Skeletal Muscle, Skin, and Bone as Three Major Nitrate Reservoirs in Mammals: Chemiluminescence and 15N-Tracer Studies in Yorkshire Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Nitrate Supplementation
2.2. Sample Collection
2.3. Determination of Total Nitrite and Nitrate Concentrations
2.4. Preparation of Samples for LC-MS/MS
2.5. Determination of 15N-Nitrate or 15N-Nitrite Percent by LC-MS/MS
2.6. Statistical Analysis
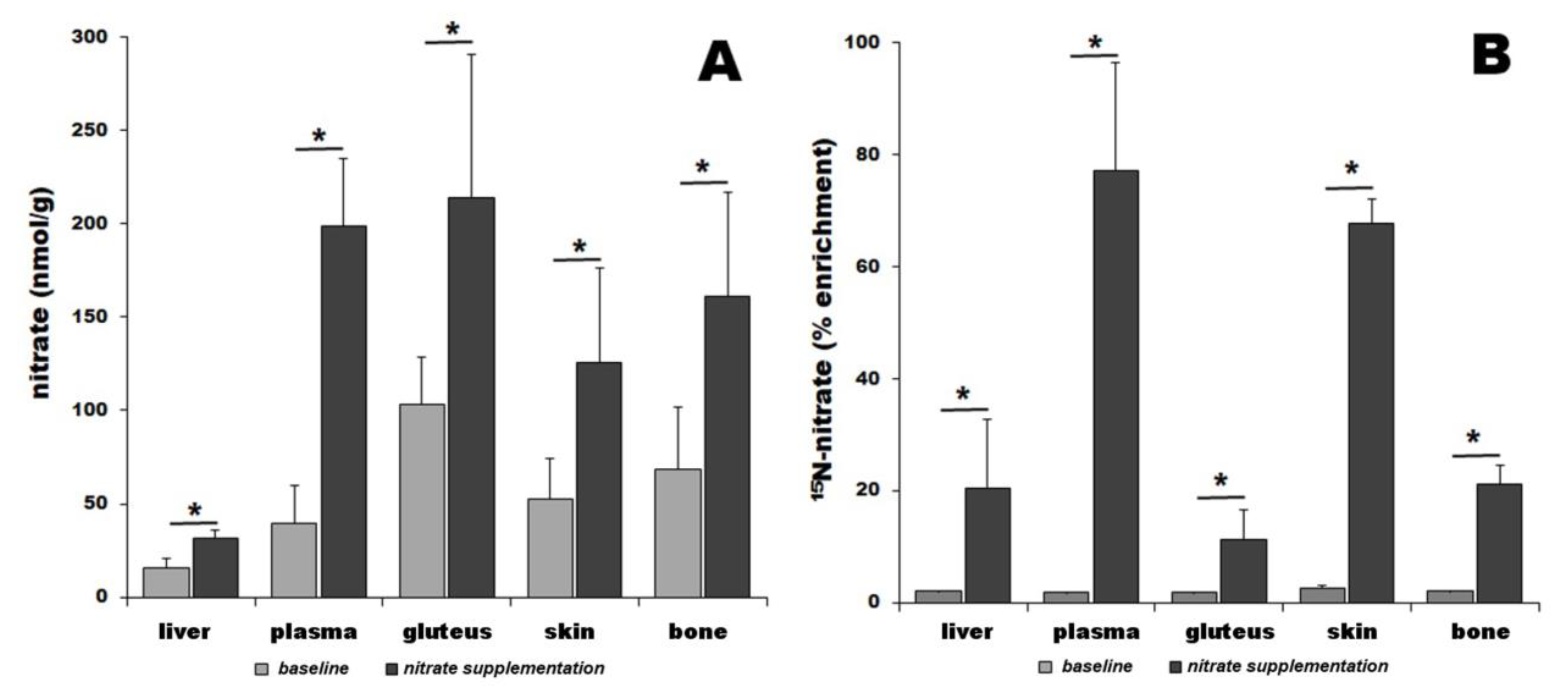
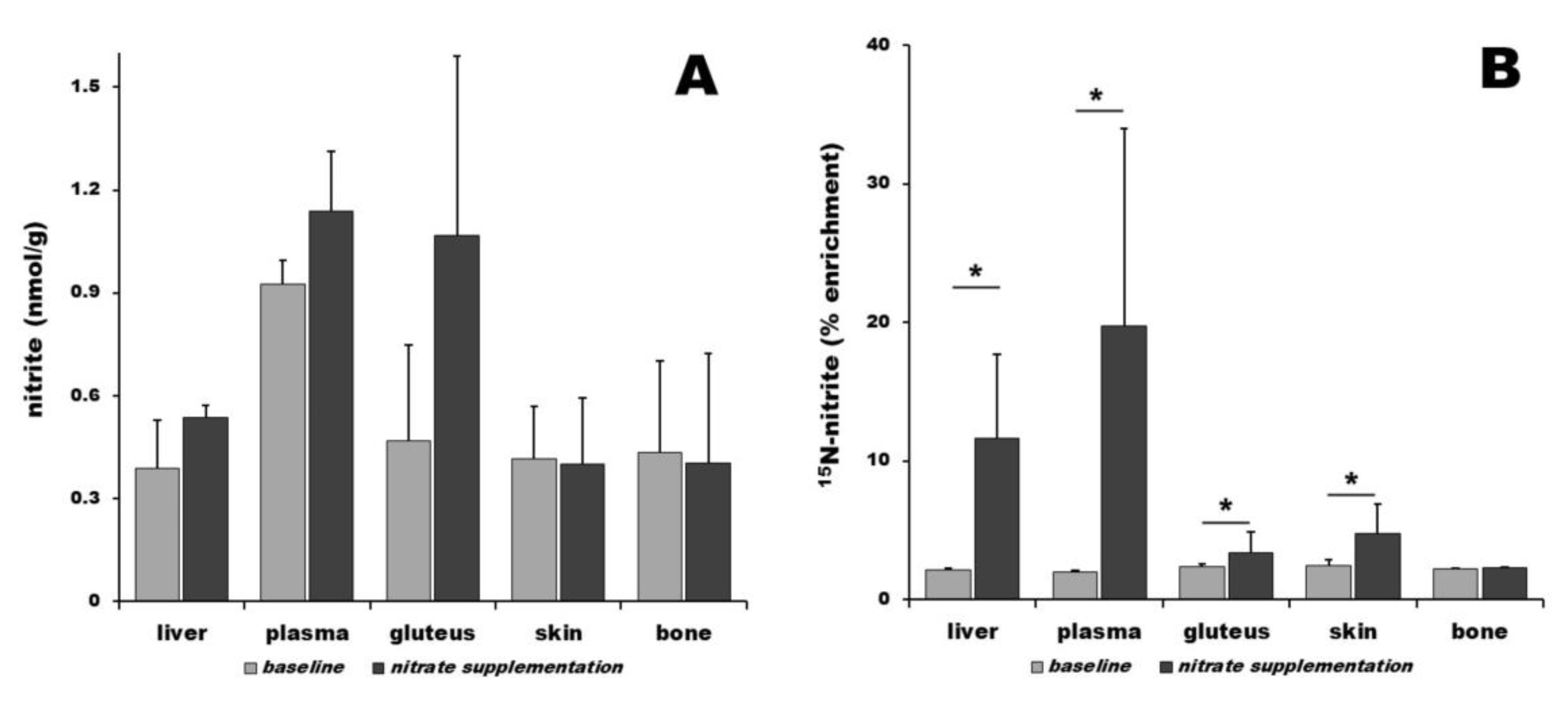
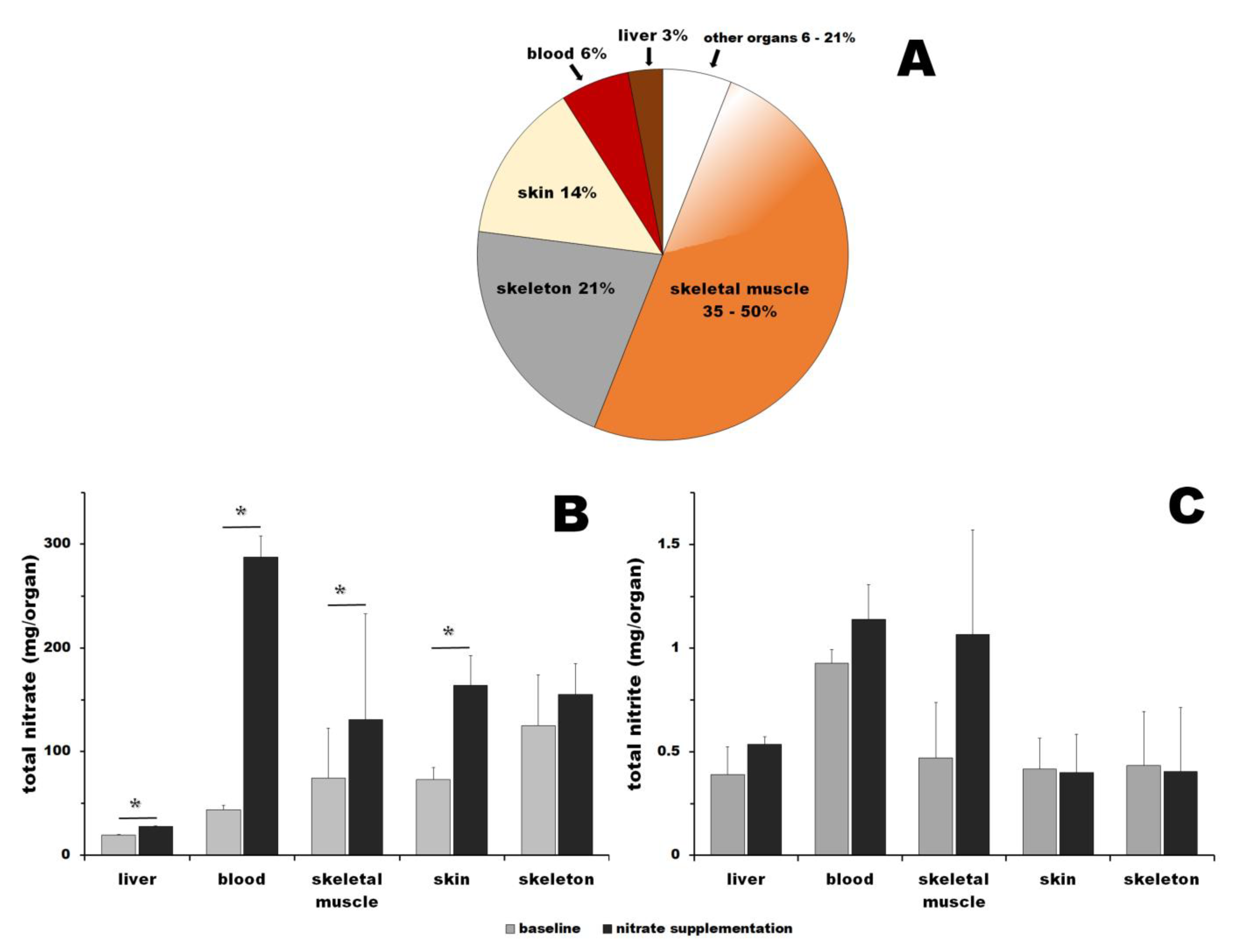
3. Results
3.1. Nitrate
3.2. Nitrite
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moncada, S.; Palmer, R.M.; Higgs, E.A. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension 1988, 12, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.R., Jr. Historical origins of the discovery of mammalian nitric oxide (nitrogen monoxide) production/physiology/pathophysiology. Biochem. Pharmacol. 2020, 176, 113793. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J.; Haque, M.M. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br. J. Pharmacol. 2019, 176, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, M.; Tanimoto, A.; Tamura, M.; Mukae, H.; Yanagihara, N.; Shimokawa, H.; Otsuji, Y. Significance of nitric oxide synthases: Lessons from triple nitric oxide synthases null mice. J. Pharmacol. Sci. 2015, 127, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Moura, J.J. Nitrite reduction by molybdoenzymes: A new class of nitric oxide-forming nitrite reductases. J. Biol. Inorg. Chem. 2015, 20, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.; Schwarz, G. Nitrite-dependent nitric oxide synthesis by molybdenum enzymes. FEBS Lett. 2018, 592, 2126–2139. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Moura, J.J.G. Putting xanthine oxidoreductase and aldehyde oxidase on the NO metabolism map: Nitrite reduction by molybdoenzymes. Redox Biol. 2018, 19, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.D.; Gladwin, M.T.; Freeman, B.A.; Lundberg, J.O.; Weitzberg, E.; Morris, A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic. Biol. Med. 2017, 105, 48–67. [Google Scholar] [CrossRef]
- Li, H.; Samouilov, A.; Liu, X.; Zweier, J.L. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: Evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry 2003, 42, 1150–1159. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. NO-synthase independent NO generation in mammals. Biochem. Biophys. Res. Commun. 2010, 396, 39–45. [Google Scholar] [CrossRef] [PubMed]
- van Faassen, E.E.; Bahrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.; Lundberg, J.O.; Mason, R.; et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef] [PubMed]
- DeMartino, A.W.; Kim-Shapiro, D.B.; Patel, R.P.; Gladwin, M.T. Nitrite and nitrate chemical biology and signalling. Br. J. Pharmacol. 2019, 176, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Suschek, C.V.; Schewe, T.; Sies, H.; Kroncke, K.D. Nitrite, a naturally occurring precursor of nitric oxide that acts like a ‘prodrug’. Biol. Chem. 2006, 387, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Hezel, M.P.; Weitzberg, E. The oral microbiome and nitric oxide homoeostasis. Oral. Dis. 2015, 21, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Tiso, M.; Schechter, A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Soltero, R.; Bailen, M.; de Lucas, B.; Ramirez-Goercke, M.I.; Pareja-Galeano, H.; Larrosa, M. Role of Oral and Gut Microbiota in Dietary Nitrate Metabolism and Its Impact on Sports Performance. Nutrients 2020, 12, 3611. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Laranjinha, J. Nitrate from diet might fuel gut microbiota metabolism: Minding the gap between redox signaling and inter-kingdom communication. Free Radic. Biol. Med. 2020, 149, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Nichols, A.L.; Honavar, J.; Dransfield, M.T.; Matalon, S.; Patel, R.P. Measuring nitrate reductase activity from human and rodent tongues. Nitric Oxide 2017, 66, 62–70. [Google Scholar] [CrossRef]
- Bryan, N.S.; Burleigh, M.C.; Easton, C. The oral microbiome, nitric oxide and exercise performance. Nitric Oxide 2022, 125–126, 23–30. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, L. Positive feedback loop between dietary nitrate intake and oral health. Nutr. Res. 2023, 115, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlen, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Borniquel, S.; Lundberg, J.O. Enhanced xanthine oxidoreductase expression and tissue nitrate reduction in germ free mice. Nitric Oxide 2010, 22, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.A.; Wilson, H.L.; Rajagopalan, K.V. Structure-based alteration of substrate specificity and catalytic activity of sulfite oxidase from sulfite oxidation to nitrate reduction. Biochemistry 2012, 51, 1134–1147. [Google Scholar] [CrossRef] [PubMed]
- Sparacino-Watkins, C.E.; Tejero, J.; Sun, B.; Gauthier, M.C.; Thomas, J.; Ragireddy, V.; Merchant, B.A.; Wang, J.; Azarov, I.; Basu, P.; et al. Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J. Biol. Chem. 2014, 289, 10345–10358. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, M.; Liu, M.; Yang, T.; Zollbrecht, C.; Huang, L.; Peleli, M.; Borniquel, S.; Kishikawa, H.; Hezel, M.; Persson, A.E.; et al. Cross-talk Between Nitrate-Nitrite-NO and NO Synthase Pathways in Control of Vascular NO Homeostasis. Antioxid. Redox Signal 2015, 23, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.T.; Strampraad, M.J.F.; Hagedoorn, P.L.; Schwarz, G. Reciprocal regulation of sulfite oxidation and nitrite reduction by mitochondrial sulfite oxidase. Nitric Oxide 2019, 89, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Jimenez, M.; Chamizo-Ampudia, A.; Calatrava, V.; Galvan, A.; Fernandez, E.; Llamas, A. From the Eukaryotic Molybdenum Cofactor Biosynthesis to the Moonlighting Enzyme mARC. Molecules 2018, 23, 3287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Krizowski, S.; Fischer-Schrader, K.; Niks, D.; Tejero, J.; Sparacino-Watkins, C.; Wang, L.; Ragireddy, V.; Frizzell, S.; Kelley, E.E.; et al. Sulfite Oxidase Catalyzes Single-Electron Transfer at Molybdenum Domain to Reduce Nitrite to Nitric Oxide. Antioxid. Redox Signal 2015, 23, 283–294. [Google Scholar] [CrossRef]
- Maia, L.B. Bringing Nitric Oxide to the Molybdenum World-A Personal Perspective. Molecules 2023, 28, 5819. [Google Scholar] [CrossRef]
- Mutus, B. The catalytic mechanism for NO production by the mitochondrial enzyme, sulfite oxidase. Biochem. J. 2019, 476, 1955–1956. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, M.; Lundberg, J.O.; Weitzberg, E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol. 2018, 224, e13080. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Easton, C.; Shepherd, A.I.; Siervo, M.; Bailey, S.J.; Clifford, T. Dietary nitrate and population health: A narrative review of the translational potential of existing laboratory studies. BMC Sports Sci. Med. Rehabil. 2021, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Woessner, M.N.; de Zevallos, J.O.; Kraus, W.E.; VanBruggen, M.D.; Schechter, A.N.; Allen, J.D. Human skeletal muscle nitrate and nitrite in individuals with peripheral arterial disease: Effect of inorganic nitrate supplementation and exercise. Physiol. Rep. 2022, 10, e15531. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Thompson, C.; Wylie, L.J.; Vanhatalo, A. Dietary Nitrate and Physical Performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Vanhatalo, A.; Seals, D.R.; Rossman, M.J.; Piknova, B.; Jonvik, K.L. Dietary Nitrate and Nitric Oxide Metabolism: Mouth, Circulation, Skeletal Muscle, and Exercise Performance. Med. Sci. Sports Exerc. 2021, 53, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Marechal, G.; Gailly, P. Effects of nitric oxide on the contraction of skeletal muscle. Cell Mol. Life Sci. 1999, 55, 1088–1102. [Google Scholar] [CrossRef]
- Haider, G.; Folland, J.P. Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med. Sci. Sports Exerc. 2014, 46, 2234–2243. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Townsend, J.R.; Pinzone, A.G.; Hoffman, J.R. Supplementation with Nitric Oxide Precursors for Strength Performance: A Review of the Current Literature. Nutrients 2023, 15, 660. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Trexler, E.T. Effects of Citrulline Supplementation on Exercise Performance in Humans: A Review of the Current Literature. J. Strength. Cond. Res. 2020, 34, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.M.; Beaver, L.M.; Reardon, P.N.; Prater, M.C.; Truong, L.; Robinson, M.M.; Tanguay, R.L.; Stevens, J.F.; Hord, N.G. Nitrate-induced improvements in exercise performance are coincident with exuberant changes in metabolic genes and the metabolome in zebrafish (Danio rerio) skeletal muscle. J. Appl. Physiol. 1985 2021, 131, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Swanson, K.M.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 2015, 47, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wylie, L.J.; Park, J.W.; Vanhatalo, A.; Kadach, S.; Black, M.I.; Stoyanov, Z.; Schechter, A.N.; Jones, A.M.; Piknova, B. Human skeletal muscle nitrate store: Influence of dietary nitrate supplementation and exercise. J. Physiol. 2019, 597, 5565–5576. [Google Scholar] [CrossRef]
- Piknova, B.; Schechter, A.N.; Park, J.W.; Vanhatalo, A.; Jones, A.M. Skeletal Muscle Nitrate as a Regulator of Systemic Nitric Oxide Homeostasis. Exerc. Sport Sci. Rev. 2022, 50, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Gilliard, C.N.; Lam, J.K.; Cassel, K.S.; Park, J.W.; Schechter, A.N.; Piknova, B. Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide 2018, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Thomas, S.M.; Schechter, A.N.; Piknova, B. Control of rat muscle nitrate levels after perturbation of steady state dietary nitrate intake. Nitric Oxide 2021, 109–110, 42–49. [Google Scholar] [CrossRef]
- Park, J.W.; Piknova, B.; Walter, P.J.; Cai, H.; Upanan, S.; Thomas, S.M.; Tunau-Spencer, K.J.; Schechter, A.N. Distribution of dietary nitrate and its metabolites in rat tissues after (15)N-labeled nitrate administration. Sci. Rep. 2023, 13, 3499. [Google Scholar] [CrossRef]
- Kadach, S.; Piknova, B.; Black, M.I.; Park, J.W.; Wylie, L.J.; Stoyanov, Z.; Thomas, S.M.; McMahon, N.F.; Vanhatalo, A.; Schechter, A.N.; et al. Time course of human skeletal muscle nitrate and nitrite concentration changes following dietary nitrate ingestion. Nitric Oxide 2022, 121, 1–10. [Google Scholar] [CrossRef]
- Kadach, S.; Park, J.W.; Stoyanov, Z.; Black, M.I.; Vanhatalo, A.; Burnley, M.; Walter, P.J.; Cai, H.; Schechter, A.N.; Piknova, B.; et al. (15) N-labelled dietary nitrate supplementation increases human skeletal muscle nitrate concentration and improves muscle torque production. Acta Physiol. 2023, 237, e13924. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Kwan Jeff Lam, K.; Schechter, A.N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide 2016, 55–56, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.; Pattullo, S.; Smith, L.; Golden, M.; Ormerod, A.; Benjamin, N. Nitric oxide is generated on the skin surface by reduction of sweat nitrate. J. Investig. Dermatol. 1996, 107, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Paunel, A.N.; Dejam, A.; Thelen, S.; Kirsch, M.; Horstjann, M.; Gharini, P.; Murtz, M.; Kelm, M.; de Groot, H.; Kolb-Bachofen, V.; et al. Enzyme-independent nitric oxide formation during UVA challenge of human skin: Characterization, molecular sources, and mechanisms. Free Radic. Biol. Med. 2005, 38, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, M.; McLintock, S.; Weerakoon, R.; Lomatschinsky, N.; Jones, S.; Rossi, A.G.; Weller, R.B. Enzyme-independent NO stores in human skin: Quantification and influence of UV radiation. J. Investig. Dermatol. 2009, 129, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Oplander, C.; Suschek, C.V. New aspects of nitrite homeostasis in human skin. J. Investig. Dermatol. 2009, 129, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Fernandez, B.O.; Hamilton, A.; Lang, N.N.; Gallagher, J.M.C.; Newby, D.E.; Feelisch, M.; Weller, R.B. UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. J. Investig. Dermatol. 2014, 134, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Byrne, S.N. An unexpected role: UVA-induced release of nitric oxide from skin may have unexpected health benefits. J. Investig. Dermatol. 2014, 134, 1791–1794. [Google Scholar] [CrossRef] [PubMed]
- Levitt, E.L.; Keen, J.T.; Wong, B.J. Augmented reflex cutaneous vasodilatation following short-term dietary nitrate supplementation in humans. Exp. Physiol. 2015, 100, 708–718. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Ridnour, L.A.; Espey, M.G.; Wink, D.A.; Roberts, D.D. Nitric oxide in wound-healing. Microsurgery 2005, 25, 442–451. [Google Scholar] [CrossRef]
- Afzali, H.; Khaksari, M.; Norouzirad, R.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Acidified nitrite improves wound healing in type 2 diabetic rats: Role of oxidative stress and inflammation. Nitric Oxide 2020, 103, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, e1801210. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, M.T.; Paganotti, A.; Seabra, A.B.; Weller, R.B. Photochemistry of nitric oxide and S-nitrosothiols in human skin. Histochem. Cell Biol. 2020, 153, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.N.; Hieda, M.; Huang, M.; Moralez, G.; Crandall, C.G. Dietary nitrate supplementation does not influence thermoregulatory or cardiovascular strain in older individuals during severe ambient heat stress. Exp. Physiol. 2020, 105, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Omori, S.; Kataoka, Y.; Maimaituxun, G.; Bailey, S.J.; Lloyd, A.B.; Arnold, J.T.; Amano, T.; Tanabe, Y.; Omi, N.; et al. Dietary nitrate supplementation increases nitrate and nitrite concentrations in human skin interstitial fluid. Nitric Oxide 2023, 134–135, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, L.; Wang, X.; Liu, H.; Zhang, C.; Wang, J.; Wang, X.; Wang, S. Salivary nitrate prevents osteoporosis via regulating bone marrow mesenchymal stem cells proliferation and differentiation. J. Orthop. Translat. 2024, 45, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, S.; Yousefzadeh, N.; Kashfi, K.; Ghasemi, A. Role of nitric oxide in type 1 diabetes-induced osteoporosis. Biochem. Pharmacol. 2022, 197, 114888. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Yuen, T.; Iqbal, J.; Rubin, M.R.; Zaidi, M. The NO-cGMP-PKG pathway in skeletal remodeling. Ann. N. Y. Acad. Sci. 2021, 1487, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Diwan, A.D.; Wang, M.X.; Jang, D.; Zhu, W.; Murrell, G.A. Nitric oxide modulates fracture healing. J. Bone Miner. Res. 2000, 15, 342–351. [Google Scholar] [CrossRef]
- Draghici, A.E.; Ely, M.R.; Hamner, J.W.; Taylor, J.A. Nitric oxide-mediated vasodilation in human bone. Microcirculation 2024, 31, e12842. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; van Oers, R.F.; Bakker, A.D.; Bacabac, R.G. Nitric oxide signaling in mechanical adaptation of bone. Osteoporos. Int. 2014, 25, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, H.; Schall, N.; Pilz, R.B. Nitric oxide and cyclic GMP functions in bone. Nitric Oxide 2018, 76, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Nitric oxide and bone. Ann. N. Y. Acad. Sci. 2010, 1192, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Kho, J.; Dawson, B.; Jiang, M.M.; Chen, Y.; Ali, S.; Burrage, L.C.; Grover, M.; Palmer, D.J.; Turner, D.L.; et al. Nitric oxide modulates bone anabolism through regulation of osteoblast glycolysis and differentiation. J. Clin. Investig. 2021, 131, e138935. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H. The Michael Mason Prize Essay 1997. Nitric oxide and bone: What a gas! Br. J. Rheumatol. 1997, 36, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ji, P.; Shang, X.; Zhou, Y. Connection between Osteoarthritis and Nitric Oxide: From Pathophysiology to Therapeutic Target. Molecules 2023, 28, 1683. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.; Boushel, R.; Hellsten, Y.; Kalliokoski, K. Regulation of bone blood flow in humans: The role of nitric oxide, prostaglandins, and adenosine. Scand. J. Med. Sci. Sports 2018, 28, 1552–1558. [Google Scholar] [CrossRef]
- Webster, J.; Dalla Via, J.; Langley, C.; Smith, C.; Sale, C.; Sim, M. Nutritional strategies to optimise musculoskeletal health for fall and fracture prevention: Looking beyond calcium, vitamin D and protein. Bone Rep. 2023, 19, 101684. [Google Scholar] [CrossRef] [PubMed]
- Draghici, A.E.; Taylor, J.A. Mechanisms of bone blood flow regulation in humans. J. Appl. Physiol. 1985 2021, 130, 772–780. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed; National Academies Press: Washington, DC, USA, 2011.
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yucel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Cassel, K.S.; Gilliard, C.N.; Schechter, A.N. Measuring Nitrite and Nitrate, Metabolites in the Nitric Oxide Pathway, in Biological Materials using the Chemiluminescence Method. J. Vis. Exp. 2016, 118, e54879. [Google Scholar] [CrossRef]
- Chao, M.R.; Shih, Y.M.; Hsu, Y.W.; Liu, H.H.; Chang, Y.J.; Lin, B.H.; Hu, C.W. Urinary nitrite/nitrate ratio measured by isotope-dilution LC-MS/MS as a tool to screen for urinary tract infections. Free Radic. Biol. Med. 2016, 93, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meininger, C.J.; Wu, G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2000, 746, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, N.E.; Ostrom, P.H. Nitrogen isotopes. In Geochemistry. Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Duncan, C.; Dougall, H.; Johnston, P.; Green, S.; Brogan, R.; Leifert, C.; Smith, L.; Golden, M.; Benjamin, N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995, 1, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Doel, J.J.; Benjamin, N.; Hector, M.P.; Rogers, M.; Allaker, R.P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral. Sci. 2005, 113, 14–19. [Google Scholar] [CrossRef] [PubMed]
- McKnight, G.M.; Smith, L.M.; Drummond, R.S.; Duncan, C.W.; Golden, M.; Benjamin, N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut 1997, 40, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, X.; Sun, Q.; Fan, Z.; Xia, D.; Ding, G.; Ong, H.L.; Adams, D.; Gahl, W.A.; Zheng, C.; et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 13434–13439. [Google Scholar] [CrossRef]
- Srihirun, S.; Park, J.W.; Teng, R.; Sawaengdee, W.; Piknova, B.; Schechter, A.N. Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric Oxide 2020, 94, 1–8. [Google Scholar] [CrossRef]
- Yousefzadeh, N.; Jeddi, S.; Zarkesh, M.; Kashfi, K.; Ghasemi, A. Altered sialin mRNA gene expression in type 2 diabetic male Wistar rats: Implications for nitric oxide deficiency. Sci. Rep. 2023, 13, 4013. [Google Scholar] [CrossRef]
- Akhtar, S.; Sagar, K.; Singh, A.; Hote, M.P.; Roy, A.; Sharma, A. Inflammation-induced sialin mediates nitrate efflux in dysfunctional endothelium affecting NO bioavailability. Nitric Oxide 2024, 146, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hu, L.; Feng, X.; Wang, S. Nitrate and Nitrite in Health and Disease. Aging Dis. 2018, 9, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Ivy, J.L. Inorganic nitrite and nitrate: Evidence to support consideration as dietary nutrients. Nutr. Res. 2015, 35, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.; Nadavade, N.; Sheikh, S.S. A review on nitrates’ health benefits and disease prevention. Nitric Oxide 2024, 142, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef] [PubMed]
- Nyakayiru, J.; Kouw, I.W.K.; Cermak, N.M.; Senden, J.M.; van Loon, L.J.C.; Verdijk, L.B. Sodium nitrate ingestion increases skeletal muscle nitrate content in humans. J. Appl. Physiol. 1985 2017, 123, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Nyakayiru, J.; van Loon, L.J.C.; Verdijk, L.B. Could intramuscular storage of dietary nitrate contribute to its ergogenic effect? A mini-review. Free Radic. Biol. Med. 2020, 152, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Troutman, A.D.; Gallardo, E.J.; Brown, M.B.; Coggan, A.R. Measurement of nitrate and nitrite in biopsy-sized muscle samples using HPLC. J. Appl. Physiol. 1985 2018, 125, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Zevallos, J.; Woessner, M.N.; Kelley, E.E. Skeletal muscle as a reservoir for nitrate and nitrite: The role of xanthine oxidase reductase (XOR). Nitric Oxide 2022, 129, 102–109. [Google Scholar] [CrossRef]
- McGarr, G.W.; King, K.E.; Saci, S.; Leduc, D.; Akerman, A.P.; Fujii, N.; Kenny, G.P. Regional variation in nitric oxide-dependent cutaneous vasodilatation during local heating in young adults. Exp. Physiol. 2021, 106, 1671–1678. [Google Scholar] [CrossRef]
- Joyner, M.J.; Dietz, N.M. Nitric oxide and vasodilation in human limbs. J. Appl. Physiol. 1985 1997, 83, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Boegli, Y.; Gremion, G.; Golay, S.; Kubli, S.; Liaudet, L.; Leyvraz, P.F.; Waeber, B.; Feihl, F. Endurance training enhances vasodilation induced by nitric oxide in human skin. J. Investig. Dermatol. 2003, 121, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Bliss, M.R. Hyperaemia. J. Tissue Viability 1998, 8, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Medow, M.S.; Taneja, I.; Stewart, J.M. Cyclooxygenase and nitric oxide synthase dependence of cutaneous reactive hyperemia in humans. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H425–H432. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Lubrano, V.; Domenici, C.; L’Abbate, A. Influence of chronic aerobic exercise on microcirculatory flow and nitric oxide in humans. Int. J. Sports Med. 2003, 24, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Schwentker, A.; Vodovotz, Y.; Weller, R.; Billiar, T.R. Nitric oxide and wound repair: Role of cytokines? Nitric Oxide 2002, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Childress, B.B.; Stechmiller, J.K. Role of nitric oxide in wound healing. Biol. Res. Nurs. 2002, 4, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Gyurko, R.; Boustany, G.; Huang, P.L.; Kantarci, A.; Van Dyke, T.E.; Genco, C.A.; Gibson, F.C., 3rd. Mice lacking inducible nitric oxide synthase demonstrate impaired killing of Porphyromonas gingivalis. Infect. Immun. 2003, 71, 4917–4924. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Haus, J.M.; Chen, L.; Wu, S.C.; Urao, N.; Koh, T.J.; Minshall, R.D. CCL28-induced CCR10/eNOS interaction in angiogenesis and skin wound healing. FASEB J. 2020, 34, 5838–5850. [Google Scholar] [CrossRef]
- Chin, L.C.; Kumar, P.; Palmer, J.A.; Rophael, J.A.; Dolderer, J.H.; Thomas, G.P.; Morrison, W.A.; Penington, A.J.; Stewart, A.G.; Mitchell, G.M. The influence of nitric oxide synthase 2 on cutaneous wound angiogenesis. Br. J. Dermatol. 2011, 165, 1223–1235. [Google Scholar] [CrossRef]
- Baldik, Y.; Talu, U.; Altinel, L.; Bilge, H.; Demiryont, M.; Aykac-Toker, G. Bone healing regulated by nitric oxide: An experimental study in rats. Clin. Orthop. Relat. Res. 2002, 404, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Gronenschild, E.H.; Muris, D.M.; Schram, M.T.; Karaca, U.; Stehouwer, C.D.; Houben, A.J. Semi-automatic assessment of skin capillary density: Proof of principle and validation. Microvasc. Res. 2013, 90, 192–198. [Google Scholar] [CrossRef] [PubMed]
- McAllister, R.M.; Amann, J.F.; Laughlin, M.H. Skeletal muscle fiber types and their vascular support. J. Reconstr. Microsurg. 1993, 9, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.H. Skeletal muscle blood flow capacity: Role of muscle pump in exercise hyperemia. Am. J. Physiol. 1987, 253, H993–H1004. [Google Scholar] [CrossRef] [PubMed]
- de la Grandmaison, G.L.; Clairand, I.; Durigon, M. Organ weight in 684 adult autopsies: New tables for a Caucasoid population. Forensic Sci. Int. 2001, 119, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.E.; Kelley, D.E.; Heshka, S.; Thornton, J.; Pi-Sunyer, F.X.; Boxt, L.; Balasubramanyam, A.; Gallagher, D.; Group, M.R.I.A.S.S.o.t.L.A.R. Skeletal muscle and organ masses differ in overweight adults with type 2 diabetes. J. Appl. Physiol. 1985 2014, 117, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Jackowski, C.; Thali, M.J.; Buck, U.; Aghayev, E.; Sonnenschein, M.; Yen, K.; Dirnhofer, R.; Vock, P. Noninvasive estimation of organ weights by postmortem magnetic resonance imaging and multislice computed tomography. Investig. Radiol. 2006, 41, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.K.; DiMaio, V.J. Normal Organ Weights in Women: Part I-The Heart. Am. J. Forensic Med. Pathol. 2015, 36, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Molina, D.K.; DiMaio, V.J. Normal organ weights in men: Part II-the brain, lungs, liver, spleen, and kidneys. Am. J. Forensic Med. Pathol. 2012, 33, 368–372. [Google Scholar] [CrossRef]
- Bell, M.D.; Long, T.; Roden, A.C.; Cooper, F.I.; Sanchez, H.; Trower, C.; Martinez, C.; Hooper, J.E.; Autopsy Committee of the College of American, P. Updating Normal Organ Weights Using a Large Current Sample Database. Arch. Pathol. Lab. Med. 2022, 146, 1486–1495. [Google Scholar] [CrossRef]
- Connor, J. List of Organs of the Human Body Functions and Pictures. Available online: https://www.adducation.info/mankind-nature-general-knowledge/all-organs-of-the-human-body/ (accessed on 17 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piknova, B.; Park, J.W.; Tunau-Spencer, K.J.; Jenkins, A.; Hellinga, D.G.; Walter, P.J.; Cai, H.; Schechter, A.N. Skeletal Muscle, Skin, and Bone as Three Major Nitrate Reservoirs in Mammals: Chemiluminescence and 15N-Tracer Studies in Yorkshire Pigs. Nutrients 2024, 16, 2674. https://doi.org/10.3390/nu16162674
Piknova B, Park JW, Tunau-Spencer KJ, Jenkins A, Hellinga DG, Walter PJ, Cai H, Schechter AN. Skeletal Muscle, Skin, and Bone as Three Major Nitrate Reservoirs in Mammals: Chemiluminescence and 15N-Tracer Studies in Yorkshire Pigs. Nutrients. 2024; 16(16):2674. https://doi.org/10.3390/nu16162674
Chicago/Turabian StylePiknova, Barbora, Ji Won Park, Khalid J. Tunau-Spencer, Audrey Jenkins, David G. Hellinga, Peter J. Walter, Hongyi Cai, and Alan N. Schechter. 2024. "Skeletal Muscle, Skin, and Bone as Three Major Nitrate Reservoirs in Mammals: Chemiluminescence and 15N-Tracer Studies in Yorkshire Pigs" Nutrients 16, no. 16: 2674. https://doi.org/10.3390/nu16162674
APA StylePiknova, B., Park, J. W., Tunau-Spencer, K. J., Jenkins, A., Hellinga, D. G., Walter, P. J., Cai, H., & Schechter, A. N. (2024). Skeletal Muscle, Skin, and Bone as Three Major Nitrate Reservoirs in Mammals: Chemiluminescence and 15N-Tracer Studies in Yorkshire Pigs. Nutrients, 16(16), 2674. https://doi.org/10.3390/nu16162674





