The Association of the Essential Amino Acids Lysine, Methionine, and Threonine with Clinical Outcomes in Patients at Nutritional Risk: Secondary Analysis of a Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Study Intervention (Randomization/Procedures)
2.4. Analysis of Blood Biomarkers
2.5. Outcomes
2.6. Definition of Low Muscle Mass and Muscle Radiodensity Based on Abdominal CT Scans
2.7. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Association of Lysine, Methionine, and Threonine with Nutritional Parameters
3.3. Association of Lysine, Methionine, and Threonine with Mortality and Further Clinical Outcomes
3.4. Association of Lysine, Methionine, and Threonine with Muscle-Specific Outcomes
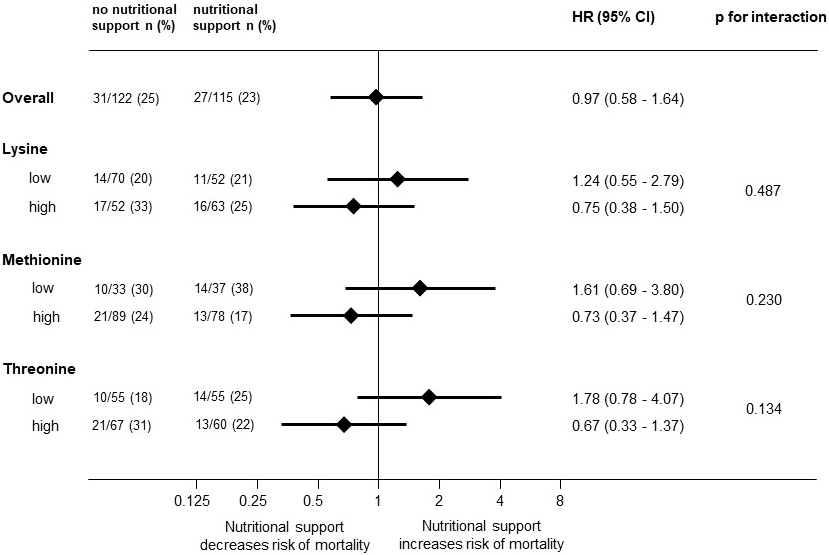
3.5. Association of Lysine, Methionine, and Threonine on Responding to Nutritional Support
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Stalder, L.; Kaegi-Braun, N.; Gressies, C.; Gregoriano, C.; Tribolet, P.; Lobo, D.N.; Gomes, F.; Hoess, C.; Pavlicek, V.; Bilz, S.; et al. Prospective validation of five malnutrition screening and assessment instruments among medical inpatients: Secondary analysis of a randomized clinical trial. Clin. Nutr. 2022, 41, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Bosaeus, I. Malnutrition in Adults. N. Engl. J. Med. 2024, 391, 155–165. [Google Scholar] [CrossRef]
- Dent, E.; Wright, O.R.; Woo, J.; Hoogendijk, E.O. Malnutrition in older adults. Lancet 2023, 401, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Karagounis, L.G.; Ng, T.P.; Carre, C.; Narang, V.; Wong, G.; Tan, C.T.Y.; Nyunt, M.S.Z.; Gao, Q.; Abel, B.; et al. Systemic and Metabolic Signature of Sarcopenia in Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 309–317. [Google Scholar] [CrossRef]
- Lv, Z.; Shi, W.; Zhang, Q. Role of Essential Amino Acids in Age-Induced Bone Loss. Int. J. Mol. Sci. 2022, 23, 11281. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Seres, D.; Lobo, D.N.; Gomes, F.; Kaegi-Braun, N.; Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet 2021, 398, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Wunderle, C.; Gomes, F.; Schuetz, P.; Stumpf, F.; Austin, P.; Ballesteros-Pomar, M.D.; Cederholm, T.; Fletcher, J.; Laviano, A.; Norman, K.; et al. ESPEN guideline on nutritional support for polymorbid medical inpatients. Clin. Nutr. 2023, 42, 1545–1568. [Google Scholar] [CrossRef]
- Kaegi-Braun, N.; Mueller, M.; Schuetz, P.; Mueller, B.; Kutz, A. Evaluation of Nutritional Support and In-Hospital Mortality in Patients With Malnutrition. JAMA Netw. Open 2021, 4, e2033433. [Google Scholar] [CrossRef]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R.; NOURISH Study Group. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef]
- Merker, M.; Felder, M.; Gueissaz, L.; Bolliger, R.; Tribolet, P.; Kagi-Braun, N.; Gomes, F.; Hoess, C.; Pavlicek, V.; Bilz, S.; et al. Association of Baseline Inflammation With Effectiveness of Nutritional Support Among Patients With Disease-Related Malnutrition: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200663. [Google Scholar] [CrossRef]
- Wunderle, C.; von Arx, D.; Mueller, S.C.; Bernasconi, L.; Neyer, P.; Tribolet, P.; Stanga, Z.; Mueller, B.; Schuetz, P. Association of Glutamine and Glutamate Metabolism with Mortality among Patients at Nutritional Risk-A Secondary Analysis of the Randomized Clinical Trial EFFORT. Nutrients 2024, 16, 222. [Google Scholar] [CrossRef]
- Stumpf, F.; Wunderle, C.; Ritz, J.; Bernasconi, L.; Neyer, P.; Tribolet, P.; Stanga, Z.; Mueller, B.; Bischoff, S.C.; Schuetz, P. Prognostic implications of the arginine metabolism in patients at nutritional risk: A secondary analysis of the randomized EFFORT trial. Clin. Nutr. 2024, 43, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Kaegi-Braun, N.; Boesiger, F.; Tribolet, P.; Gomes, F.; Kutz, A.; Hoess, C.; Pavlicek, V.; Bilz, S.; Sigrist, S.; Brandle, M.; et al. Validation of modified GLIM criteria to predict adverse clinical outcome and response to nutritional treatment: A secondary analysis of a randomized clinical trial. Clin. Nutr. 2022, 41, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Ritz, J.; Wunderle, C.; Stumpf, F.; Laager, R.; Tribolet, P.; Neyer, P.; Bernasconi, L.; Stanga, Z.; Mueller, B.; Schuetz, P. Association of tryptophan pathway metabolites with mortality and effectiveness of nutritional support among patients at nutritional risk: Secondary analysis of a randomized clinical trial. Front. Nutr. 2024, 11, 1335242. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Adegoke, O.A.; Abdullahi, A.; Tavajohi-Fini, P. mTORC1 and the regulation of skeletal muscle anabolism and mass. Appl. Physiol. Nutr. Metab. 2012, 37, 395–406. [Google Scholar] [CrossRef]
- Bodine, S.C. The role of mTORC1 in the regulation of skeletal muscle mass. Fac. Rev. 2022, 11, 32. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef]
- Wang, X.; Proud, C.G. The mTOR pathway in the control of protein synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, J.; Song, T.; Peng, J.; Wei, H. Methionine Regulates mTORC1 via the T1R1/T1R3-PLCbeta-Ca2+-ERK1/2 Signal Transduction Process in C2C12 Cells. Int. J. Mol. Sci. 2016, 17, 1684. [Google Scholar] [CrossRef] [PubMed]
- Cavuoto, P.; Fenech, M.F. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat. Rev. 2012, 38, 726–736. [Google Scholar] [CrossRef]
- Matthews, D.E. Review of Lysine Metabolism with a Focus on Humans. J. Nutr. 2020, 150 (Suppl. S1), 2548S–2555S. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.J.; McGeachie, M.; Baron, R.M.; Gazourian, L.; Haspel, J.A.; Nakahira, K.; Fredenburgh, L.E.; Hunninghake, G.M.; Raby, B.A.; Matthay, M.A.; et al. Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS ONE 2014, 9, e87538. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc, E.W.G. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Bendavid, I.; Lobo, D.N.; Barazzoni, R.; Cederholm, T.; Coeffier, M.; de van der Schueren, M.; Fontaine, E.; Hiesmayr, M.; Laviano, A.; Pichard, C.; et al. The centenary of the Harris-Benedict equations: How to assess energy requirements best? Recommendations from the ESPEN expert group. Clin. Nutr. 2021, 40, 690–701. [Google Scholar] [CrossRef]
- Weinberger, K.M. Metabolomics in diagnosing metabolic diseases. Ther. Umsch. 2008, 65, 487–491. [Google Scholar] [CrossRef]
- Illig, T.; Gieger, C.; Zhai, G.; Römisch-Margl, W.; Wang-Sattler, R.; Prehn, C.; Altmaier, E.; Kastenmüller, G.; Kato, B.S.; Mewes, H.W.; et al. A genome-wide perspective of genetic variation in human metabolism. Nat. Genet. 2010, 42, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Yet, I.; Menni, C.; Shin, S.Y.; Mangino, M.; Soranzo, N.; Adamski, J.; Suhre, K.; Spector, T.D.; Kastenmüller, G.; Bell, J.T. Genetic Influences on Metabolite Levels: A Comparison across Metabolomic Platforms. PLoS ONE 2016, 11, e0153672. [Google Scholar] [CrossRef]
- Trabado, S.; Al-Salameh, A.; Croixmarie, V.; Masson, P.; Corruble, E.; Fève, B.; Colle, R.; Ripoll, L.; Walther, B.; Boursier-Neyret, C.; et al. The human plasma-metabolome: Reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS ONE 2017, 12, e0173615. [Google Scholar] [CrossRef]
- Lau, C.E.; Siskos, A.P.; Maitre, L.; Robinson, O.; Athersuch, T.J.; Want, E.J.; Urquiza, J.; Casas, M.; Vafeiadi, M.; Roumeliotaki, T.; et al. Determinants of the urinary and serum metabolome in children from six European populations. BMC Med. 2018, 16, 202. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Abdel Rahman, A.M. Metabolomics toward personalized medicine. Mass. Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Baumgartner, A.; Olpe, T.; Griot, S.; Mentil, N.; Staub, N.; Burn, F.; Schindera, S.; Kaegi-Braun, N.; Tribolet, P.; Hoess, C.; et al. Association of CT-based diagnosis of sarcopenia with prognosis and treatment response in patients at risk of malnutrition—A secondary analysis of the Effect of early nutritional support on Frailty, Functional Outcomes, and Recovery of malnourished medical inpatients Trial (EFFORT) trial. Clin. Nutr. 2023, 42, 199–207. [Google Scholar] [PubMed]
- Mueller, L.; Mentil, N.; Staub, N.; Griot, S.; Olpe, T.; Burn, F.; Schindera, S.; Mueller, B.; Schuetz, P.; Stanga, Z.; et al. Association of Thoracic Skeletal Muscle Index with Clinical Outcome and Response to Nutritional Interventions in Patients at Risk of Malnutrition-Secondary Analysis of a Randomized Trial. Nutrients 2023, 15, 817. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Liu, R.; Qiu, Z.; Zhang, L.; Ma, W.; Zi, L.; Wang, K.; Kuang, T.; Zhao, K.; Wang, W. High intramuscular adipose tissue content associated with prognosis and postoperative complications of cancers. J. Cachexia Sarcopenia Muscle 2023, 14, 2509–2519. [Google Scholar] [CrossRef]
- Liu, X. Classification accuracy and cut point selection. Stat. Med. 2012, 31, 2676–2686. [Google Scholar] [CrossRef]
- Ciolino, J.D.; Martin, R.H.; Zhao, W.; Jauch, E.C.; Hill, M.D.; Palesch, Y.Y. Covariate imbalance and adjustment for logistic regression analysis of clinical trial data. J. Biopharm. Stat. 2013, 23, 1383–1402. [Google Scholar] [CrossRef]
- Su, L.; Li, H.; Xie, A.; Liu, D.; Rao, W.; Lan, L.; Li, X.; Li, F.; Xiao, K.; Wang, H.; et al. Dynamic changes in amino acid concentration profiles in patients with sepsis. PLoS ONE 2015, 10 (Suppl. S6), e0121933. [Google Scholar] [CrossRef]
- Ji, M.; Xu, Q.; Li, X. Dietary methionine restriction in cancer development and antitumor immunity. Trends Endocrinol. Metab. 2024, 35, 400–412. [Google Scholar] [CrossRef]
- Lee, B.C.; Kaya, A.; Gladyshev, V.N. Methionine restriction and life-span control. Ann. N. Y. Acad. Sci. 2016, 1363, 116–124. [Google Scholar] [CrossRef]
- Yuan, S.; Mason, A.M.; Carter, P.; Burgess, S.; Larsson, S.C. Homocysteine, B vitamins, and cardiovascular disease: A Mendelian randomization study. BMC Med. 2021, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J. Toxicity of methionine in humans. J. Nutr. 2006, 136, 1722s–1725s. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Deng, J.P.; Zhang, L.; Zhang, W.W.; Sun, J.Y.; Chi, F.; Zhang, J.; Wu, S.G.; He, Z.Y. Prognostic significance of the skeletal muscle index and systemic inflammatory index in patients with lymph node-positive breast cancer after radical mastectomy. BMC Cancer 2022, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, H.; Gong, Y.; Kuang, J.; Yan, L.; Ruan, G.; Gao, F.; Gan, J. The value of L3 skeletal muscle index in evaluating preoperative nutritional risk and long-term prognosis in colorectal cancer patients. Sci. Rep. 2020, 10, 8153. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino acid-dependent control of mTORC1 signaling: A variety of regulatory modes. J. Biomed. Sci. 2020, 27, 87. [Google Scholar] [CrossRef] [PubMed]
- Growdon, J.H.; Nader, T.M.; Schoenfeld, J.; Wurtman, R.J. L-threonine in the treatment of spasticity. Clin. Neuropharmacol. 1991, 14, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Patterson, V. A double-blind study of L-threonine in patients with spinal spasticity. Acta Neurol. Scand. 1993, 88, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Nerstedt, A.; Cansby, E.; Andersson, C.X.; Laakso, M.; Stancakova, A.; Bluher, M.; Smith, U.; Mahlapuu, M. Serine/threonine protein kinase 25 (STK25): A novel negative regulator of lipid and glucose metabolism in rodent and human skeletal muscle. Diabetologia 2012, 55, 1797–1807. [Google Scholar] [CrossRef]
- Hoppe, P.E.; Chau, J.; Flanagan, K.A.; Reedy, A.R.; Schriefer, L.A. Caenorhabditis elegans unc-82 encodes a serine/threonine kinase important for myosin filament organization in muscle during growth. Genetics 2010, 184, 79–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papadopoulou, S.K.; Voulgaridou, G.; Kondyli, F.S.; Drakaki, M.; Sianidou, K.; Andrianopoulou, R.; Rodopaios, N.; Pritsa, A. Nutritional and Nutrition-Related Biomarkers as Prognostic Factors of Sarcopenia, and Their Role in Disease Progression. Diseases 2022, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Van den Berghe, G. Nutrition in the acute phase of critical illness. N. Engl. J. Med. 2014, 370, 2450–2451. [Google Scholar] [CrossRef] [PubMed]
- Vanderheyden, S.; Casaer, M.P.; Kesteloot, K.; Simoens, S.; De Rijdt, T.; Peers, G.; Wouters, P.J.; Coenegrachts, J.; Grieten, T.; Polders, K.; et al. Early versus late parenteral nutrition in ICU patients: Cost analysis of the EPaNIC trial. Crit. Care 2012, 16, R96. [Google Scholar] [CrossRef] [PubMed]
- Allingstrup, M.J.; Esmailzadeh, N.; Wilkens Knudsen, A.; Espersen, K.; Hartvig Jensen, T.; Wiis, J.; Perner, A.; Kondrup, J. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin. Nutr. 2012, 31, 462–468. [Google Scholar] [CrossRef] [PubMed]
| Overall | No 30-Day Mortality | 30-Day Mortality | p-Value | |
|---|---|---|---|---|
| n = 237 | n = 179 | n = 58 | ||
| Sociodemographic | ||||
| Male sex, n (%) | 136 (57.4%) | 94 (52.5%) | 42 (72.4%) | 0.008 |
| Age, years, mean (SD) | 73.4 (13.6) | 72.5 (13.9) | 76.1 (12.4) | 0.083 |
| Nutritional assessment, mean (SD) | ||||
| BMI, kg/m2 | 24.3 (5.0) | 24.4 (5.2) | 24.0 (4.0) | 0.60 |
| Weight, kg | 68.7 (14.9) | 68.7 (15.4) | 68.8 (13.7) | 0.96 |
| Height, cm | 168.1 (8.6) | 167.8 (8.4) | 169.1 (9.2) | 0.33 |
| Admission diagnosis, n (%) | ||||
| Infection | 64 (27.0%) | 50 (27.9%) | 14 (24.1%) | 0.57 |
| Cancer | 75 (31.6%) | 50 (27.9%) | 25 (43.1%) | 0.031 |
| Cardiovascular disease | 24 (10.1%) | 17 (9.5%) | 7 (12.1%) | 0.57 |
| Frailty | 13 (5.5%) | 10 (5.6%) | 3 (5.2%) | 0.90 |
| Lung disease | 11 (4.6%) | 6 (3.4%) | 5 (8.6%) | 0.097 |
| Gastrointestinal disease | 13 (5.5%) | 13 (7.3%) | 0 (0.0%) | 0.035 |
| Neurological disease | 4 (1.7%) | 4 (2.2%) | 0 (0.0%) | 0.25 |
| Renal disease | 15 (6.3%) | 14 (7.8%) | 1 (1.7%) | 0.097 |
| Metabolic disease | 6 (2.5%) | 5 (2.8%) | 1 (1.7%) | 0.65 |
| Other | 3 (1.3%) | 2 (1.1%) | 1 (1.7%) | 0.72 |
| Comorbidities, n (%) | ||||
| Hypertension | 138 (58.2%) | 107 (59.8%) | 31 (53.4%) | 0.40 |
| Malignant disease | 113 (47.7%) | 78 (43.6%) | 35 (60.3%) | 0.026 |
| Chronic kidney disease | 81 (34.2%) | 63 (35.2%) | 18 (31.0%) | 0.56 |
| Coronary heart disease | 54 (22.8%) | 41 (22.9%) | 13 (22.4%) | 0.94 |
| Diabetes | 43 (18.1%) | 33 (18.4%) | 10 (17.2%) | 0.84 |
| Congestive heart failure | 45 (19.0%) | 30 (16.8%) | 15 (25.9%) | 0.12 |
| Chronic obstructive pulmonary disease | 28 (11.8%) | 17 (9.5%) | 11 (19.0%) | 0.052 |
| Peripheral arterial disease | 26 (11.0%) | 19 (10.6%) | 7 (12.1%) | 0.76 |
| Cerebrovascular disease | 27 (11.4%) | 21 (11.7%) | 6 (10.3%) | 0.77 |
| Dementia | 11 (4.6%) | 7 (3.9%) | 4 (6.9%) | 0.35 |
| Amino acids | ||||
| Low lysine, n (%) | 122 (51.5%) | 97 (54.2%) | 25 (43.1%) | 0.14 |
| Low methionine, n (%) | 70 (29.5%) | 46 (25.7%) | 24 (41.4%) | 0.023 |
| Low threonine, n (%) | 110 (46.4%) | 86 (48.0%) | 24 (41.4%) | 0.38 |
| Lysine | Methionine | Threonine | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Coef. | p-Value | 95%-CI | Mean | Coef. | p-Value | 95%-CI | Mean | Coef. | p-Value | 95%-CI | |
| NRS 2002 score | ||||||||||||
| 3 | 211.1 | reference | 25.3 | reference | 103.0 | reference | ||||||
| 4 | 199.1 | −12.0 | 0.23 | −31.4 to 7.4 | 22.5 | −2.8 | 0.08 | −6.0 to 0.4 | 96.8 | −6.2 | 0.34 | −19.0 to 6.6 |
| ≥5 | 186.7 | −24.4 | 0.01 | −43.0 to −5.8 | 22.6 | −2.7 | 0.09 | −5.7 to 0.4 | 96.4 | −6.6 | 0.29 | −18.9 to 5.7 |
| Food intake | ||||||||||||
| >75% | 215.9 | reference | 25.8 | reference | 104.2 | reference | ||||||
| 50–75% | 185.7 | −30.2 | 0.03 | −58.0 to −2.4 | 22.9 | −2.9 | 0.22 | −7.4 to 1.7 | 94.8 | −9.5 | 0.31 | −27.8 to 8.9 |
| 25–50% | 202.0 | −13.9 | 0.30 | −40.4 to 12.6 | 22.8 | −2.9 | 0.19 | −7.2 to 1.4 | 97.2 | −7.1 | 0.43 | −24.5 to 10.4 |
| 0–25% | 193.7 | −22.2 | 0.14 | −51.6 to 7.1 | 23.4 | −2.3 | 0.34 | −7.1 to 2.5 | 102.7 | −1.5 | 0.88 | −20.8 to 17.8 |
| BMI | ||||||||||||
| <18.5 | 178.7 | reference | 23.2 | reference | 91.4 | reference | ||||||
| 18.5–25 | 200.1 | 21.4 | 0.11 | −4.9 to 47.7 | 22.9 | −0.3 | 0.88 | −4.7 to 4.0 | 98.2 | 6.8 | 0.44 | −10.4 to 24.1 |
| 25–30 | 195.2 | 16.5 | 0.25 | −11.8 to 44.9 | 22.9 | −0.3 | 0.90 | −4.9 to 4.4 | 95.2 | 3.8 | 0.69 | −14.8 to 22.5 |
| >30 | 205.0 | 26.3 | 0.09 | −3.7 to 56.2 | 24.6 | 1.4 | 0.58 | −3.5 to 6.3 | 106.9 | 15.5 | 0.12 | −4.2 to 35.2 |
| Weight loss | ||||||||||||
| 1 (none) | 201.0 | reference | 23.1 | reference | 97.6 | reference | ||||||
| 2 (>5% in 3 mts) | 197.4 | −3.6 | 0.74 | −24.4 to 17.3 | 23.8 | 0.7 | 0.70 | −2.7 to 4.1 | 100.6 | 3.0 | 0.67 | −10.7 to 16.6 |
| 3 (>5% in 2 mts) | 201.7 | 0.7 | 0.95 | −20.9 to 22.3 | 24.9 | 1.8 | 0.31 | −1.7 to 5.3 | 104.5 | 6.9 | 0.34 | −7.3 to 21.0 |
| 4 (>5% in 1 mts) | 184.6 | −16.4 | 0.11 | −36.6 to 3.8 | 21.7 | −1.5 | 0.38 | −4.8 to 1.8 | 92.4 | −5.2 | 0.44 | −18.5 to 8.0 |
| Disease severity | ||||||||||||
| 0 | 182.0 | reference | 27.2 | reference | 132.0 | reference | ||||||
| 1 | 198.4 | 16.4 | 0.63 | −51.1 to 83.9 | 23.4 | −3.8 | 0.49 | −14.8 to 7.1 | 99.3 | −32.7 | 0.14 | −76.7 to 11.3 |
| 2 | 194.6 | 12.6 | 0.72 | −55.5 to 80.6 | 23.0 | −4.2 | 0.45 | −15.3 to 6.8 | 95.3 | −36.7 | 0.10 | −81.1 to 7.7 |
| 3 | 274.0 | 92.0 | 0.18 | −41.6 to 225.6 | 14.0 | −13.2 | 0.23 | −34.9 to 8.5 | 84.3 | −47.7 | 0.28 | −134.8 to 39.5 |
| All-Cause Mortality | Low Plasma Levels | High Plasma Levels | HR (95% CI) | p-Value | |
| 30-day mortality | |||||
| Lysine | 25/122 (20.5%) | 33/115 (28.7%) | 0.69 (0.40–1.18) | 0.17 | |
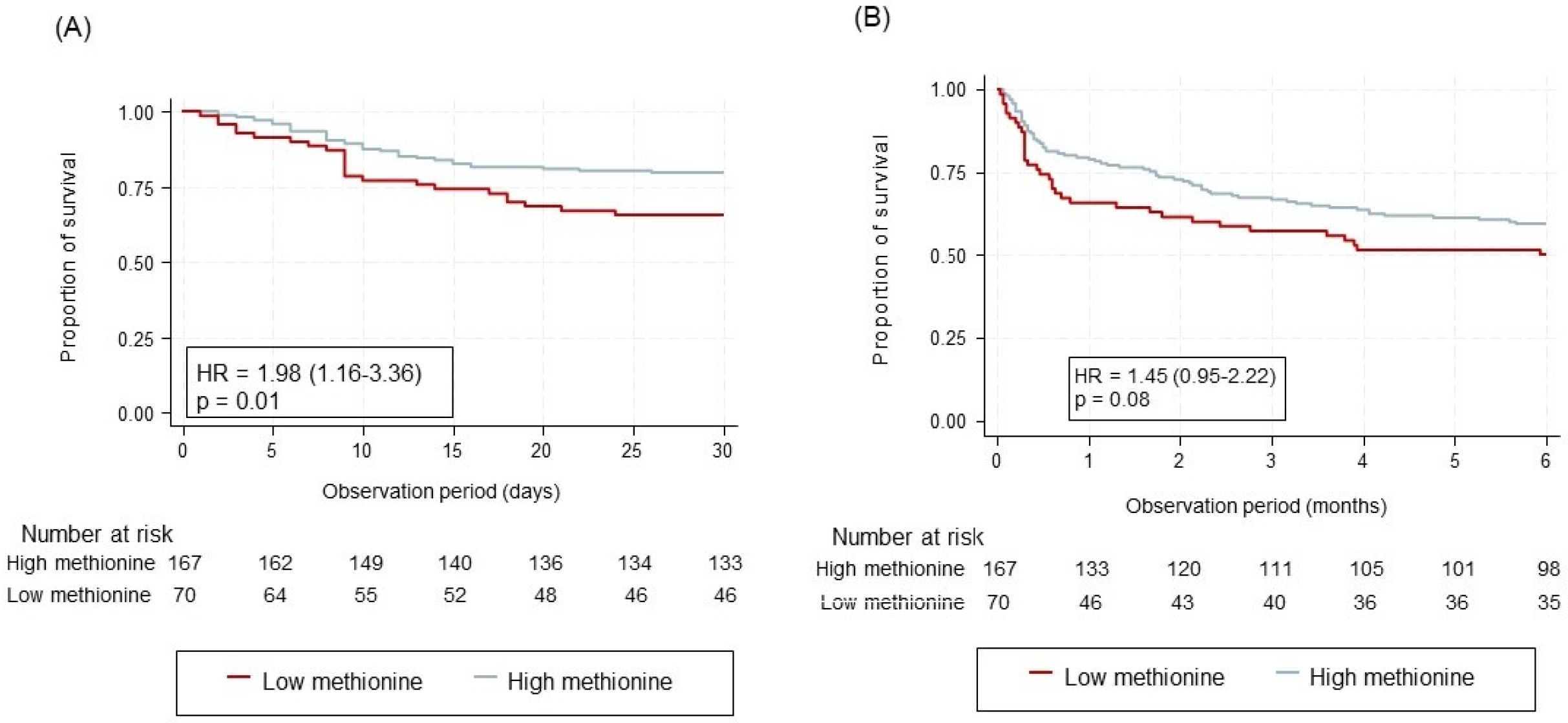
| Methionine | 24/70 (34.3%) | 34/167 (20.4%) | 1.98 (1.16–3.36) | 0.01 | |
| Threonine | 24/110 (21.8%) | 34/127 (26.8%) | 0.77 (0.45–1.30) | 0.33 | |
| 180-day mortality | |||||
| Lysine | 54/122 (44.3%) | 48/115 (41.7%) | 1.02 (0.68–1.53) | 0.93 | |
| Methionine | 35/70 (50.0%) | 67/167 (40.1%) | 1.45 (0.95–2.22) | 0.08 | |
| Threonine | 48/110 (43.6%) | 54/127 (42.5%) | 0.85 (0.57–1.27) | 0.43 | |
| Other Clinical Outcomes | Low Plasma Levels | High Plasma Levels | OR (95% CI) | p-Value | |
| Adverse event 30 days | |||||
| Lysine | 44/122 (36.0%) | 48/115 (41.7%) | 0.72 (0.41–1.27) | 0.26 | |
| Methionine | 36/70 (51.4%) | 56/167 (33.5%) | 2.21 (1.21–4.05) | 0.01 | |
| Threonine | 42/110 (38.2%) | 50/127 (39.4%) | 0.86(0.09–8.49) | 0.60 | |
| Barthel Decline > 10 | |||||
| Lysine | 31/122 (25.4%) | 34/115 (29.6%) | 0.77 (0.41–1.46) | 0.42 | |
| Methionine | 26/70 (37.1%) | 39/167 (23.6%) | 2.06 (1.06–4.01) | 0.03 | |
| Threonine | 29/110 (26.4%) | 36/127 (28.4%) | 0.79 (0.42–1.48) | 0.46 | |
| Falls 180 days | |||||
| Lysine | 14/121 (11.6%) | 9/115 (7.8%) | 1.47 (0.59–3.62) | 0.41 | |
| Methionine | 8/70 (11.4%) | 15/166 (9.0%) | 1.26 (0.50–3.20) | 0.62 | |
| Threonine | 15/109 (13.8%) | 8/127 (6.3%) | 2.46 (0.98–6.19) | 0.05 | |
| Muscle-Specific Outcomes | Low Plasma Levels | High Plasma Levels | OR (95% CI) | p-Value | |
| Sarcopenia | |||||
| Lysine | 17/72 (23.6%) | 22/81 (27.2%) | 0.37 (0.14–0.95) | 0.04 | |
| Methionine | 12/43 (27.9%) | 27/110 (24.5%) | 0.53 (0.19–1.47) | 0.22 | |
| Threonine | 23/78 (29.5%) | 16/75 (21.3%) | 0.96 (0.39–2.35) | 0.93 | |
| Low skeletal muscle index | |||||
| Lysine | 26/33 (78.8%) | 20/28 (71.4%) | 1.46 (0.40–5.27) | 0.57 | |
| Methionine | 15/18 (83.3%) | 31/43 (72.1%) | 2.05 (0.46–9.08) | 0.35 | |
| Threonine | 28/33 (84.8%) | 18/28 (64.3%) | 3.14 (0.89–11.02) | 0.07 | |
| Low muscle radiodensity | |||||
| Lysine | 27/33 (81.8%) | 15/28 (53.6%) | 2.48 (0.63–9.85) | 0.20 | |
| Methionine | 15/18 (83.3%) | 27/43 (62.8%) | 2.88 (0.48–17.29) | 0.25 | |
| Threonine | 26/33 (78.8%) | 16/28 (57.1%) | 2.93 (0.72–12.00) | 0.13 | |
| High intramuscular adipose tissue | |||||
| Lysine | 11/33 (33.3%) | 4/28 (14.3%) | 2.37 (0.14–10.06) | 0.24 | |
| Methionine | 4/18 (22.2%) | 11/43 (25.5%) | 0.54 (0.12–2.34) | 0.41 | |
| Threonine | 8/33 (24.2%) | 7/28 (25.0%) | 0.69 (0.19–2.54) | 0.58 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunderle, C.; Haller, L.; Laager, R.; Bernasconi, L.; Neyer, P.; Stumpf, F.; Tribolet, P.; Stanga, Z.; Mueller, B.; Schuetz, P. The Association of the Essential Amino Acids Lysine, Methionine, and Threonine with Clinical Outcomes in Patients at Nutritional Risk: Secondary Analysis of a Randomized Clinical Trial. Nutrients 2024, 16, 2608. https://doi.org/10.3390/nu16162608
Wunderle C, Haller L, Laager R, Bernasconi L, Neyer P, Stumpf F, Tribolet P, Stanga Z, Mueller B, Schuetz P. The Association of the Essential Amino Acids Lysine, Methionine, and Threonine with Clinical Outcomes in Patients at Nutritional Risk: Secondary Analysis of a Randomized Clinical Trial. Nutrients. 2024; 16(16):2608. https://doi.org/10.3390/nu16162608
Chicago/Turabian StyleWunderle, Carla, Luana Haller, Rahel Laager, Luca Bernasconi, Peter Neyer, Franziska Stumpf, Pascal Tribolet, Zeno Stanga, Beat Mueller, and Philipp Schuetz. 2024. "The Association of the Essential Amino Acids Lysine, Methionine, and Threonine with Clinical Outcomes in Patients at Nutritional Risk: Secondary Analysis of a Randomized Clinical Trial" Nutrients 16, no. 16: 2608. https://doi.org/10.3390/nu16162608
APA StyleWunderle, C., Haller, L., Laager, R., Bernasconi, L., Neyer, P., Stumpf, F., Tribolet, P., Stanga, Z., Mueller, B., & Schuetz, P. (2024). The Association of the Essential Amino Acids Lysine, Methionine, and Threonine with Clinical Outcomes in Patients at Nutritional Risk: Secondary Analysis of a Randomized Clinical Trial. Nutrients, 16(16), 2608. https://doi.org/10.3390/nu16162608






