Beneficial Effects of Ginger Extract on Eye Fatigue and Shoulder Stiffness: A Randomized, Double-Blind, and Placebo-Controlled Parallel Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Subjects
2.3. Study Design
2.4. Ocular Blood Flow
2.5. Peripheral Blood Flow
2.6. Visual Analog Scales (VAS)
2.7. Shoulder Muscle Stiffness
2.8. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Ginger Enhances Peripheral Blood Flow Velocity
3.3. Ginger Alleviates Eye Fatigue and Shoulder Stiffness
3.4. Safety of Ginger
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M.U.R. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar] [CrossRef]
- Li, F.; Nitteranon, V.; Tang, X.; Liang, J.; Zhang, G.; Parkin, K.L.; Hu, Q. In vitro antioxidant and anti-inflammatory activities of 1-dehydro-[6]-gingerdione, 6-shogaol, 6-dehydroshogaol and hexahydrocurcumin. Food Chem. 2012, 135, 332–337. [Google Scholar] [CrossRef]
- Danwilai, K.; Konmun, J.; Sripanidkulchai, B.-O.; Subongkot, S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: A pilot study. Cancer Manag. Res. 2017, 9, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Luo, D.; Ma, Y.; Zhang, J.; Li, M.; Yao, L.; Shi, X.; Liu, X.; Yang, K. Ginger for health care: An overview of systematic reviews. Complement. Ther. Med. 2019, 45, 114–123. [Google Scholar] [CrossRef]
- Borrelli, F.; Capasso, R.; Aviello, G.; Pittler, M.H.; Izzo, A.A. Effectiveness and Safety of Ginger in the Treatment of Pregnancy-Induced Nausea and Vomiting. Obstet. Gynecol. 2005, 105, 849–856. [Google Scholar] [CrossRef]
- Ding, M.; Leach, M.; Bradley, H. The effectiveness and safety of ginger for pregnancy-induced nausea and vomiting: A systematic review. Women Birth 2013, 26, e26–e30. [Google Scholar] [CrossRef] [PubMed]
- Stanisiere, J.; Mousset, P.-Y.; Lafay, S. How Safe Is Ginger Rhizome for Decreasing Nausea and Vomiting in Women during Early Pregnancy? Foods 2018, 7, 50. [Google Scholar] [CrossRef]
- Thomson, M.; Corbin, R.; Leung, L. Effects of Ginger for Nausea and Vomiting in Early Pregnancy: A Meta-Analysis. J. Am. Board Fam. Med. 2014, 27, 115–122. [Google Scholar] [CrossRef]
- Viljoen, E.; Visser, J.; Koen, N.; Musekiwa, A. A systematic review and meta-analysis of the effect and safety of ginger in the treatment of pregnancy-associated nausea and vomiting. Nutr. J. 2014, 13, 20. [Google Scholar] [CrossRef]
- Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients 2020, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Attari, V.E.; Mahdavi, A.M.; Javadivala, Z.; Mahluji, S.; Vahed, S.Z.; Ostadrahimi, A. A systematic review of the anti-obesity and weight lowering effect of ginger (Zingiber officinale Roscoe) and its mechanisms of action. Phytother. Res. 2018, 32, 577–585. [Google Scholar] [CrossRef]
- Maharlouei, N.; Tabrizi, R.; Lankarani, K.B.; Rezaianzadeh, A.; Akbari, M.; Kolahdooz, F.; Rahimi, M.; Keneshlou, F.; Asemi, Z. The effects of ginger intake on weight loss and metabolic profiles among overweight and obese subjects: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Gao, H.-K.; Rezaie, P.; Ferns, G.A. The effect of ginger supplementation on serum C-reactive protein, lipid profile and glycaemia: A systematic review and meta-analysis. Food Nutr. Res. 2016, 60, 32613. [Google Scholar] [CrossRef]
- Pourmasoumi, M.; Hadi, A.; Rafie, N.; Najafgholizadeh, A.; Mohammadi, H.; Rouhani, M.H. The effect of ginger supplementation on lipid profile: A systematic review and meta-analysis of clinical trials. Phytomedicine 2018, 43, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, H.; Song, Z.; Wang, X.; Sun, Z. Effects of Ginger (Zingiber officinale Roscoe) on Type 2 Diabetes Mellitus and Components of the Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid.-Based Complement. Altern. Med. 2018, 2018, 5692962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Zhang, X.; Feng, Q.; Guo, X.; Li, S.; Li, R.; Chu, D.; Ma, Y. Evaluation of daily ginger consumption for the prevention of chronic diseases in adults: A cross-sectional study. Nutrition 2017, 36, 79–84. [Google Scholar] [CrossRef]
- Hasani, H.; Arab, A.; Hadi, A.; Pourmasoumi, M.; Ghavami, A.; Miraghajani, M. Does ginger supplementation lower blood pressure? A systematic review and meta-analysis of clinical trials. Phytother. Res. 2019, 33, 1639–1647. [Google Scholar] [CrossRef]
- Chen, C.X.; Barrett, B.; Kwekkeboom, K. Efficacy of oral ginger (Zingiber officinale) for dysmenorrhea: A systematic review and meta-analysis. Evid. Based Compl. Altern. Med. 2016, 2016, 6295737. [Google Scholar] [CrossRef]
- Daily, J.W.; Zhang, X.; Kim, D.S.; Park, S. Efficacy of Ginger for Alleviating the Symptoms of Primary Dysmenorrhea: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Pain Med. 2015, 16, 2243–2255. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Ford, C.T.; Tepper, D. Zingiberaceae extracts for pain: A systematic review and meta-analysis. Nutr. J. 2015, 14, 50. [Google Scholar] [CrossRef]
- Terry, R.; Posadzki, P.; Watson, L.K.; Ernst, E. The Use of Ginger (Zingiber officinale) for the Treatment of Pain: A Systematic Review of Clinical Trials. Pain Med. 2011, 12, 1808–1818. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.B. Ginger (Zingiber officinale) as an Analgesic and Ergogenic Aid in Sport: A Systemic Review. J. Strength Cond. Res. 2015, 29, 2980–2995. [Google Scholar] [CrossRef]
- Fjærvoll, H.; Fjærvoll, K.; Magno, M.; Moschowits, E.; Vehof, J.; Dartt, D.A.; Utheim, T.P. The association between visual display terminal use and dry eye: A review. Acta Ophthalmol. 2022, 100, 357–375. [Google Scholar] [CrossRef]
- Kawashima, M.; Yamatsuji, M.; Yokoi, N.; Fukui, M.; Ichihashi, Y.; Kato, H.; Nishida, M.; Uchino, M.; Kinoshita, S.; Tsubota, K. Screening of dry eye disease in visual display terminal workers during occupational health examinations: The Moriguchi study. J. Occup. Health 2015, 57, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Miljanović, B.; Dana, R.; Sullivan, D.A.; Schaumberg, D.A. Impact of Dry Eye Syndrome on Vision-Related Quality of Life. Am. J. Ophthalmol. 2007, 143, 409–415. [Google Scholar] [CrossRef]
- Zeitz, O.; Galambos, P.; Wagenfeld, L.; Wiermann, A.; Wlodarsch, P.; Praga, R.; Matthiessen, E.T.; Richard, G.; Klemm, M. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br. J. Ophthalmol. 2006, 90, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Shiga, Y.; Aizawa, N.; Tsuda, S.; Yokoyama, Y.; Omodaka, K.; Kunikata, H.; Yasui, T.; Kato, K.; Kurashima, H.; Miyamoto, E.; et al. Preperimetric Glaucoma Prospective Study (PPGPS): Predicting Visual Field Progression with Basal Optic Nerve Head Blood Flow in Normotensive PPG Eyes. Transl. Vis. Sci. Technol. 2018, 7, 11. [Google Scholar] [CrossRef]
- Shimada, N.; Ohno-Matsui, K.; Harino, S.; Yoshida, T.; Yasuzumi, K.; Kojima, A.; Kobayashi, K.; Futagami, S.; Tokoro, T.; Mochizuki, M. Reduction of retinal blood flow in high myopia. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K.; Kraemer, R.; Lichtenberg, A.; Jagodzinski, M.; Gosling, T.; Richter, M.; Krettek, C. Microcirculation of the Ankle after Cryo/Cuff Application in Healthy Volunteers. Int. J. Sports Med. 2006, 27, 250–255. [Google Scholar] [CrossRef]
- Li, H.; Yoshizaki, T.; Liang, L.; Iwahashi, M.; Kawahara, A.; Shirai, A.; Arimitsu, J.; Ito, M.; Tsumura, N.; Ogawa-Ochiai, K. Assessing the effects of Kampo medicine on human skin texture and microcirculation. Artif. Life Robot. 2022, 27, 64–69. [Google Scholar] [CrossRef]
- Wu, H.; Horng, C.; Tsai, S.; Lee, Y.; Hsu, S.; Tsai, Y.; Tsai, F.; Chiang, J.; Kuo, D.; Yang, J. Relaxant and vasoprotective effects of ginger extracts on porcine coronary arteries. Int. J. Mol. Med. 2018, 41, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.N.; Raya, I.; Yasmin, R.; Dirpan, A.; Arsyad, A.; Permatasari, A.E.; Sumidarti, A.; Umami, N. Ginger honey affects cortisol, estrogen and glutathione levels; preliminary study to target preconceptional women. Gac. Sanit. 2021, 35, S251–S253. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.N.; Duckles, S.P.; Pelligrino, D.A. Influence of sex steroid hormones on cerebrovascular function. J. Appl. Physiol. 2006, 101, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Van Buren, G.A.; Yang, D.-S.; Clark, K.E. Estrogen-induced uterine vasodilatation is antagonized by L-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am. J. Obstet. Gynecol. 1992, 167, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Weiner, C.P.; Lizasoain, I.; A Baylis, S.; Knowles, R.G.; Charles, I.G.; Moncada, S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc. Natl. Acad. Sci. USA 1994, 91, 5212–5216. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Butterworth, J.; Malecaze, F.; Calvas, P. Axial Length of Myopia: A Review of Current Research. Ophthalmologica 2011, 225, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.W.; de Vries, M.M.; Montolio, F.G.J.; Jansonius, N.M. Myopia as a Risk Factor for Open-Angle Glaucoma: A Systematic Review and Meta-Analysis. Ophthalmology 2011, 118, 1989–1994.e2. [Google Scholar] [CrossRef]
- Lee, S.S.-Y.; Lingham, G.; Sanfilippo, P.G.; Hammond, C.J.; Saw, S.-M.; Guggenheim, J.A.; Yazar, S.; Mackey, D.A. Incidence and Progression of Myopia in Early Adulthood. JAMA Ophthalmol. 2022, 140, 162–169. [Google Scholar] [CrossRef] [PubMed]
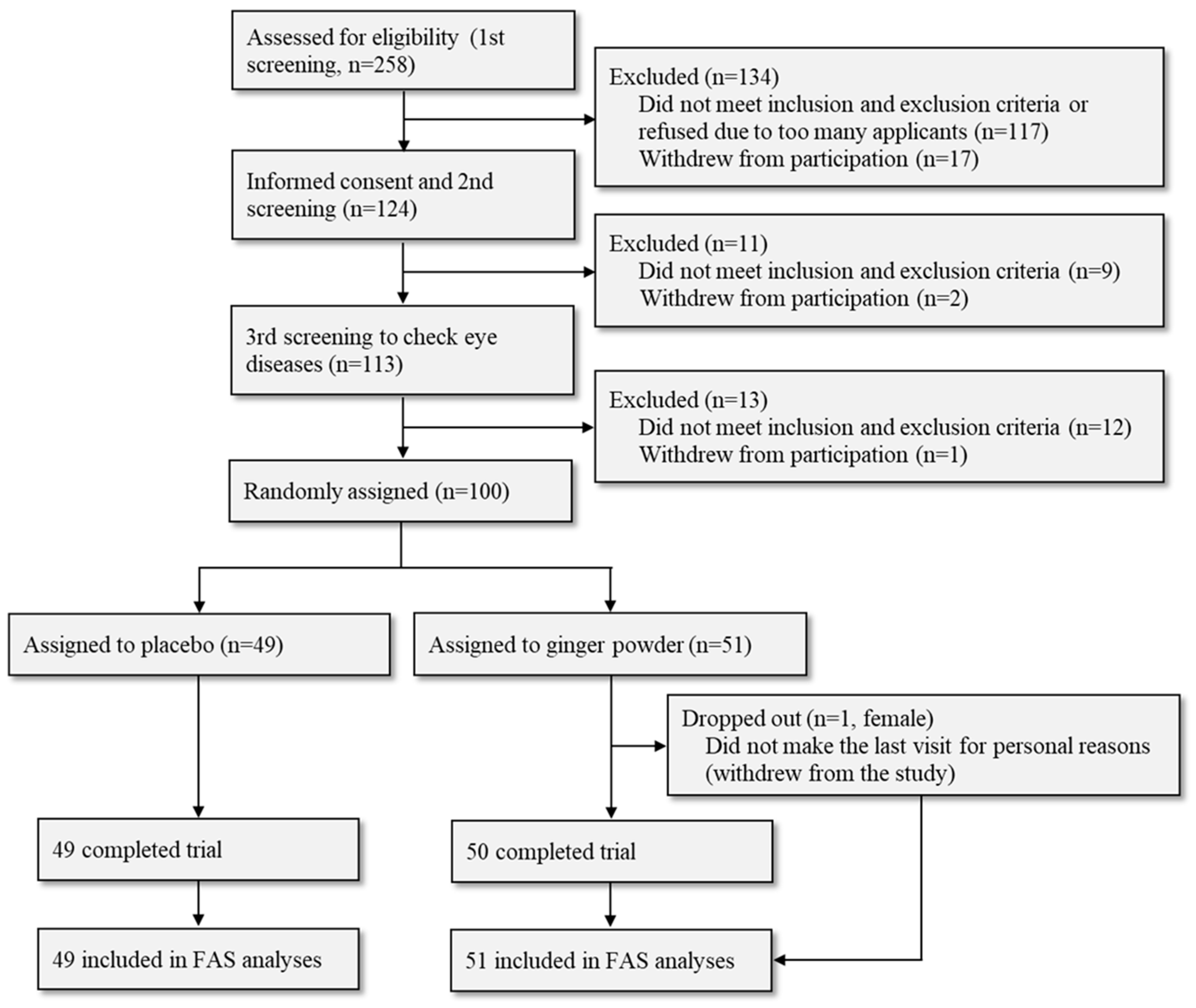

| Placebo (n = 49) | Ginger (n = 51) | p-Value | |
|---|---|---|---|
| Age (years) | 51.1 ± 12.0 | 52.0 ± 10.2 | 0.694 |
| Male/Female | 12/37 | 13/38 | - |
| Height (cm) | 159.7 ± 7.8 | 159.5 ± 6.8 | 0.902 |
| Body weight (kg) | 55.3 ± 11.9 | 56.6 ± 11.6 | 0.626 |
| BMI (kg/m2) | 21.5 ± 3.3 | 22.1 ± 3.8 | 0.429 |
| Body fat percentage (%) | 24.8 ± 7.5 | 26.1 ± 8.1 | 0.482 |
| Systolic blood pressure (mmHg) | 109.9 ± 18.0 | 112.4 ± 15.2 | 0.673 |
| Diastolic blood pressure (mmHg) | 66.8 ± 12.9 | 68.3 ± 10.5 | 0.902 |
| Heart rate (beats per min) | 68.8 ± 12.5 | 69.7 ± 9.0 | 0.469 |
| White blood cell count (×103/mL) | 5.23 ± 1.55 | 5.45 ± 1.19 | 0.438 |
| Red blood cell count (×106/mL) | 4.57 ± 0.43 | 4.64 ± 0.45 | 0.480 |
| Hemoglobin (g/dL) | 13.6 ± 1.4 | 14.0 ± 1.3 | 0.102 |
| Hematocrit (%) | 42.3 ± 3.9 | 43.4 ± 3.7 | 0.151 |
| Platelet count (×104/mL) | 24.6 ± 6.3 | 25.4 ± 6.0 | 0.532 |
| AST (IU/L) | 21.3 ± 5.0 | 21.0 ± 6.0 | 0.797 |
| ALT (IU/L) | 18.9 ± 9.2 | 19.6 ± 12.9 | 0.746 |
| γ-GTP (IU/L) | 21.4 ± 8.7 | 28.2 ± 33.8 | 0.176 |
| LDH(IU/L) | 188 ± 26 | 188 ± 24 | 0.968 |
| Choline esterase (IU/L) | 316 ± 68 | 345 ± 70 * | 0.041 |
| Alkaline phosphatase (IU/L) | 65.9 ± 19.1 | 67.5 ± 16.9 | 0.676 |
| Amylase (IU/L) | 80.1 ± 28.9 | 96.9 ± 92.1 | 0.227 |
| Total protein (g/dL) | 7.44 ± 0.45 | 7.41 ± 0.37 | 0.708 |
| Total bilirubin (mg/dL) | 0.79 ± 0.28 | 0.73 ± 0.22 | 0.233 |
| Albumin (g/dL) | 4.57 ± 0.28 | 4.53 ± 0.25 | 0.473 |
| Uric acid (mg/dL) | 4.69 ± 1.10 | 4.89 ± 1.39 | 0.436 |
| Blood urea nitrogen (mg/dL) | 13.5 ± 3.3 | 13.2 ± 4.4 | 0.720 |
| Creatinine (mg/dL) | 0.691 ± 0.131 | 0.690 ± 0.165 | 0.968 |
| eGFR (mL/min/1.73 m2) | 98.9 ± 21.0 | 99.5 ± 21.9 | 0.886 |
| Total cholesterol (mg/dL) | 228 ± 42 | 232 ± 40 | 0.613 |
| LDL cholesterol (mg/dL) | 136 ± 35 | 141 ± 33 | 0.523 |
| HDL cholesterol (mg/dL) | 69.4 ± 20.4 | 70.8 ± 17.2 | 0.728 |
| LDL/HDL ratio | 2.13 ± 0.82 | 2.10 ± 0.65 | 0.846 |
| Triglycerides (mg/dL) | 110.9 ± 97.9 | 107.9 ± 63.6 | 0.856 |
| Fasting blood glucose (mg/dL) | 97.3 ± 8.7 | 95.5 ± 9.0 | 0.327 |
| Placebo | Ginger | p-Value | |
|---|---|---|---|
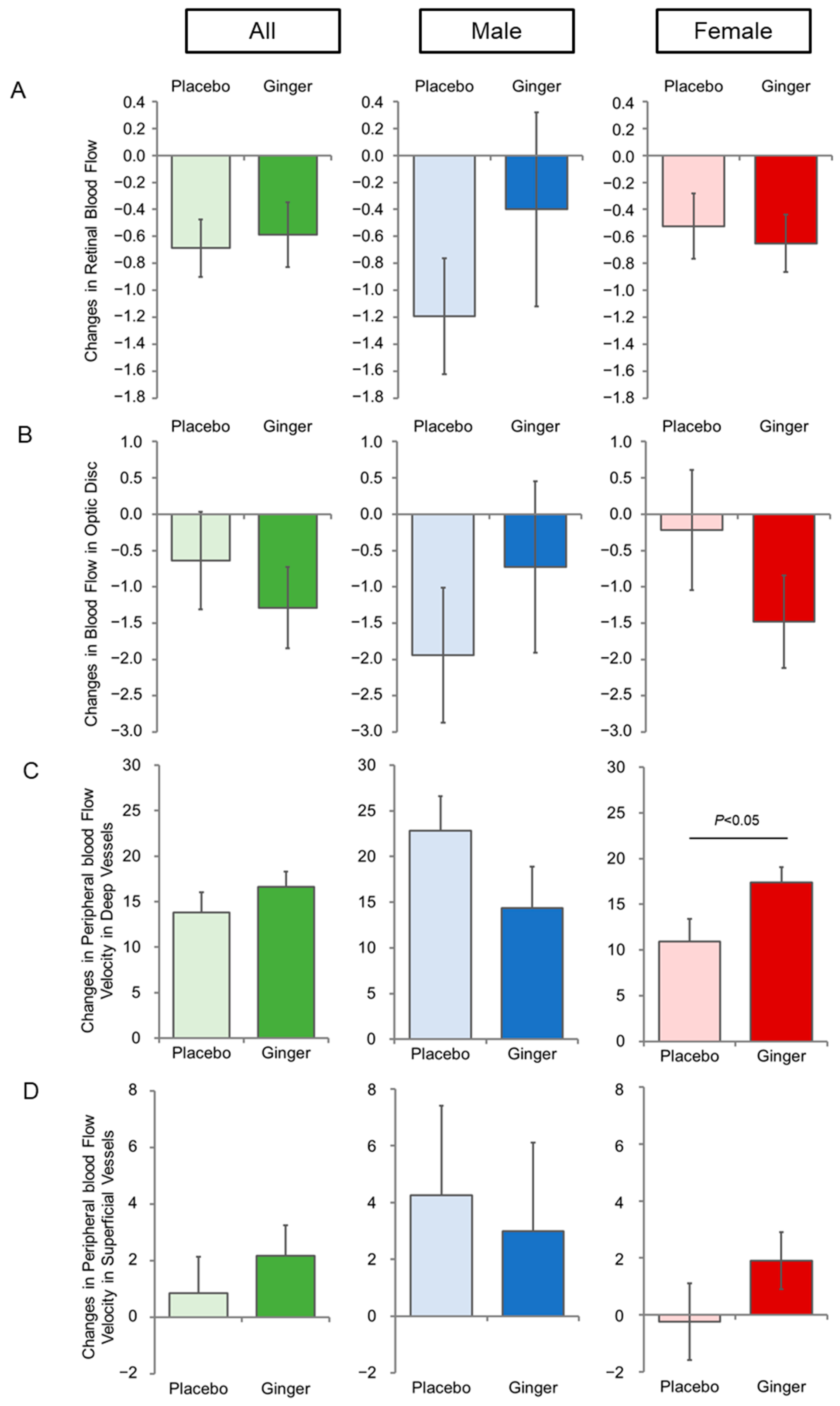
| Retinal blood flow | |||
| All (n = 49, 51) | 10.4 ± 3.9 | 9.4 ± 3.3 | 0.143 |
| Male (n = 12, 13) | 10.1 ± 3.0 | 9.5 ± 3.8 | 0.662 |
| Female (n = 37, 38) | 10.6 ± 4.2 | 9.4 ± 3.2 | 0.160 |
| Blood flow in optic disc | |||
| All (n = 49, 51) | 34.7 ± 6.3 | 34.1 ± 6.3 | 0.588 |
| Male (n = 12, 13) | 30.6 ± 4.8 | 31.1 ± 5.8 | 0.819 |
| Female (n = 37, 38) | 36.1 ± 6.2 | 35.1 ± 6.2 | 0.480 |
| Peripheral blood Flow Velocity in Deep Vessels | |||
| All (n = 49, 51) | 38.9 ± 15.1 | 36.6 ± 13.4 | 0.438 |
| Male (n = 12, 13) | 31.8 ± 13.5 | 38.0 ± 17.0 | 0.322 |
| Female (n = 37, 38) | 41.2 ± 15.1 | 36.2 ± 12.2 | 0.118 |
| Peripheral blood Flow Velocity in Superficial Vessels | |||
| All (n = 49, 51) | 33.3 ± 7.7 | 31.5 ± 7.7 | 0.226 |
| Male (n = 12, 13) | 30.3 ± 7.8 | 30.6 ± 9.3 | 0.917 |
| Female (n = 37, 38) | 34.4 ± 7.5 | 31.8 ± 7.2 | 0.131 |
| VAS of Eye Fatigue (mm) | |||
| All (n = 49, 51) | 57.8 ± 17.7 | 64.7 ± 14.4 * | 0.035 |
| Male (n = 12, 13) | 58.9 ± 19.3 | 66.1 ± 16.5 | 0.328 |
| Female (n = 37, 38) | 57.4 ± 17.5 | 64.2 ± 13.8 | 0.065 |
| VAS of Shoulder Stiffness (mm) | |||
| All (n = 49, 51) | 65.6 ± 20.2 | 68.6 ± 17.2 | 0.429 |
| Male (n = 12, 13) | 63.5 ± 24.2 | 67.0 ± 16.2 | 0.680 |
| Female (n = 37, 38) | 66.3 ± 19.1 | 69.2 ± 17.8 | 0.502 |
| VAS of Body Warmth (mm) | |||
| All (n = 49, 51) | 52.4 ± 14.2 | 53 ± 16.5 | 0.834 |
| Male (n = 12, 13) | 54.5 ± 12.2 | 14.4 ± 4.2 | 0.999 |
| Female (n = 37, 38) | 51.7 ± 14.8 | 53 ± 17.4 | 0.740 |
| Shoulder Muscle Stiffness | |||
| All (n = 49, 51) | 32.1 ± 4.8 | 32.4 ± 6.4 | 0.788 |
| Male (n = 12, 13) | 29.9 ± 2.6 | 29.1 ± 5.4 | 0.647 |
| Female (n = 37, 38) | 32.8 ± 5.1 | 33.5 ± 6.3 | 0.591 |
| Male | Female | |||
|---|---|---|---|---|
| ≥51 Years Old | <51 Years Old | ≥51 Years Old | <51 Years Old | |
| (n; Placebo, Ginger) | (n = 9, 9) | (n = 3, 4) | (n = 21, 20) | (n = 16, 18) |
| Retinal blood flow | −0.0 (−2.4, 2.4) | 3.0 (1.5, 4.5) ** | −0.2 (−1.1, 0.7) | −0.0 (−0.9, 0.8) |
| Blood flow in optic disc | 0.7 (−3.2, 4.6) | 2.4 (−1.9, 6.6) | −0.9 (−3.9, 2.1) | −1.7 (−4.5, 1.0) |
| Peripheral blood Flow Velocity in Deep Vessels | −12.4 (−27.9, 3.1) | 2.0 (−9.9, 13.9) | 2.8 (−5.2, 10.9) | 10.9 (2.4, 19.5) * |
| Peripheral blood Flow Velocity in Superficial Vessels | −0.3 (−12.1, 11.5) | −3.1 (−12.1, 5.9) | 4.3 (0.5, 8.1) * | −0.3 (−5.9, 5.4) |
| VAS of Eye Fatigue (mm) | −7.4 (−27.2, 12.4) | −4.6 (−31.4, 22.2) | −1.2 (−13.8, 11.3) | −16.8 (−30.9, −2.6) * |
| VAS of Shoulder Stiffness (mm) | −5.5 (−28.5, 17.5) | 6.6 (−18.3, 31.6) | −5.8 (−19.2, 7.7) | −12.9 (−24.2, −1.7) * |
| VAS of Body Warmth (mm) | 3.6 (−15.4, 22.5) | 33.3 (9.6, 57.1) * | 4.7 (−8.2, 17.7) | 1.5 (−14.0, 17.0) |
| Shoulder Muscle Stiffness | −1.6 (−8.3, 5.1) | −3.3 (−6.7, 0.1) | 0.8 (−3.4, 5.0) | −1.3 (−4.8, 2.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashikawa, F.; Nakaniida, Y.; Li, H.; Liang, L.; Kanno, K.; Ogawa-Ochiai, K.; Kiuchi, Y. Beneficial Effects of Ginger Extract on Eye Fatigue and Shoulder Stiffness: A Randomized, Double-Blind, and Placebo-Controlled Parallel Study. Nutrients 2024, 16, 2715. https://doi.org/10.3390/nu16162715
Higashikawa F, Nakaniida Y, Li H, Liang L, Kanno K, Ogawa-Ochiai K, Kiuchi Y. Beneficial Effects of Ginger Extract on Eye Fatigue and Shoulder Stiffness: A Randomized, Double-Blind, and Placebo-Controlled Parallel Study. Nutrients. 2024; 16(16):2715. https://doi.org/10.3390/nu16162715
Chicago/Turabian StyleHigashikawa, Fumiko, Yuta Nakaniida, Hongyang Li, Lian Liang, Keishi Kanno, Keiko Ogawa-Ochiai, and Yoshiaki Kiuchi. 2024. "Beneficial Effects of Ginger Extract on Eye Fatigue and Shoulder Stiffness: A Randomized, Double-Blind, and Placebo-Controlled Parallel Study" Nutrients 16, no. 16: 2715. https://doi.org/10.3390/nu16162715
APA StyleHigashikawa, F., Nakaniida, Y., Li, H., Liang, L., Kanno, K., Ogawa-Ochiai, K., & Kiuchi, Y. (2024). Beneficial Effects of Ginger Extract on Eye Fatigue and Shoulder Stiffness: A Randomized, Double-Blind, and Placebo-Controlled Parallel Study. Nutrients, 16(16), 2715. https://doi.org/10.3390/nu16162715








