Acute Dosing Strategy with Vistula Tart Cherries for Recovery of Strenuous Exercise—A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Damage Protocol
2.4. Supplementation
2.5. Functional Performance and Perceptual Variables
2.5.1. Maximal Voluntary Contraction (MVC)
2.5.2. Muscle Soreness
2.5.3. Limb Girth
2.5.4. Range of Motion (ROM)
2.6. Data Analysis
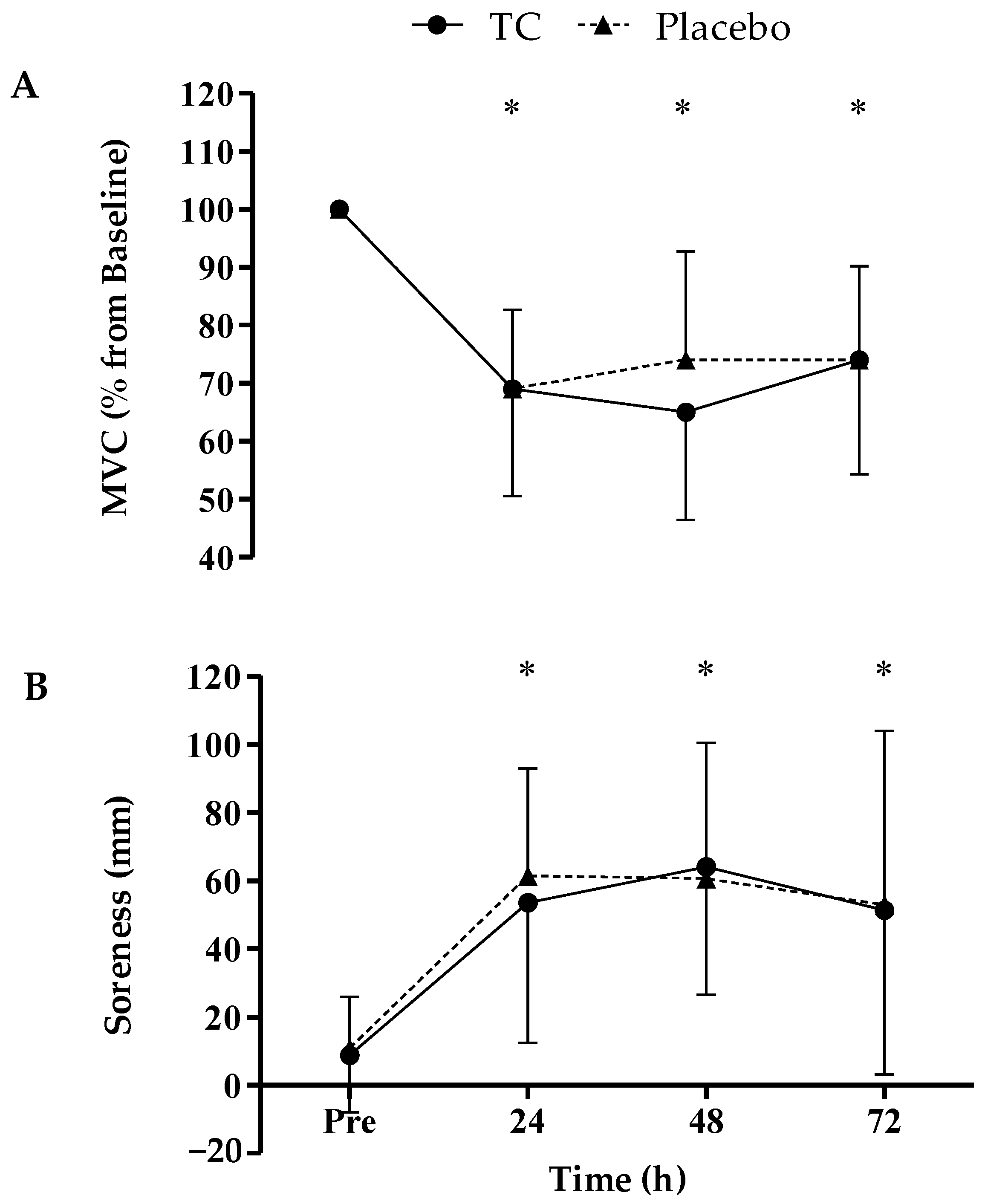
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fatouros, I.G.; Jamurtas, A.Z. Insights into the molecular etiology of exercise-induced inflammation: Opportunities for optimizing performance. J. Inflamm. Res. 2016, 9, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Sayers, S.P. Etiology of exercise-induced muscle damage. Can. J. Appl. Physiol. 1999, 24, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Garner, S.; Nelson, N.; Simper, T.N.; Hall, A.C.; Ranchordas, M.K. Effect of bilberry juice on indices of muscle damage and inflammation in runners completing a half-marathon: A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- McLeay, Y.; Barnes, M.J.; Mundel, T.; Hurst, S.M.; Hurst, R.D.; Stannard, S.R. Effect of New Zealand blueberry consumption on recovery from eccentric exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2012, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Kaltsatou, A.; Cicchella, A. Effect of cocoa products and its polyphenolic constituents on exercise performance and exercise-induced muscle damage and inflammation: A review of clinical trials. Nutrients 2019, 11, 1471. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.; Machado, Á.S.; Souza, M.A.; Mello-Carpes, P.B.; Carpes, F.P. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol. Behav. 2018, 194, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.L.; Ranchordas, M.K.; Johnson, E.; Denning, J.; Downing, F.; Lynn, A. No effect of tart cherry juice or pomegranate juice on recovery from exercise-induced muscle damage in non-resistance trained men. Nutrients 2019, 11, 1593. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Keane, K.M.; Quinlan, R.; Howatson, G. Tart Cherry Supplementation and Recovery From Strenuous Exercise: A Systematic Review and Meta-Analysis. Int. J. Sport. Nutr. Exerc. Metab. 2021, 31, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Ginsburg, I.; Kohen, R.; Koren, E. Microbial and host cells acquire enhanced oxidant-scavenging abilities by binding polyphenols. Arch. Biochem. Biophys. 2011, 506, 12–23. [Google Scholar] [CrossRef]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: Molecular mechanisms of action as anti-inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.A.J.; McHugh, M.P.; Padilla-Zakour, O.I. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef]
- Wangdi, J.T.; O’Leary, M.F.; Kelly, V.G.; Jackman, S.R.; Tang, J.C.; Dutton, J.; Bowtell, J.L. Tart cherry supplement enhances skeletal muscle glutathione peroxidase expression and functional recovery after muscle damage. Med. Sci. Sports Exerc. 2022, 54, 609–621. [Google Scholar] [CrossRef]
- Bowtell, J.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef]
- Quinlan, R.; Hill, J.A. The Efficacy of Tart Cherry Juice in Aiding Recovery After Intermittent Exercise. Int. J. Sports Physiol. Perform. 2019, 15, 368–374. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Levers, K.; Dalton, R.; Galvan, E.; O’Connor, A.; Goodenough, C.; Simbo, S.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; Riechman, S.; et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J. Int. Soc. Sports Nutr. 2016, 13, 22. [Google Scholar] [CrossRef]
- Levers, K.; Dalton, R.; Galvan, E.; Goodenough, C.; O’Connor, A.; Simbo, S.; Barringer, N.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; et al. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J. Int. Soc. Sports Nutr. 2015, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, K.S.; Perrier, E.T.; Elliot, D.L.; Chesnutt, J.C. Efficacy of tart cherry juice in reducing muscle pain during running: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2010, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.; Peeling, P.; Binnie, M.; Dawson, B.; Sim, M. Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players. J. Int. Soc. Sports Nutr. 2016, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Morehen, J.C.; Clarke, J.; Batsford, J.; Barrow, S.; Brown, A.D.; Stewart, C.E.; Morton, J.P.; Close, G.L. Montmorency tart cherry juice does not reduce markers of muscle soreness, function and inflammation following professional male rugby League match-play. Eur. J. Sport. Sci. 2021, 21, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Kupusarevic, J.; McShane, K.; Clifford, T. Cherry Gel Supplementation Does Not Attenuate Subjective Muscle Soreness or Alter Wellbeing Following a Match in a Team of Professional Rugby Union players: A Pilot Study. Sports 2019, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.; Brashill, C.; Brett, A.; Clifford, T. Tart Cherry Juice: No Effect on Muscle Function Loss or Muscle Soreness in Professional Soccer Players After a Match. Int. J. Sports Physiol. Perform. 2020, 15, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.M.; Wolforth, J.; McNish, R.; Kaufman, P.B.; Bolling, S.F. Tissue bioavailability of anthocyanins from whole tart cherry in healthy rats. Food Chem. 2015, 171, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2016, 55, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Gaze, D.C.; Davison, G.W.; George, T.W.; Scotter, M.J.; Howatson, G. Montmorency tart cherry (Prunus cerasus L.) concentrate lowers uric acid, independent of plasma cyanidin-3-O-glucosiderutinoside. J. Funct. Foods 2014, 11, 82–90. [Google Scholar] [CrossRef]
- Squires, E.; Walshe, I.H.; Cheung, W.; Bowerbank, S.L.; Dean, J.R.; Wood, J.; McHugh, M.P.; Plattner, S.; Howatson, G. Plasma-Induced Changes in the Metabolome Following Vistula Tart Cherry Consumption. Nutrients 2024, 16, 1023. [Google Scholar] [CrossRef]
- McKay, A.; Stellingwerff, T.; Smith, E.; Martin, D.; Mujika, I.; Goosey-Tolfrey, V.; Sheppard, J.; Burke, L. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2021, 17, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, N.P.; Mercer, T.H. The utility of isokinetic dynamometry in the assessment of human muscle function. Sports Med. 1996, 21, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Van Someren, K.; Hortobagyi, T. Repeated bout effect after maximal eccentric exercise. Int. J. Sports Med. 2007, 28, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Kastello, G.; Bretl, M.; Clark, E.; Delvaux, C.; Hoeppner, J.; McNea, L.; Strauss, J. The effect of cherry supplementation on Exercise induced oxidative stress. Int. J. Food Sci. Nutr. 2014, 1, 20–26. [Google Scholar] [CrossRef][Green Version]
- Howatson, G.; van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Baltzopoulos, V.; Williams, J.G.; Brodie, D.A. Sources of error in isokinetic dynamometry: Effects of visual feedback on maximum torque. J. Orthop. Sports Phys. Ther. 1991, 13, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Tokmakidis, S.P.; Kokkinidis, E.A.; Smilios, I.; Douda, H. The effects of ibuprofen on delayed muscle soreness and muscular performance after eccentric exercise. J. Strength Cond. Res. 2003, 17, 53–59. [Google Scholar] [PubMed]
- Hooper, D.R.; Orange, T.; Gruber, M.T.; Darakjian, A.A.; Conway, K.L.; Hausenblas, H. Broad spectrum polyphenol supplementation from tart cherry extract on markers of recovery from intense resistance exercise. J. Int. Soc. Sports Nutr. 2021, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Quero-García, J.; Iezzoni, A.; Pulawska, J.; Lang, G.A. Cherries: Botany, Production and Uses; CABI: Wallingford, UK, 2017. [Google Scholar]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and health: A review. Crit. Rev. Food Sci. Nutr. 2010, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Ramer Mikkelsen, U.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar] [PubMed]
- Seymour, E.M.; Warber, S.M.; Kirakosyan, A.; Noon, K.R.; Gillespie, B.; Uhley, V.E.; Wunder, J.; Urcuyo, D.E.; Kaufman, P.B.; Bolling, S.F. Anthocyanin pharmacokinetics and dose-dependent plasma antioxidant pharmacodynamics following whole tart cherry intake in healthy humans. J. Funct. Foods 2014, 11, 509–516. [Google Scholar] [CrossRef]
- Beals, K.; Allison, K.F.; Darnell, M.; Lovalekar, M.; Baker, R.; Nieman, D.C.; Vodovotz, Y.; Lephart, S.M. The effects of a tart cherry beverage on reducing exercise-induced muscle soreness. Isokinet. Exerc. Sci. 2017, 25, 53–63. [Google Scholar] [CrossRef]
- Nosaka, K.; Clarkson, P.M. Variability in serum creatine kinase response after eccentric exercise of the elbow flexors. Int. J. Sports Med. 1996, 17, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.C.; Hueglin, S.; Broad, E. Tart Cherry Juice in Athletes: A Literature Review and Commentary. Curr. Sports Med. Rep. 2017, 16, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Lanier, A.B. Use of Nonsteroidal Anti-Inflammatory Drugs Following Exercise-Induced Muscle Injury. Sports Med. 2003, 33, 177–186. [Google Scholar] [CrossRef] [PubMed]
| Group | Sex (n) | Age (Years) | Height (cm) | Mass (kg) |
|---|---|---|---|---|
| TC | Males (7) | 22 ± 1 | 178 ± 5 | 87 ± 20 |
| Females (5) | 23 ± 3 | 170 ± 5 | 68 ± 8 | |
| Placebo | Males (5) | 23 ± 3 | 181 ± 6 | 68 ± 14 |
| Females (5) | 26 ± 4 | 159 ± 7 | 60 ± 13 |
| Pre | 24 | 48 | 72 | ||
|---|---|---|---|---|---|
| Pain Pressure Threshold (N) * | TC | 35 ± 14 | 20 ± 8 | 19 ± 8 | 21 ± 7 |
| PLC | 28 ± 10 | 20 ± 7 | 18 ± 6 | 19 ± 7 | |
| 95% CI | 28.93–44.24 | 27.12–51.13 | 19.59–49.59 | −44.24–28.93 | |
| Limb Girth (mm) * | TC | 322 ± 58 | 326 ± 58 | 325 ± 56 | 326 ± 56 |
| PLC | 312 ± 44 | 316 ± 45 | 317 ± 44 | 316 ± 46 | |
| 95% CI | −0.59–−0.17 | −0.62–−0.10 | −0.68–−0.01 | 0.17–0.59 | |
| Range of Motion (%) * | TC | 100 ± 0 | 90 ± 4 | 88 ± 10 | 89 ± 11 |
| PLC | 100 ± 0 | 93 ± 3 | 91 ± 6 | 89 ± 12 | |
| 95% CI | 7.37–11.88 | 7.71–16.06 | 6.75–17.25 | −11.89–−7.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squires, E.; Walshe, I.H.; Dodd, A.; Broadbelt, E.; Hayman, O.; McHugh, M.P.; Howatson, G. Acute Dosing Strategy with Vistula Tart Cherries for Recovery of Strenuous Exercise—A Feasibility Study. Nutrients 2024, 16, 2709. https://doi.org/10.3390/nu16162709
Squires E, Walshe IH, Dodd A, Broadbelt E, Hayman O, McHugh MP, Howatson G. Acute Dosing Strategy with Vistula Tart Cherries for Recovery of Strenuous Exercise—A Feasibility Study. Nutrients. 2024; 16(16):2709. https://doi.org/10.3390/nu16162709
Chicago/Turabian StyleSquires, Emma, Ian H. Walshe, Alex Dodd, Edward Broadbelt, Oliver Hayman, Malachy P. McHugh, and Glyn Howatson. 2024. "Acute Dosing Strategy with Vistula Tart Cherries for Recovery of Strenuous Exercise—A Feasibility Study" Nutrients 16, no. 16: 2709. https://doi.org/10.3390/nu16162709
APA StyleSquires, E., Walshe, I. H., Dodd, A., Broadbelt, E., Hayman, O., McHugh, M. P., & Howatson, G. (2024). Acute Dosing Strategy with Vistula Tart Cherries for Recovery of Strenuous Exercise—A Feasibility Study. Nutrients, 16(16), 2709. https://doi.org/10.3390/nu16162709







