Objectively Measured Sleep Duration and Health-Related Quality of Life in Older Adults with Metabolic Syndrome: A One-Year Longitudinal Analysis of the PREDIMED-Plus Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Principal Predictor Variable: Objectively Assessed Sleep by Accelerometry
2.3. Outcome Variable: Health-Related Quality of Life (HRQoL)
2.4. Covariates
2.5. Statistical Analysis
2.5.1. Cross-Sectional Analysis
2.5.2. Longitudinal Analysis
3. Results
3.1. Baseline Descriptive Characteristics
3.2. Cross-Sectional Analysis
3.2.1. Night-Time Sleep Duration
3.2.2. Daytime Sleep Duration
3.3. Longitudinal Analysis
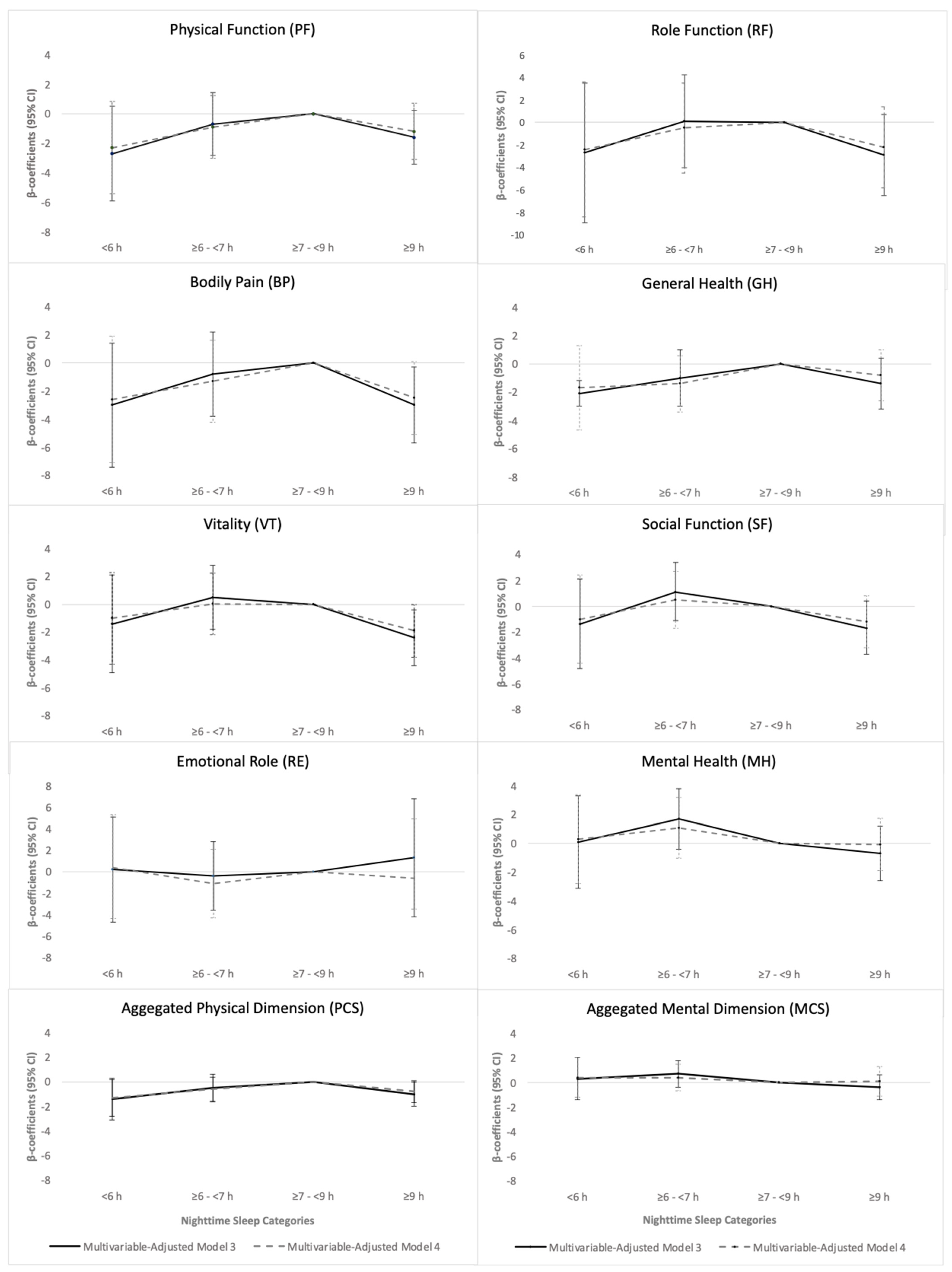
3.3.1. Night-Time Sleep Duration
3.3.2. Daytime Sleep Duration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakour, C.; Nieto, F.J.; Petersen, D.J. Foundations of Sleep Health, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2022; Available online: https://www.thensf.org/foundations-of-sleep-health/ (accessed on 13 June 2023).
- Böhmer, M.N.; Hamers, P.C.M.; Bindels, P.J.E.; Oppewal, A.; Someren, E.J.W.; van Festen, D.A.M. Are we still in the dark? A systematic review on personal daily light exposure, sleep-wake rhythm, and mood in healthy adults from the general population. Sleep Health 2021, 7, 610–630. [Google Scholar] [CrossRef] [PubMed]
- Samson, D.R.; Crittenden, A.N.; Mabulla, I.A.; Mabulla, A.Z.P.; Nunn, C.L. Chronotype variation drives night-time sentinel-like behaviour in hunter–gatherers. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170967. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.M.; Dijk, D.J.; Drake, R.J.; Bee, P.E. Adherence and acceptability of light therapies to improve sleep in intrinsic circadian rhythm sleep disorders and neuropsychiatric illness: A systematic review. Sleep Health 2020, 6, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Ray, M.A.; Hébert, J.R.; Youngstedt, S.D.; Zhang, H.; Steck, S.E.; Bogan, R.K.; Burch, J.B. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000–2010. Sleep 2016, 39, 1399–1410. [Google Scholar] [CrossRef]
- Ferrie, J.E.; Kumari, M.; Salo, P.; Singh-Manoux, A.; Kivimaki, M. Sleep epidemiology—A rapidly growing field. Int. J. Epidemiol. 2011, 40, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, C.; Glozier, N.; Marshall, N.S. Recent Evidence on Worldwide Trends on Sleep Duration. Curr. Sleep Med. Rep. 2015, 1, 195–204. [Google Scholar] [CrossRef]
- Kerkhof, G.A. Epidemiology of sleep and sleep disorders in The Netherlands. Sleep Med. 2017, 30, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Madrid-Valero, J.J.; Martínez-Selva, J.M.; Ribeiro do Couto, B.; Sánchez-Romera, J.F.; Ordoñana, J.R. Age and gender effects on the prevalence of poor sleep quality in the adult population. Gac. Sanit. 2017, 31, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Vieira, P.; Gomes, A.A.; Roth, T.; de Azevedo, M.H.P.; Marques, D.R. Sleep difficulties and use of prescription and non-prescription sleep aids in Portuguese higher education students. Sleep Epidemiol. 2021, 1, 100012. [Google Scholar] [CrossRef]
- Andréu, M.M.; de Larrinaga, A.R.; Pérez, J.A.M.; Martínez, M.M.; Cuesta, F.J.P.; Guerra, A.J.A.; Santo-Tomás, O.R.; Luque, M.J.J.; Isern, F.J.S.; Sanz, T.C.; et al. Sueño saludable: Evidencias y guías de actuación. Documento oficial de la Sociedad Española de Sueño. Rev. Neurol. 2016, 63, 1. [Google Scholar] [CrossRef]
- Fan, M.; Sun, D.; Zhou, T.; Heianza, Y.; Lv, J.; Li, L.; Qi, L. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385,292 UK biobank participants. Eur. Heart J. 2020, 41, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Rosique-Esteban, N.; Papandreou, C.; Romaguera, D.; Warnberg, J.; Corella, D.; Martínez-González, M.; Díaz-López, A.; Estruch, R.; Vioque, J.; Arós, F.; et al. Cross-sectional associations of objectively-measured sleep characteristics with obesity and type 2 diabetes in the PREDIMED-Plus trial. Sleep 2018, 41, zsy190. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.T.; Thiel, D.; Al Sayah, F.; Mundt, C.; Qiu, W.; Buman, M.P.; Vallance, J.K.; Johnson, J.A. Objectively measured sleep and health-related quality of life in older adults with type 2 diabetes: A cross-sectional study from the Alberta’s Caring for Diabetes Study. Sleep Health 2017, 3, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Smiley, A.; King, D.; Bidulescu, A. The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014. Nutrients 2019, 11, 2582. [Google Scholar] [CrossRef] [PubMed]
- Stickley, A.; Leinsalu, M.; DeVylder, J.E.; Inoue, Y.; Koyanagi, A. Sleep problems and depression among 237 023 community-dwelling adults in 46 low- and middle-income countries. Sci. Rep. 2019, 9, 12011. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zhang, H.; Zhang, D. Sleep Duration and Depression Among Adults: A Meta-Analysis of Prospective Studies. Depress. Anxiety 2015, 32, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Hale, L. An introduction and invitation to join our sleep health community. Sleep Health 2015, 1, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wheaton, A.G.; Croft, J.B.; Xu, F.; Cunningham, T.J.; Greenlund, K.J. Relationship between sleep duration and self-reported health-related quality of life among US adults with or without major chronic diseases, 2014. Sleep Health 2018, 4, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.E.; Stone, K.L.; Ancoli-Israel, S.; Blackwell, T.; Ewing, S.K.; Boudreau, R.; Cauley, J.A.; Hall, M.; Matthews, K.A.; Newman, A.B. Poor Sleep is Associated with Poorer Physical Performance and Greater Functional Limitations in Older Women. Sleep 2007, 30, 1317–1326. [Google Scholar] [CrossRef]
- Faubel, R.; Lopez-Garcia, E.; Guallar-Castillón, P.; Balboa-Castillo, T.; Gutiérrez-Fisac, J.L.; Banegas, J.R.; Rodríguez-Artalejo, F. Sleep Duration and Health-Related Quality of Life among Older Adults: A Population-Based Cohort in Spain. Sleep 2009, 32, 1059–1068. [Google Scholar]
- Xiao, Q.; Chaput, J.-P.; Olds, T.; Fogelholm, M.; Hu, G.; Lambert, E.V.; Maher, C.; Maia, J.; Onywera, V.; Sarmiento, O.L.; et al. Sleep characteristics and health-related quality of life in 9- to 11-year-old children from 12 countries. Sleep Health 2020, 6, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kondracki, A.J.; Sun, N.; Gautam, P.; Kalan, M.E.; Jebai, R.; Gbadamosi, S.O.; Sun, W. Nighttime sleep duration, daytime napping, and metabolic syndrome: Findings from the China Health and Retirement Longitudinal Study. Sleep Breath 2022, 26, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Díaz-López, A.; Babio, N.; Martínez-González, M.A.; Bulló, M.; Corella, D.; Fitó, M.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Long Daytime Napping Is Associated with Increased Adiposity and Type 2 Diabetes in an Elderly Population with Metabolic Syndrome. J. Clin. Med. 2019, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, F.; Danini, B.; Bagheri, R.; Fantini, M.L.; Pereira, B.; Moustafa, F.; Trousselard, M.; Navel, V. Effects of a Short Daytime Nap on the Cognitive Performance: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 10212. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; McPhillips, M.V.; Li, J. 0720 Daytime Napping and Cognition in Older Adults. Sleep 2018, 41 (Suppl. S1), A267–A268. [Google Scholar] [CrossRef]
- Faraut, B.; Andrillon, T.; Vecchierini, M.F.; Leger, D. Napping: A public health issue. From epidemiological to laboratory studies. Sleep Med. Rev. 2017, 35, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Buil-Cosiales, P.; Corella, D.; Bulló, M.; Fitó, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; López-Miranda, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2019, 48, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Migueles, J.H.; Rowlands, A.V.; Huber, F.; Sabia, S.; van Hees, V.T. GGIR: A Research Community–Driven Open Source R Package for Generating Physical Activity and Sleep Outcomes From Multi-Day Raw Accelerometer Data. J. Meas. Phys. Behav. 2019, 2, 188–196. [Google Scholar] [CrossRef]
- van Hees, V.T.; Sabia, S.; Jones, S.E.; Wood, A.R.; Anderson, K.N.; Kivimäki, M.; Frayling, T.M.; Pack, A.I.; Bucan, M.; Trenell, M.I.; et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci. Rep. 2018, 8, 12975. [Google Scholar] [CrossRef]
- Plekhanova, T.; Rowlands, A.V.; Yates, T.; Hall, A.; Brady, E.M.; Davies, M.; Khunti, K.; Edwardson, C.L. Equivalency of Sleep Estimates: Comparison of Three Research-Grade Accelerometers. J. Meas. Phys. Behav. 2020, 3, 294–303. [Google Scholar] [CrossRef]
- Alonso, J.; Regidor, E.; Barrio, G.; Prieto, L.; Rodríguez, C.; De La Fuente, L. Population reference values of the Spanish version of the Health Questionnaire SF-36. Med. Clin. 1998, 111, 410–416. [Google Scholar]
- Vilagut, G.; Ferrer, M.; Rajmil, L.; Rebollo, P.; Permanyer-Miralda, G.; Quintana, J.M.; Santed, R.; Valderas, J.M.; Ribera, A.; Domingo-Salvany, A.; et al. The Spanish version of the Short Form 36 Health Survey: A decade of experience and new developments. Gac. Sanit. 2005, 19, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J. Manual de Puntuación de la Versión Española del Cuestionario SF-36. Published Online 2000. Available online: http://www.imim.es (accessed on 1 May 2024).
- López-García, E.; Banegas, J.R.; Graciani Pérez-Regadera, A.; Gutiérrez-Fisac, J.L.; Alonso, J.; Rodríguez-Artalejo, F. Population-based reference values for the Spanish version of the SF-36 Health Survey in the elderly. Med. Clin. 2003, 120, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.J.; Munro, J.F.; Brazier, J.E. Using the SF-36 with older adults: A cross-sectional community-based survey. Age Ageing 2001, 30, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Balboa-Castillo, T.; León-Muñoz, L.M.; Graciani, A.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Longitudinal association of physical activity and sedentary behavior during leisure time with health-related quality of life in community-dwelling older adults. Health Qual. Life Outcomes 2011, 9, 47. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.; Hansen, B.H.; van Hees, V.T.; Ekelund, U. Evaluation of raw acceleration sedentary thresholds in children and adults. Scand. J. Med. Sci. Sports 2017, 27, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud. Actividad Física. Available online: https://www.who.int/es/news-room/fact-sheets/detail/physical-activity (accessed on 29 November 2021).
- Sleep Foundation. How Much Sleep Do We Really Need? Sleep Foundation. 9 March 2021. Available online: https://www.sleepfoundation.org/how-sleep-works/how-much-sleep-do-we-really-need (accessed on 13 December 2021).
- Ghafouri, M.; Teymourzadeh, A.; Nakhostin-Ansari, A.; Sepanlou, S.G.; Dalvand, S.; Moradpour, F.; Bavarsad, A.H.; Boogar, S.S.; Dehghan, M.; Ostadrahimi, A.; et al. Prevalence and predictors of low back pain among the Iranian population: Results from the Persian cohort study. Ann. Med. Surg. 2022, 74, 103243. [Google Scholar] [CrossRef]
- Park, H.M.; Kwon, Y.J.; Kim, H.S.; Lee, Y.J. Relationship between Sleep Duration and Osteoarthritis in Middle-Aged and Older Women: A Nationwide Population-Based Study. J. Clin. Med. 2019, 8, 356. [Google Scholar] [CrossRef]
- Chen, X.; Gelaye, B.; Williams, M.A. Sleep characteristics and health-related quality of life among a national sample of American young adults: Assessment of possible health disparities. Qual. Life Res. 2014, 23, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Gribble, A.K.; Sayón-Orea, C.; Bes-Rastrollo, M.; Kales, S.N.; Shirahama, R.; Martínez-González, M.; Fernandez-Montero, A. Risk of Developing Metabolic Syndrome Is Affected by Length of Daily Siesta: Results from a Prospective Cohort Study. Nutrients 2021, 13, 4182. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Bulló, M.; Díaz-López, A.; Martínez-González, M.A.; Corella, D.; Castañer, O.; Vioque, J.; Romaguera, D.; Martínez, A.J.; Pérez-Farinós, N.; et al. High sleep variability predicts a blunted weight loss response and short sleep duration a reduced decrease in waist circumference in the PREDIMED-Plus Trial. Int. J. Obes. 2020, 44, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Afolalu, E.F.; Ramlee, F.; Tang, N.K.Y. Effects of sleep changes on pain-related health outcomes in the general population: A systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med. Rev. 2018, 39, 82–97. [Google Scholar] [CrossRef]
- Jung, K.I.; Song, C.H.; Ancoli-Israel, S.; Barrett-Connor, E. Gender differences in nighttime sleep and daytime napping as predictors of mortality in older adults: The Rancho Bernardo Study. Sleep Med. 2013, 14, 12–19. [Google Scholar] [CrossRef]


| Categories of Night-Time Sleep Duration (h/d) | p-Value | |||||
|---|---|---|---|---|---|---|
| Total | <6 | ≥6–<7 | ≥7–<9 | ≥9 | ||
| n = 2119 | n = 129 | n = 316 | n = 1247 | n = 427 | ||
| Sleep parameters | ||||||
| Night-time sleep duration, Min–max, h/d | 3.1–14.2 | 3.1–5.9 | 6.0–6.9 | 7.0–8.0 | 9.0–14.2 | <0.001 |
| Night-time sleep duration, mean (SD), h/d | 8.0 (1.3) | 5.2 (0.7) | 6.6 (0.3) | 8.0 (0.5) | 9.8 (0.7) | <0.001 |
| Napping duration, median (IQ), min/d | 61.2 (37.8–91.2) | 90 (69.0–139.8) | 68.7 (42.0–103.2) | 55.8 (33.0–81.6) | 64.8 (40.8–97.2) | <0.001 |
| Age, mean (SD), years | 65.0 (4.9) | 64.7 (5.3) | 64.0 (5.1) | 64.9 (4.9) | 66.3 (4.4) | <0.001 |
| Female, n (%) | 1005 (47.4) | 25 (19.4) | 106 (33.5) | 616 (33.5) | 258 (60.4) | <0.001 |
| Labor status, n (%) | ||||||
| Retired | 1219 (57.5) | 77 (59.7) | 162 (51.3) | 698 (56.0) | 282 (66.0) | <0.001 |
| Educational level, n (%) | ||||||
| ≤Primary education | 1062 (50.1) | 56 (43.4) | 129 (40.8) | 611 (49.0) | 66 (15.5) | <0.001 |
| University education | 463 (21.9) | 40 (31.0) | 95 (30.1) | 262 (21.0) | 266 (62.3) | |
| Smoking, n (%) | ||||||
| Never | 919 (43.4) | 36 (27.9) | 112 (35.4) | 555 (44.5) | 216 (50.6) | <0.001 |
| Caffeine drinks/day, median (IQ), mg/day | 21.4 (0–50) | 21.4 (0–125) | 7.1 (0–50) | 21.4 (0–50) | 3.3 (0–50) | <0.001 |
| Alcohol drinks/day, median (IQ), g/day | 5.1 (0.7–14.8) | 7.4 (1.5–28.4) | 7.3 (1.5–18.6) | 5.1 (0.7–14.7) | 2.9 (0.0–11.8) | <0.001 |
| Leisure time spent watching TV, mean (SD), h/day | ||||||
| Non-labor days | 3.9 (3.3) | 4.6 (7.7) | 4.2 (5.2) | 3.7 (2.0) | 3.8 (1.9) | 0.01 |
| Sedative treatment, n (%) | 514 (24.3) | 29 (22.5) | 52 (16.5) | 295 (23.7) | 138 (32.3) | <0.001 |
| Depression, n (%) | 472 (22.3) | 23 (17.8) | 51 (16.1) | 274 (22.0) | 124 (29.0) | <0.001 |
| BMI, mean (SD), kg/m2 | 32.6 (3.5) | 33.4 (3.5) | 32.9 (3.4) | 32.4 (3.4) | 32.8 (3.6) | 0.004 |
| PA, mean (SD) | ||||||
| IPA | 7.2 (1.7) | 8.9 (2.6) | 7.6 (1.7) | 7.1 (1.6) | 6.6 (1.4) | <0.001 |
| LPA | 2.6 (1.1) | 2.6 (1.3) | 2.8 (1.1) | 2.6 (1.1) | 2.2 (0.9) | <0.001 |
| MVPA | 40.2 (32.2) | 40.1 (34.6) | 42.1 (33.4) | 41.8 (32.5) | 34.0 (28.9) | <0.001 |
| HRQoL, SF-36 score, mean (SD), points (1-year follow-up) | ||||||
| PF | 79.3 (18.6) | 78.3 (19.2) | 80.9 (17.5) | 80.2 (17.9) | 75.7 (20.9) | <0.001 |
| RF | 81.3 (33.3) | 82.2 (32.4) | 84.2 (30.6) | 82.1 (32.6) | 76.6 (36.9) | 0.01 |
| BP | 65.9 (25.1) | 66.3 (24.6) | 67.7 (23.6) | 66.9 (24.9) | 61.6 (26.5) | 0.001 |
| GH | 64.3 (17.0) | 63.8 (16.7) | 64.9 (16.4) | 64.9 (17.0) | 62.0 (17.2) | 0.02 |
| VT | 65.1 (19.3) | 65.2 (17.6) | 67.1 (17.7) | 65.7 (19.1) | 61.6 (20.9) | <0.001 |
| SF | 85.6 (19.3) | 85.9 (20.4) | 88.1 (17.5) | 86.0 (19.2) | 82.7 (20.0) | 0.001 |
| RE | 90.3 (26.0) | 92.2 (22.6) | 91.1 (24.0) | 90.6 (25.8) | 88.2 (28.9) | 0.27 |
| MH | 75.4 (17.8) | 77.1 (16.0) | 78.0 (15.5) | 75.4 (17.7) | 73.2 (19.6) | 0.002 |
| PCS | 46.3 (8.4) | 45.9 (8.5) | 46.9 (8.0) | 46.7 (8.3) | 44.8 (9.1) | <0.001 |
| MCS | 51.5 (9.3) | 52.3 (8.7) | 52.4 (8.2) | 51.4 (9.3) | 50.6 (10.2) | 0.05 |
| Categories of Night-Time Sleep Duration (h/d) | ||||
|---|---|---|---|---|
| <6 | ≥6–<7 | ≥7–<9 | ≥9 | |
| n = 129 | n = 316 | n = 1247 | n = 427 | |
| HRQoL | β-Coefficients (95% CI) p-Value | β-Coefficients (95% CI) p-Value | β-Coefficients (95% CI) p-Value | β-Coefficients (95% CI) p-Value |
| PF | −5.4 (−8.6 to −2.3) 0.001 | −1.7 (−3.9 to 0.5) 0.12 | 0 (ref.) | −2.2 (−4.1 to −0.2) 0.03 |
| RF | −4.3 (−10.2 to 1.6) 0.16 | −0.6 (−4.7 to 3.4) 0.77 | 0 (ref.) | −3.2 (−6.8 to 0.4) 0.08 |
| BP | −5.0 (−9.4 to −0.6) 0.03 | −1.8 (−4.8 to 1.2) 0.25 | 0 (ref.) | −3.4 (−6.1 to −0.7) 0.01 |
| GH | −3.6 (−6.6 to −0.5) 0.02 | −1.6 (−3.6 to 0.5) 0.14 | 0 (ref.) | −1.7 (−3.5 to 0.1) 0.07 |
| VT | −4.1 (−7.5 to −0.7) 0.02 | −0.5 (−2.8 to 1.8) 0.68 | 0 (ref.) | −2.9 (−5.9 to −0.9) 0.005 |
| SF | −3.4 (−6.7 to 0.03) 0.05 | 0.4 (−1.9 to 2.7) 0.74 | 0 (ref.) | −2.1 (−4.2 to −0.08) 0.04 |
| RE | −0.8 (−5.5 to 3.9) 0.73 | −0.7 (−3.9 to 2.5) 0.66 | 0 (ref.) | −1.6 (−4.4 to 1.3) 0.29 |
| MH | −1.5 (−4.6 to 1.6) 0.36 | 1.1 (−1.0 to 3.2) 0.30 | 0 (ref.) | −1.2 (−3.1 to 0.7) 0.23 |
| PCS | −2.3 (−3.8 to −0.8) 0.002 | −0.8 (−1.8 to 0.2) 0.10 | 0 (ref.) | −1.1 (−2.0 to −0.3) 0.01 |
| MCS | −0.3 (−2.0 to 1.3) 0.70 | 0.5 (−0.6 to 1.6) 0.40 | 0 (ref.) | −0.6 (−1.6 to 0.4) 0.26 |
| Categories of Daytime Sleep Duration (min/d) | |||||||
|---|---|---|---|---|---|---|---|
| <15 | ≥15 to <30 | ≥30 to <60 | ≥60 | ||||
| β-Coefficients | p-Value | β-Coefficients | p-Value | β-Coefficients | p-Value | ||
| Night-time Sleep Duration <7 h/d (n = 445) | |||||||
| HRQoL, SF-36 Score | |||||||
| PCS | |||||||
| Model 3 | 0 (ref.) | 0.9 (−4.1 to 5.9) | 0.73 | 2.3 (−2.4 to 7.0) | 0.34 | 0.70 (−3.8 to 5.2) | 0.76 |
| Model 4 | 0 (ref.) | 0.9 (−4.1 to 5.9) | 0.74 | 2.3 (−2.4 to 7.0) | 0.34 | 0.98 (−3.6 to 5.5) | 0.67 |
| MCS | |||||||
| Model 3 | 0 (ref.) | 6.2 (0.8 to 11.6) | 0.02 | 6.8 (1.8 to 11.9) | 0.008 | 5.0 (0.1 to 9.9) | 0.05 |
| Model 4 | 0 (ref.) | 5.5 (0.1 to 10.9) | 0.04 | 6.3 (1.3 to 11.3) | 0.01 | 4.8 (−0.1 to 9.7) | 0.06 |
| Night-time Sleep Duration ≥7 to <9 h/d (n = 1247) | |||||||
| HRQoL, SF-36 Score | |||||||
| PCS | |||||||
| Model 3 | 0 (ref.) | 1.4 (−0.6 to 3.4) | 0.18 | 1.8 (−0.1 to 3.6) | 0.06 | 1.4 (−0.5 to 3.2) | 0.15 |
| Model 4 | 0 (ref.) | 1.4 (−0.6 to 3.4) | 0.17 | 1.9 (−0.1 to 3.7) | 0.05 | 1.6 (−0.2 to 3.5) | 0.08 |
| MCS | |||||||
| Model 3 | 0 (ref.) | 1.0 (−1.4 to 3.4) | 0.41 | −0.1 (−2.3 to 2.1) | 0.95 | −0.5 (−2.6 to 1.7) | 0.68 |
| Model 4 | 0 (ref.) | 1.2 (−1.2 to 3.5) | 0.33 | 0.2 (−1.9 to 2.3) | 0.87 | 0.2 (−1.9 to 2.3) | 0.86 |
| Night-time Sleep Duration ≥9 h/d (n = 427) | |||||||
| HRQoL, SF-36 Score | |||||||
| PCS | |||||||
| Model 3 | 0 (ref.) | −1.4 (−6.2 to 3.4) | 0.56 | −2.7 (−7.0 to 1.6) | 0.41 | −1.8 (−6.0 to 2.5) | 0.41 |
| Model 4 | 0 (ref.) | −0.7 (−5.4 to 5.4) | 0.76 | −2.2 (−6.5 to 2.0) | 0.29 | −0.7 (−4.9 to 3.4) | 0.73 |
| MCS | |||||||
| Model 3 | 0 (ref.) | −0.5 (−6.2 to 5.2) | 0.87 | −1.1 (−6.2 to 4.0) | 0.67 | −1.6 (−6.7 to 3.4) | 0.52 |
| Model 4 | 0 (ref.) | −0.2 (−5.8 to 5.3) | 0.94 | −0.1 (−5.0 to 4.9) | 0.98 | −0.7 (−5.6 to 4.2) | 0.77 |
| Categories of Daytime Sleep Duration (min/d) | |||||||
|---|---|---|---|---|---|---|---|
| <15 | ≥15 to <30 | ≥30 to <60 | ≥60 | ||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Night-time Sleep Duration <7 h/d (n = 445) | |||||||
| HRQoL, SF-36 Score | |||||||
| PCS | |||||||
| Model 3 | 1.00 (ref.) | 0.47 (0.11–1.92) | 0.29 | 0.51 (0.14–1.90) | 0.32 | 0.68 (0.19–2.44) | 0.56 |
| Model 4 | 1.00 (ref.) | 0.43 (0.10–1.80) | 0.25 | 0.50 (0.13–1.88) | 0.31 | 0.73 (0.20–2.62) | 0.63 |
| MCS | |||||||
| Model 3 | 1.00 (ref.) | 0.66 (0.16–2.68) | 0.56 | 0.64 (0.17–2.33) | 0.50 | 0.59 (0.17–2.07) | 0.41 |
| Model 4 | 1.00 (ref.) | 0.75 (0.18–3.19) | 0.70 | 0.60 (0.16–2.30) | 0.46 | 0.54 (0.15–1.95) | 0.34 |
| Night-time Sleep Duration ≥7 to <9 h/d (n = 1247) | |||||||
| HRQoL, SF-36 Score | |||||||
| PCS | |||||||
| Model 3 | 1.00 (ref.) | 0.54 (0.31–0.93) | 0.03 | 0.73 (0.44–1.19) | 0.21 | 0.88 (0.54–1.44) | 0.62 |
| Model 4 | 1.00 (ref.) | 0.53 (0.31–0.93) | 0.03 | 0.72 (0.43–1.18) | 0.20 | 0.87 (0.53–1.43) | 0.59 |
| MCS | |||||||
| Model 3 | 1.00 (ref.) | 0.98 (0.54–1.77) | 0.94 | 0.97 (0.57–1.67) | 0.92 | 0.99 (0.58–1.68) | 0.96 |
| Model 4 | 1.00 (ref.) | 1.01 (0.55–1.84) | 0.99 | 1.00 (0.58–1.68) | 0.93 | 0.98 (0.57–1.68) | 0.93 |
| Night-time Sleep Duration ≥9 h/d (n = 427) | |||||||
| HRQoL, SF-36 Score | |||||||
| PCS | |||||||
| Model 3 | 1.00 (ref.) | 0.53 (0.15–1.84) | 0.32 | 0.28 (0.09–0.87) | 0.03 | 0.33 (0.11–1.03) | 0.06 |
| Model 4 | 1.00 (ref.) | 0.52 (0.14–1.89) | 0.32 | 0.27 (0.08–0.88) | 0.03 | 0.36 (0.11–1.15) | 0.08 |
| MCS | |||||||
| Model 3 | 1.00 (ref.) | 1.80 (0.51–6.36) | 0.36 | 1.11 (0.35–3.49) | 0.86 | 0.97 (0.31–3.02) | 0.96 |
| Model 4 | 1.00 (ref.) | 1.87 (0.52–6.70) | 0.34 | 1.09 (0.34–3.51) | 0.88 | 0.94 (0.29–2.97) | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Delgado, A.; Martín-Sánchez, V.; Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Schröder, H.; Martínez, A.; Alonso-Gómez, Á.M.; Wärnberg, J.; Vioque, J.; et al. Objectively Measured Sleep Duration and Health-Related Quality of Life in Older Adults with Metabolic Syndrome: A One-Year Longitudinal Analysis of the PREDIMED-Plus Cohort. Nutrients 2024, 16, 2631. https://doi.org/10.3390/nu16162631
Marcos-Delgado A, Martín-Sánchez V, Martínez-González MÁ, Corella D, Salas-Salvadó J, Schröder H, Martínez A, Alonso-Gómez ÁM, Wärnberg J, Vioque J, et al. Objectively Measured Sleep Duration and Health-Related Quality of Life in Older Adults with Metabolic Syndrome: A One-Year Longitudinal Analysis of the PREDIMED-Plus Cohort. Nutrients. 2024; 16(16):2631. https://doi.org/10.3390/nu16162631
Chicago/Turabian StyleMarcos-Delgado, Alba, Vicente Martín-Sánchez, Miguel Ángel Martínez-González, Dolores Corella, Jordi Salas-Salvadó, Helmut Schröder, Alfredo Martínez, Ángel M. Alonso-Gómez, Julia Wärnberg, Jesús Vioque, and et al. 2024. "Objectively Measured Sleep Duration and Health-Related Quality of Life in Older Adults with Metabolic Syndrome: A One-Year Longitudinal Analysis of the PREDIMED-Plus Cohort" Nutrients 16, no. 16: 2631. https://doi.org/10.3390/nu16162631
APA StyleMarcos-Delgado, A., Martín-Sánchez, V., Martínez-González, M. Á., Corella, D., Salas-Salvadó, J., Schröder, H., Martínez, A., Alonso-Gómez, Á. M., Wärnberg, J., Vioque, J., Romaguera, D., López-Miranda, J., Estruch, R., Tinahones, F. J., Santos-Lozano, J. M., Álvarez-Pérez, J., Bueno-Cavanillas, A., Cano-Ibáñez, N., Amezcua-Prieto, C., ... Nieto, J. (2024). Objectively Measured Sleep Duration and Health-Related Quality of Life in Older Adults with Metabolic Syndrome: A One-Year Longitudinal Analysis of the PREDIMED-Plus Cohort. Nutrients, 16(16), 2631. https://doi.org/10.3390/nu16162631























