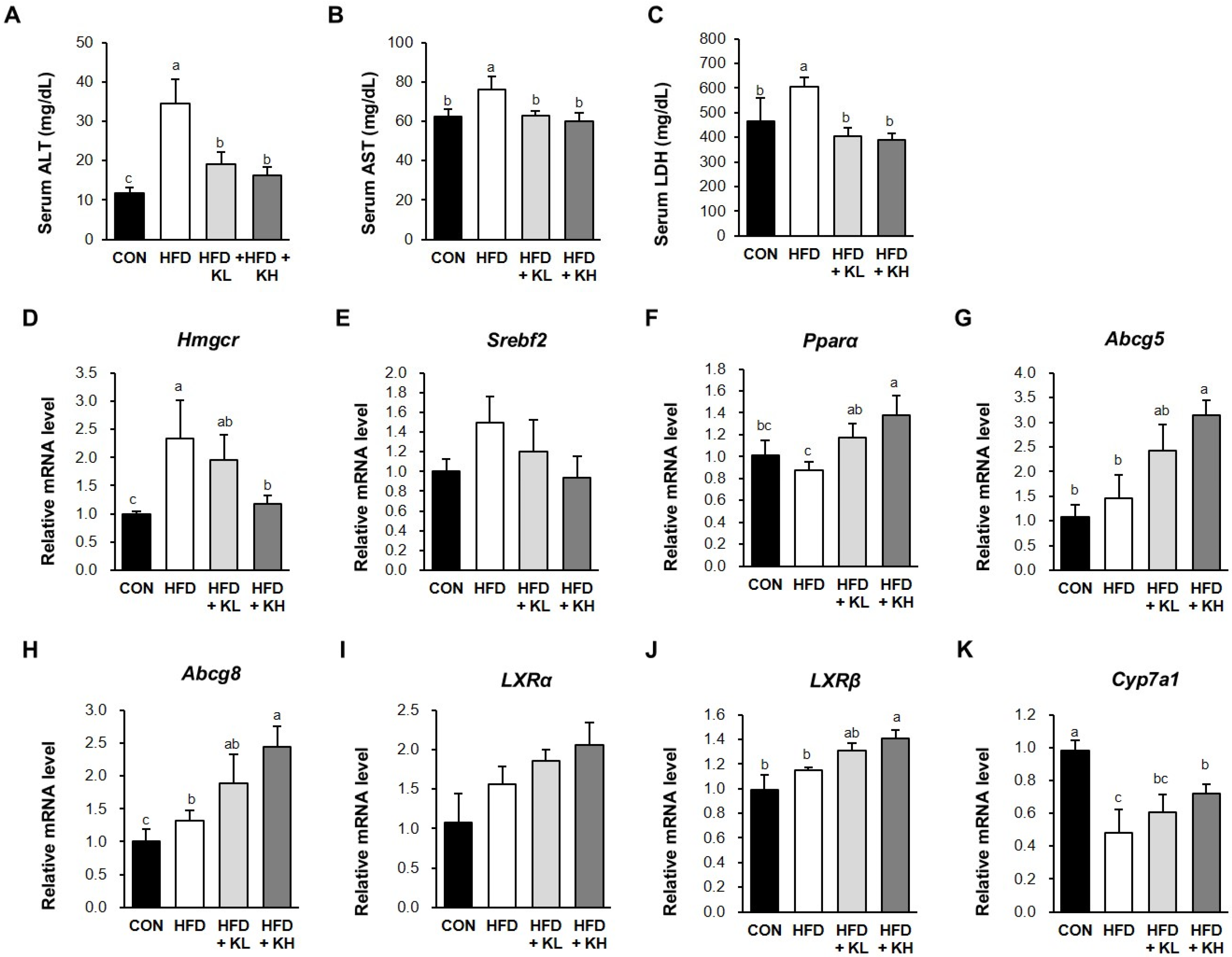
2.5. Biochemical Analyses
2.6. Western Blot
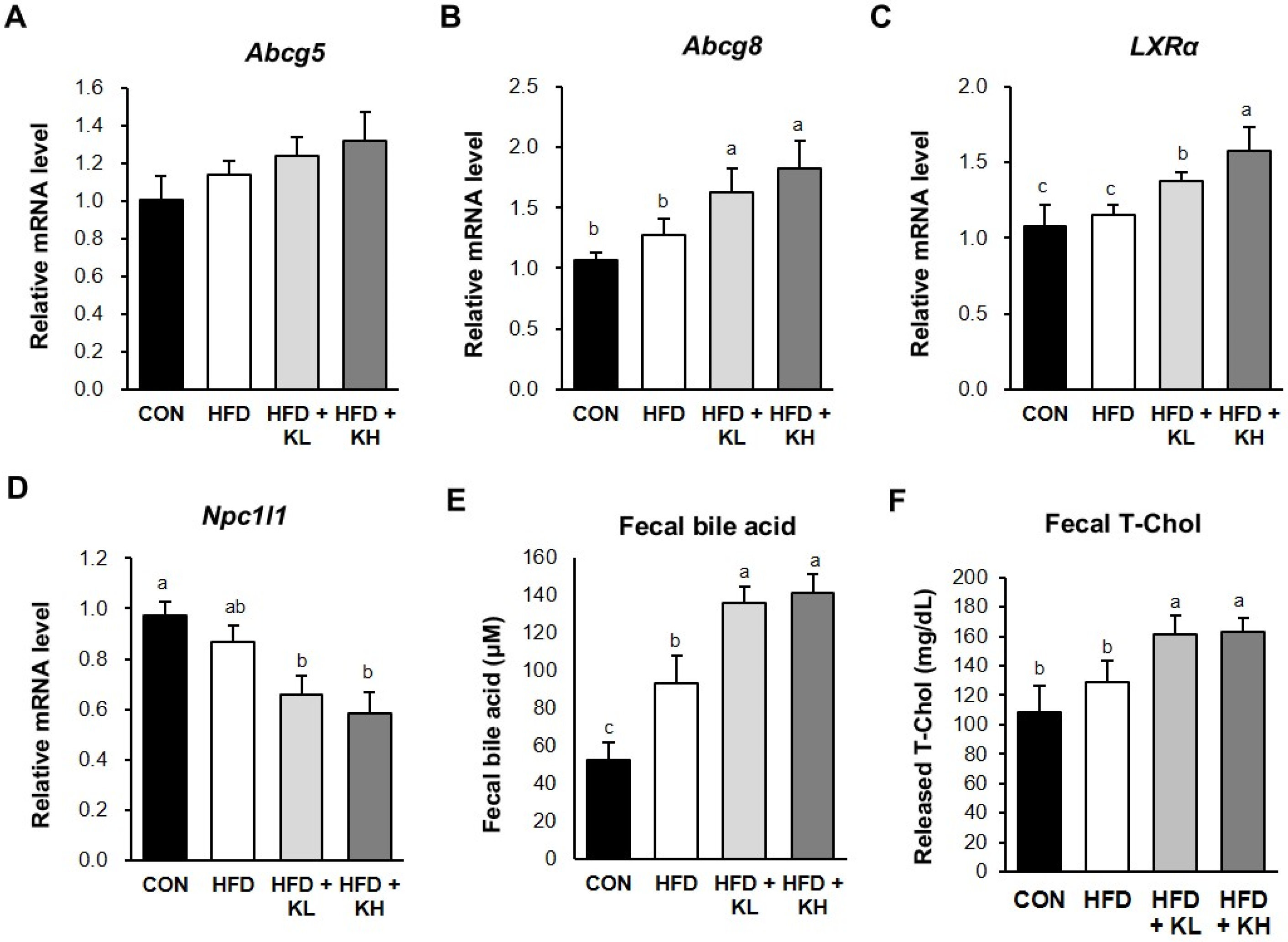
2.7. RNA Extraction and Gene Expression Analysis
2.8. Fecal Cholesterol and Bile Acid Analysis
2.9. Statistical Analysis
3. Results
3.1. A Lactobacillus HY7601 and KY1032 Mixture Ameliorates HFD-Induced Obesity
3.2. The Lactobacillus HY7601 and KY1032 Mixture Reduces the Expansion of Adipose Tissue in HFD-Fed Mice
3.3. The Lactobacillus HY7601 and KY1032 Mixture Affects the Serum Lipid Profile of the Mice
3.4. The Lactobacillus HY7601 and KY1032 Mixture Affects the Energy Metabolism of the Mice
3.5. The Lactobacillus HY7601 and KY1032 Mixture Increases UCP1 Activation in Adipose Tissue
3.6. The Lactobacillus HY7601 and KY1032 Mixture Increases the Expression of Thermogenic Factors in Adipose Tissue
3.7. The Lactobacillus HY7601 and KY1032 Mixture Induces Cholesterol Disposal and the Fatty Acids’ β-Oxidation in the Liver
3.8. The Lactobacillus HY7601 and KY1032 Mixture Stimulates Cholesterol Excretion from the Jejunum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grundy, S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, type 2 diabetes, and cancer risk. Front. Oncol. 2021, 10, 615375. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Löffler, M.C.; Betz, M.J.; Blondin, D.P.; Augustin, R.; Sharma, A.K.; Tseng, Y.-H.; Scheele, C.; Zimdahl, H.; Mark, M.; Hennige, A.M. Challenges in tackling energy expenditure as obesity therapy: From preclinical models to clinical application. Mol. Metab. 2021, 51, 101237. [Google Scholar] [CrossRef] [PubMed]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Kim, W.K.; Bae, K.-H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 2014, 6, 33. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Swarbrick, M.M.; Ho, K.K. Brown adipose tissue in adult humans: A metabolic renaissance. Endocr. Rev. 2013, 34, 413–438. [Google Scholar] [CrossRef]
- Schirinzi, V.; Poli, C.; Berteotti, C.; Leone, A. Browning of adipocytes: A potential therapeutic approach to obesity. Nutrients 2023, 15, 2229. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q. Brown and beige adipose tissue: A novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.; Park, S.; Kim, S.K.; Lee, J.S.; Kim, K.W.; Kwon, O. Hypothalamic control of energy expenditure and thermogenesis. Exp. Mol. Med. 2022, 54, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Diet induced thermogenesis. Nutr. Metab. 2004, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: From mice to men. Front. Endocrinol. 2020, 11, 533838. [Google Scholar] [CrossRef]
- Mele, L.; Bidault, G.; Mena, P.; Crozier, A.; Brighenti, F.; Vidal-Puig, A.; Del Rio, D. Dietary (Poly) phenols, brown adipose tissue activation, and energy expenditure: A narrative review. Adv. Nutr. 2017, 8, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Spicer, E.G.; Gavini, C.K.; Goudjo-Ako, A.J.; Novak, C.M.; Shi, H. Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation). Physiol. Behav. 2014, 125, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Herndon, D.N.; Chondronikola, M.; Chao, T.; Annamalai, P.; Bhattarai, N.; Saraf, M.K.; Capek, K.D.; Reidy, P.T.; Daquinag, A.C. Human and mouse brown adipose tissue mitochondria have comparable UCP1 function. Cell Metab. 2016, 24, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Soler-Vázquez, M.C.; del Mar Romero, M.; Todorcevic, M.; Delgado, K.; Calatayud, C.; Benitez-Amaro, A.; Lhoëst, M.T.L.C.; Mera, P.; Zagmutt, S.; Bastías-Pérez, M. Implantation of CPT1AM-expressing adipocytes reduces obesity and glucose intolerance in mice. Metab. Eng. 2023, 77, 256–272. [Google Scholar] [CrossRef]
- Lizcano, F. The beige adipocyte as a therapy for metabolic diseases. Int. J. Mol. Sci. 2019, 20, 5058. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of adipose tissue browning as a strategy to combat obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, J.M.; Chiang, J.Y. Understanding bile acid signaling in diabetes: From pathophysiology to therapeutic targets. Diabetes Metab. J. 2019, 43, 257. [Google Scholar] [CrossRef] [PubMed]
- Gianfrancesco, M.A.; Paquot, N.; Piette, J.; Legrand-Poels, S. Lipid bilayer stress in obesity-linked inflammatory and metabolic disorders. Biochem. Pharmacol. 2018, 153, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Hampton, R.Y. Proteolysis and sterol regulation. Annu. Rev. Cell Dev. Biol. 2002, 18, 345–378. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Asuka, E.; Jialal, I. Hypercholesterolemia; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care Clin. Off. Pract. 2013, 40, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dahlman-Wright, K. Liver X receptor in cholesterol metabolism. J. Endocrinol. 2009, 204, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.L.; Volle, D.H.; Zhang, Y.; McDonald, J.G.; Sion, B.; Lefrançois-Martinez, A.-M.; Caira, F.; Veyssière, G.; Mangelsdorf, D.J.; Lobaccaro, J.-M.A. Liver X receptors regulate adrenal cholesterol balance. J. Clin. Investig. 2006, 116, 1902–1912. [Google Scholar] [CrossRef]
- Chiang, J.Y.; Ferrell, J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xie, Z.; Gao, W.; Pu, L.; Wei, J.; Guo, C. Quercetin regulates hepatic cholesterol metabolism by promoting cholesterol-to-bile acid conversion and cholesterol efflux in rats. Nutr. Res. 2016, 36, 271–279. [Google Scholar] [CrossRef]
- Korach-André, M.; Archer, A.; Barros, R.P.; Parini, P.; Gustafsson, J.-Å. Both liver-X receptor (LXR) isoforms control energy expenditure by regulating brown adipose tissue activity. Proc. Natl. Acad. Sci. USA 2011, 108, 403–408. [Google Scholar] [CrossRef]
- Dib, L.; Bugge, A.; Collins, S. LXRα fuels fatty acid-stimulated oxygen consumption in white adipocytes. J. Lipid Res. 2014, 55, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Lee, E.-S.; Song, E.-J.; Shin, D.-U.; Eom, J.-E.; Shin, H.S.; Kim, J.E.; Oh, J.Y.; Nam, Y.-D.; Lee, S.-Y. Lacticaseibacillus paracasei AO356 ameliorates obesity by regulating adipogenesis and thermogenesis in C57BL/6J male mice. J. Funct. Foods 2023, 101, 105404. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, G.; Noh, M.-g.; Park, J.-h.; Jang, M.; Fang, S.; Park, H. Lactobacillus fermentum promotes adipose tissue oxidative phosphorylation to protect against diet-induced obesity. Exp. Mol. Med. 2020, 52, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Park, D.-S.; Rahman, M.; Ki, S.-J.; Lee, Y.R.; Imran, K.; Yoon, D.; Heo, J.; Lee, T.-J.; Kim, Y.-S. Bifidobacterium longum DS0956 and Lactobacillus rhamnosus DS0508 culture-supernatant ameliorate obesity by inducing thermogenesis in obese-mice. Benef. Microbes 2020, 11, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, Y.J.; Kim, M.; Kim, M.; Kwak, J.H.; Lee, J.-W.; Ahn, Y.-T.; Sim, J.-H.; Lee, J.H. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduced body adiposity and Lp-PLA2 activity in overweight subjects. J. Funct. Foods 2015, 19, 744–752. [Google Scholar] [CrossRef]
- Park, D.-Y.; Ahn, Y.-T.; Park, S.-H.; Huh, C.-S.; Yoo, S.-R.; Yu, R.; Sung, M.-K.; McGregor, R.A.; Choi, M.-S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose tissue: Physiology to metabolic dysfunction. In Endotext [Internet]; National Institutes of Health (NIH): Stapleton, NY, USA, 2020. [Google Scholar]
- Branca, R.T.; He, T.; Zhang, L.; Floyd, C.S.; Freeman, M.; White, C.; Burant, A. Detection of brown adipose tissue and thermogenic activity in mice by hyperpolarized xenon MRI. Proc. Natl. Acad. Sci. USA 2014, 111, 18001–18006. [Google Scholar] [CrossRef] [PubMed]
- Oelkrug, R.; Krause, C.; Herrmann, B.; Resch, J.; Gachkar, S.; El Gammal, A.T.; Wolter, S.; Mann, O.; Oster, H.; Kirchner, H. Maternal brown fat thermogenesis programs glucose tolerance in the male offspring. Cell Rep. 2020, 33, 108351. [Google Scholar] [CrossRef]
- Crawford, S.O.; Hoogeveen, R.C.; Brancati, F.L.; Astor, B.C.; Ballantyne, C.M.; Schmidt, M.I.; Young, J.H. Association of blood lactate with type 2 diabetes: The Atherosclerosis Risk in Communities Carotid MRI Study. Int. J. Epidemiol. 2010, 39, 1647–1655. [Google Scholar] [CrossRef]
- Klaus, S.; Keipert, S.; Rossmeisl, M.; Kopecky, J. Augmenting energy expenditure by mitochondrial uncoupling: A role of AMP-activated protein kinase. Genes Nutr. 2012, 7, 369–386. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef] [PubMed]
- AL-Janabi, A.; Ali, Z.Q.; Noree, Z.M. Lactate dehydrogenase as an indicator of liver, muscular and cancer diseases. J. Coast. Life Med. 2015, 3, 543–546. [Google Scholar]
- Marchesini, G.; Moscatiello, S.; Di Domizio, S.; Forlani, G. Obesity-associated liver disease. J. Clin. Endocrinol. Metab. 2008, 93, s74–s80. [Google Scholar] [CrossRef] [PubMed]
- Klett, E.L.; Lee, M.-H.; Adams, D.B.; Chavin, K.D.; Patel, S.B. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol. 2004, 4, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Breevoort, S.R.; Angdisen, J.; Fu, M.; Schmidt, D.R.; Holmstrom, S.R.; Kliewer, S.A.; Mangelsdorf, D.J.; Schulman, I.G. Liver LXRα expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J. Clin. Investig. 2012, 122, 1688–1699. [Google Scholar] [CrossRef]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef]
- Heo, W.; Lee, E.S.; Cho, H.T.; Kim, J.H.; Lee, J.H.; Yoon, S.M.; Kwon, H.T.; Yang, S.; Kim, Y.-J. Lactobacillus plantarum LRCC 5273 isolated from Kimchi ameliorates diet-induced hypercholesterolemia in C57BL/6 mice. Biosci. Biotechnol. Biochem. 2018, 82, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, G.; Zheng, L.; Chen, Z.; Liu, X. Hypocholesterolemic effect of capsaicinoids in rats fed diets with or without cholesterol. J. Agric. Food Chem. 2013, 61, 4287–4293. [Google Scholar] [CrossRef]
- Banno, A.; Wang, J.; Okada, K.; Mori, R.; Mijiti, M.; Nagaoka, S. Identification of a novel cholesterol-lowering dipeptide, phenylalanine-proline (FP), and its down-regulation of intestinal ABCA1 in hypercholesterolemic rats and Caco-2 cells. Sci. Rep. 2019, 9, 19416. [Google Scholar] [CrossRef]
- Wirth, A.; Wabitsch, M.; Hauner, H. The prevention and treatment of obesity. Dtsch. Ärzteblatt Int. 2014, 111, 705. [Google Scholar] [CrossRef]
- Herz, C.T.; Kiefer, F.W. Adipose tissue browning in mice and humans. J. Endocrinol. 2019, 241, R97–R109. [Google Scholar] [CrossRef]
- Calderon-Dominguez, M.; Mir, J.F.; Fucho, R.; Weber, M.; Serra, D.; Herrero, L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 2016, 5, 98–118. [Google Scholar] [CrossRef]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary factors promoting brown and beige fat development and thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Bodke, H.; Jogdand, S. Role of probiotics in human health. Cureus 2022, 14, e31313. [Google Scholar] [CrossRef]
- Giraffa, G.; Chanishvili, N.; Widyastuti, Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010, 161, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Dong, H.J.; Jeong, H.U.; Ryu, D.W.; Song, S.M.; Kim, Y.R.; Jung, H.H.; Kim, T.H.; Kim, Y.-H. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci. Rep. 2020, 10, 869. [Google Scholar] [CrossRef]
- Xie, N.; Cui, Y.; Yin, Y.-N.; Zhao, X.; Yang, J.-W.; Wang, Z.-G.; Fu, N.; Tang, Y.; Wang, X.-H.; Liu, X.-W. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement. Altern. Med. 2011, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Koppula, S.; Wankhede, N.L.; Sammeta, S.S.; Shende, P.V.; Pawar, R.S.; Chimthanawala, N.; Umare, M.D.; Taksande, B.G.; Upaganlawar, A.B.; Umekar, M.J. Modulation of Cholesterol metabolism with Phytoremedies in Alzheimer’s disease: A comprehensive Review. Ageing Res. Rev. 2024, 99, 102389. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.-J.; Lee, K.; Hong, H.-J.; Hong, D.-K.; Jung, S.-H.; Park, S.-D.; Shim, J.-J.; Lee, J.-L. Effects of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 on overweight and the gut microbiota in humans: Randomized, double-blinded, placebo-controlled clinical trial. Nutrients 2022, 14, 2484. [Google Scholar] [CrossRef]
- Labbé, S.M.; Caron, A.; Chechi, K.; Laplante, M.; Lecomte, R.; Richard, D. Metabolic activity of brown, “beige,” and white adipose tissues in response to chronic adrenergic stimulation in male mice. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E260–E268. [Google Scholar] [CrossRef]
- Kozak, L.; Anunciado-Koza, R. UCP1: Its involvement and utility in obesity. Int. J. Obes. 2008, 32, S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Castro Cabezas, M. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Brochu, M.; Mathieu, M.E.; Karelis, A.D.; Doucet, É.; Lavoie, M.E.; Garrel, D.; Rabasa-Lhoret, R. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: A Monet study. Obesity 2008, 16, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Pratley, R.E.; Salbe, A.D.; Bogardus, C.; Ravussin, E.; Tataranni, P.A. Energy expenditure, fat oxidation, and body weight regulation: A study of metabolic adaptation to long-term weight change. J. Clin. Endocrinol. Metab. 2000, 85, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chiang, J.Y. Regulation of bile acid and cholesterol metabolism by PPARs. PPAR Res. 2009, 2009, 501739. [Google Scholar] [CrossRef]
- Di Gregorio, M.C.; Cautela, J.; Galantini, L. Physiology and physical chemistry of bile acids. Int. J. Mol. Sci. 2021, 22, 1780. [Google Scholar] [CrossRef]




| Antibody Target | Catalog No. |
|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | CS 2188 |
| Uncoupling protein 1 | CS 77298 |
| Phosphorylated adenosine monophosphate-activated protein kinase (Thr172) | CS 50081 |
| Adenosine monophosphate-activated protein kinase | CS 2535 |
| Peroxisome proliferator-activated receptor gamma coactivator 1-alpha | CS 2178 |
| Sirtuin-1 | CS 2028 |
| Gene | Product Name | Catalog No. |
|---|---|---|
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | Mm99999915_g1 |
| Pparα | Peroxisome proliferator-activated receptor alpha | Mm00440939_m1 |
| Hmgcr | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | Mm01282499_m1 |
| Srebf2 | Sterol regulatory element-binding factor 2 | Mm01306292_m1 |
| Abcg5 | ATP-binding cassette, sub-family G (WHITE), member 5 | Mm00446241_m1 |
| Abcg8 | ATP-binding cassette, sub-family G (WHITE), member 8 | Mm00445980_m1 |
| Nr1h3 (Lxrα) | Nuclear receptor subfamily 1, group H, member 3 | Mm00443451_m1 |
| Nr1h21 (Lxrβ) | Nuclear receptor subfamily 1, group H, member 2 | Mm00437265_g1 |
| Cyp7a1 | Cytochrome P450, family 7, subfamily a, polypeptide 1 | Mm00484150_m1 |
| Npc1l1 | NPC1-like 1 | Mm01191972_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Kim, H.-J.; Kim, J.-Y.; Shim, J.-J.; Lee, J.-H. A Mixture of Lactobacillus HY7601 and KY1032 Regulates Energy Metabolism in Adipose Tissue and Improves Cholesterol Disposal in High-Fat-Diet-Fed Mice. Nutrients 2024, 16, 2570. https://doi.org/10.3390/nu16152570
Lee K, Kim H-J, Kim J-Y, Shim J-J, Lee J-H. A Mixture of Lactobacillus HY7601 and KY1032 Regulates Energy Metabolism in Adipose Tissue and Improves Cholesterol Disposal in High-Fat-Diet-Fed Mice. Nutrients. 2024; 16(15):2570. https://doi.org/10.3390/nu16152570
Chicago/Turabian StyleLee, Kippeum, Hyeon-Ji Kim, Joo-Yun Kim, Jae-Jung Shim, and Jae-Hwan Lee. 2024. "A Mixture of Lactobacillus HY7601 and KY1032 Regulates Energy Metabolism in Adipose Tissue and Improves Cholesterol Disposal in High-Fat-Diet-Fed Mice" Nutrients 16, no. 15: 2570. https://doi.org/10.3390/nu16152570
APA StyleLee, K., Kim, H.-J., Kim, J.-Y., Shim, J.-J., & Lee, J.-H. (2024). A Mixture of Lactobacillus HY7601 and KY1032 Regulates Energy Metabolism in Adipose Tissue and Improves Cholesterol Disposal in High-Fat-Diet-Fed Mice. Nutrients, 16(15), 2570. https://doi.org/10.3390/nu16152570





