Anticancer Effects of Secoiridoids—A Scoping Review of the Molecular Mechanisms behind the Chemopreventive Effects of the Olive Tree Components Oleocanthal, Oleacein, and Oleuropein
Abstract
1. Introduction
1.1. Global Burden of Cancer
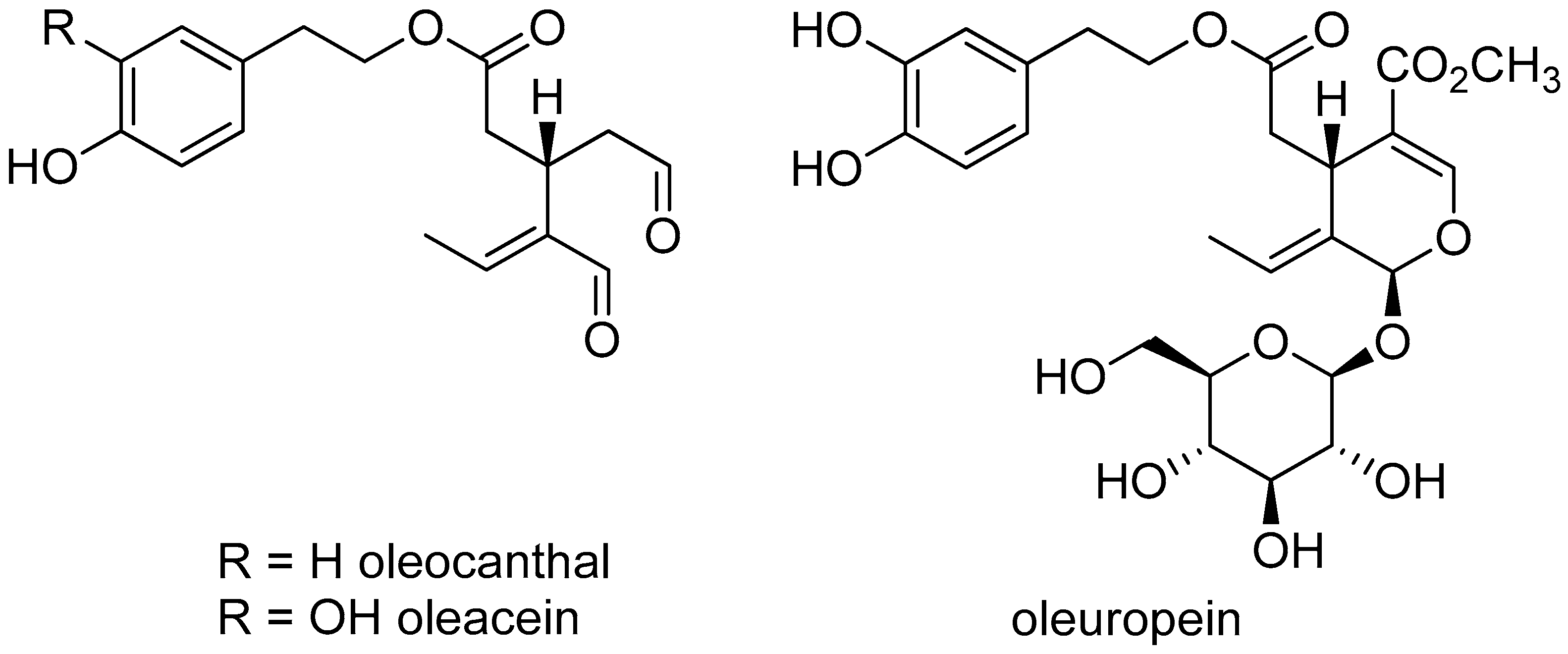
1.2. The History and Compounds of Olive Oil
1.3. Epidemiological Data about the Anti-Cancer Effects of Olive Oil Consumption
1.4. The Relevance of Secoiridoids
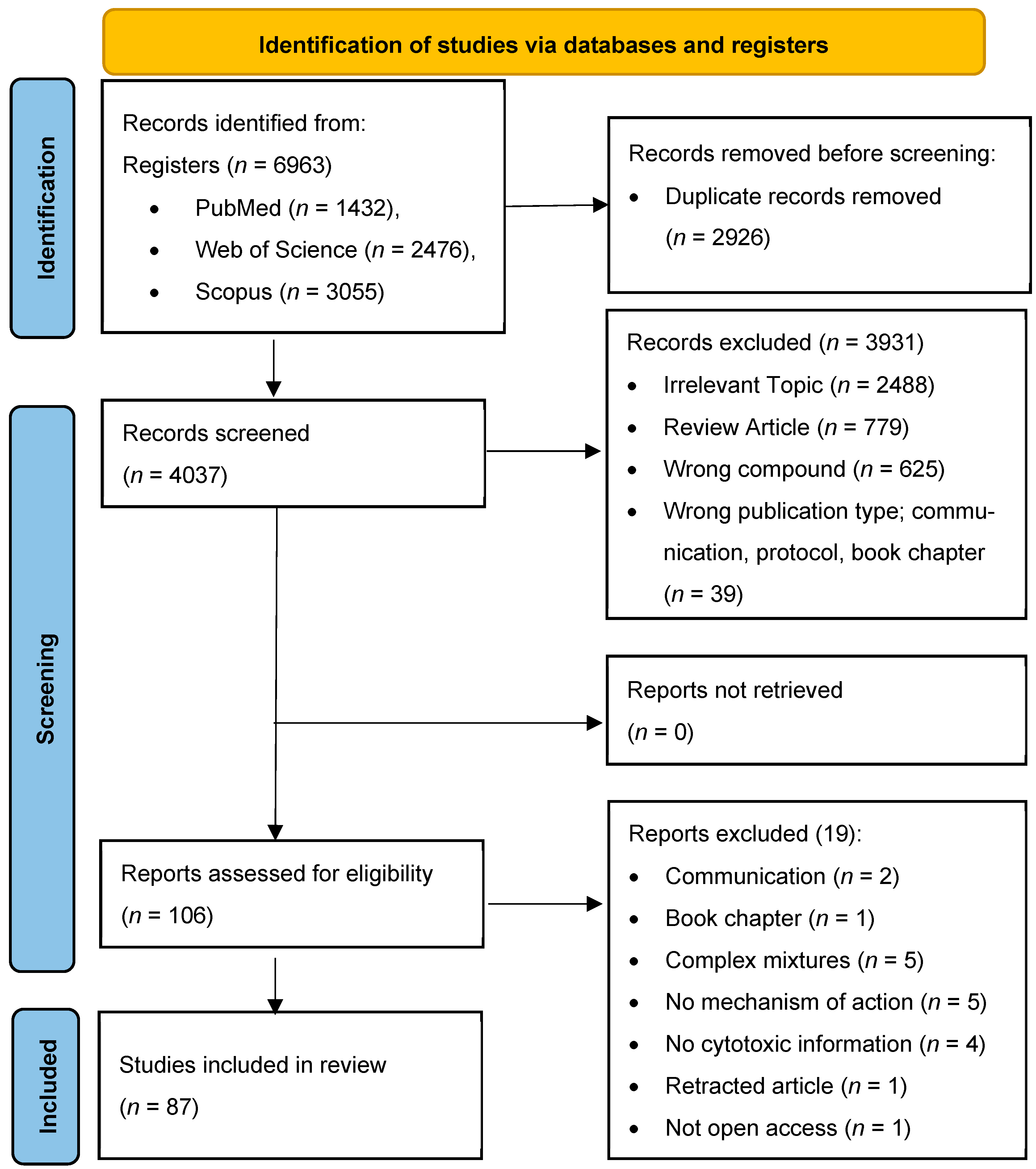
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
3. Results
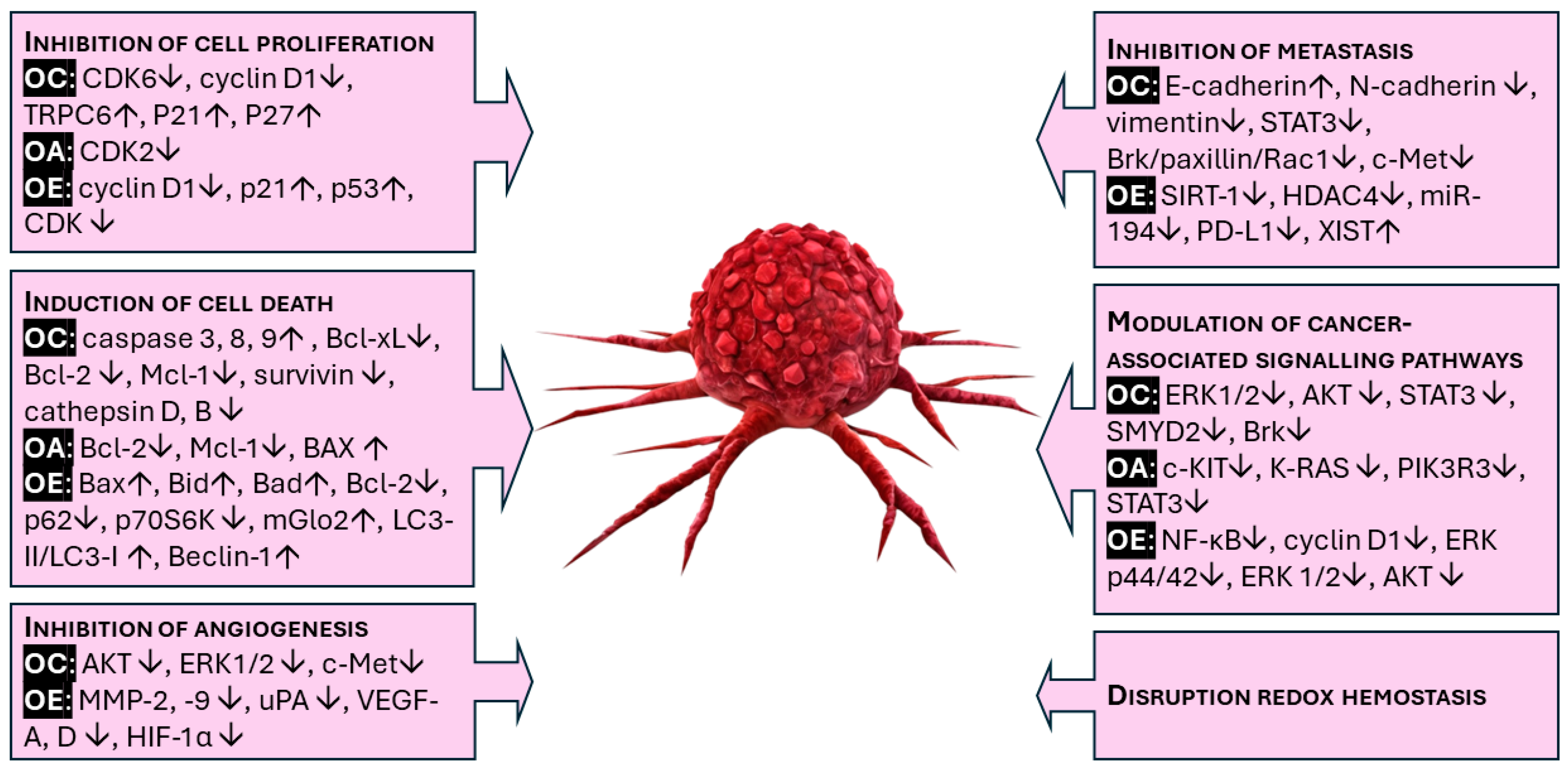
3.1. Chemopreventive Mechanism of Oleocanthal, Oleuropein, and Oleacein
3.1.1. Inhibition of Cell Proliferation (Cell Cycle Arrest)
- Oleocanthal
- b.
- Oleacein
- c.
- Oleuropein
3.1.2. Induction of Cell Death (Apoptosis, Autophagy, and Necrosis)
- Oleocanthal
- b.
- Oleacein
- c.
- Oleuropein
3.1.3. Inhibition of Angiogenesis
- Oleocanthal
- b.
- Oleacein
- c.
- Oleuropein
3.1.4. Inhibition of Metastasis
- Oleocanthal
- b.
- Oleacein
- c.
- Oleuropein
3.1.5. Modulation of Cancer-Associated Signaling Pathways
- Oleocanthal
- b.
- Oleacein
- c.
- Oleuropein
3.1.6. Disruption of Redox Hemostasis and Endoplasmic Reticulum Stress
- Oleocanthal
- b.
- Oleuropein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Cancer Burden Growing, Amidst Mounting Need for Services; WHO: Lyon, France; Geneva, Switzerland, 2024; Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 10 May 2024).
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef]
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar]
- Fabiani, R. Anti-Cancer Properties of Olive Oil Secoiridoid Phenols: A Systematic Review of in Vivo Studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef]
- Emma, M.R.; Augello, G.; Stefano, V.; Azzolina, A.; Giannitrapani, L.; Montalto, G.; Cervello, M.; Cusimano, A. Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 1234. [Google Scholar] [CrossRef]
- Quiles, J.L.; Ramírez-Tortosa, M.C.; Yaqoob, P. Olive Oil and Health; CABI Publishing: Wallingford, UK, 2006. [Google Scholar]
- Albini, A.; Albini, F.; Corradino, P.; Dugo, L.; Calabrone, L.; Noonan, D.M. From Antiquity to Contemporary Times: How Olive Oil by-Products and Waste Water Can Contribute to Health. Front. Nutr. 2023, 10, 1254947. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The Diet and 15-Year Death Rate in the Seven Countries Study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.; Forastiere, F.; Farchi, S.; Mallone, S.; Trequattrinni, T.; Anatra, F.; Schmid, G.; Perucci, C.A. The Protective Effect of the Mediterranean Diet on Lung Cancer. Nutr. Cancer 2003, 46, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Bosetti, C.; Negri, E.; Lipworth, L.; La Vecchia, C. Olive Oil and Cancer Risk: An Update of Epidemiological Findings through 2010. Curr. Pharm. Des. 2011, 17, 805–812. [Google Scholar] [CrossRef]
- Bosetti, C.; La Vecchia, C.; Talamini, R.; Negri, E.; Levi, F.; Maso, L.D.; Franceschi, S. Food Groups and Laryngeal Cancer Risk: A Case-control Study from Italy and Switzerland. Int. J. Cancer 2002, 100, 355–360. [Google Scholar] [CrossRef]
- Markellos, C.; Ourailidou, M.-E.; Gavriatopoulou, M.; Halvatsiotis, P.; Sergentanis, T.N.; Psaltopoulou, T. Olive Oil Intake and Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0261649. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, M.T.; Buntinx, F.; Kellen, E.; Dongen, M.C.; Dagnelie, P.C.; Muls, E.; Zeegers, M.P. Consumption of Animal Products, Olive Oil and Dietary Fat and Results from the Belgian Case–Control Study on Bladder Cancer Risk. Eur. J. Cancer 2011, 47, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, J.P.; Villalobos, M.A.; Carmona, J.A.; Martín-Romero, M.; Smith-Agreda, J.M.; de la Cuesta, F.S. Antithrombotic Potential of Olive Oil Administration in Rabbits with Elevated Cholesterol. Thromb. Res. 2000, 100, 305–315. [Google Scholar] [CrossRef]
- Larsen, L.F.; Jespersen, J.; Marckmann, P. Are Olive Oil Diets Antithrombotic? Diets Enriched with Olive, Rapeseed, or Sunflower Oil Affect Postprandial Factor VII Differently. Am. J. Clin. Nutr. 1999, 70, 976–982. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and Hydrolyzable Compounds in Virgin Olive Oil. 3. Spectroscopic Characterizations of the Secoiridoid Derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q. Phytochemistry: Ibuprofen-like Activity in Extra-Virgin Olive Oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Adsersen, A.; Christensen, S.B.; Jensen, S.R.; Nyman, U.; Smitt, U.W. Isolation of an Angiotensin Converting Enzyme (ACE) Inhibitor from Olea Europaea and Olea Lancea. Phytomedicine 1996, 2, 319–325. [Google Scholar] [CrossRef]
- Ruiz-García, I.; Ortíz-Flores, R.; Badía, R.; García-Borrego, A.; García-Fernández, M.; Lara, E.; Martín-Montañez, E.; García-Serrano, S.; Valdés, S.; Gonzalo, M.; et al. Rich Oleocanthal and Oleacein Extra Virgin Olive Oil and Inflammatory and Antioxidant Status in People with Obesity and Prediabetes. The APRIL Study: A Randomised, Controlled Crossover Study. Clin. Nutr. 2023, 42, 1389–1398. [Google Scholar] [CrossRef]
- HMPC. Assessment Report on Olea Europaea L., Folium; European Medicines Agency: London, UK, 2016. [Google Scholar]
- Lozano-Castellón, J.; López-Yerena, A.; Rinaldi De Alvarenga, J.F.; Romero Del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Health-Promoting Properties of Oleocanthal and Oleacein: Two Secoiridoids from Extra-Virgin Olive Oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 2532–2548. [Google Scholar] [CrossRef]
- Szabo, L.; Molnar, R.; Tomesz, A.; Deutsch, A.; Darago, R.; Varjas, T.; Ritter, Z.; Szentpeteri, J.L.; Andreidesz, K.; Mathe, D.; et al. Olive Oil Improves While Trans Fatty Acids Further Aggravate the Hypomethylation of LINE-1 Retrotransposon DNA in an Environmental Carcinogen Model. Nutrients 2022, 14, 908. [Google Scholar] [CrossRef] [PubMed]
- Goren, L.; Zhang, G.; Kaushik, S.; Breslin, P.A.S.; Du, Y.-C.N.; Foster, D.A. (−)-Oleocanthal and (−)-Oleocanthal-Rich Olive Oils Induce Lysosomal Membrane Permeabilization in Cancer Cells. PLoS ONE 2019, 14, e0216024. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.B.; Ebrahim, H.Y.; Tajmim, A.; King, J.A.; Abdelwahed, K.S.; Abd Elmageed, Z.Y.; El Sayed, K.A. Oleocanthal Attenuates Metastatic Castration-Resistant Prostate Cancer Progression and Recurrence by Targeting SMYD2. Cancers 2022, 14, 3542. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C.; et al. Cytotoxic Activity of Oleocanthal Isolated from Virgin Olive Oil on Human Melanoma Cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Pastorio, C.; Torres-Rusillo, S.; Ortega-Vidal, J.; Jiménez-López, M.C.; Iañez, I.; Salido, S.; Santamaría, M.; Altarejos, J.; Molina, I.J. (−)-Oleocanthal Induces Death Preferentially in Tumor Hematopoietic Cells through Caspase Dependent and Independent Mechanisms. Food Funct. 2022, 13, 11334–11341. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The Olive Oil Phenolic (−)-Oleocanthal Modulates Estrogen Receptor Expression in Luminal Breast Cancer in Vitro and in Vivo and Synergizes with Tamoxifen Treatment. Eur. J. Pharmacol. 2017, 810, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Diez-Bello, R.; Jardin, I.; Lopez, J.J.; El Haouari, M.; Ortega-Vidal, J.; Altarejos, J.; Salido, G.; Salido, S.; Rosado, J. (−)-Oleocanthal Inhibits Proliferation and Migration by Modulating Ca2+ Entry through TRPC6 in Breast Cancer Cells. Biochim. Biophys. Acta BBA Mol. Cell Res. 2019, 1866, 474–485. [Google Scholar] [CrossRef]
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; Mccubrey, J.A.; Montalto, G.; et al. Oleocanthal Exerts Antitumor Effects on Human Liver and Colon Cancer Cells through ROS Generation. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (−)-Oleocanthal Inhibits Growth and Metastasis by Blocking Activation of STAT3 in Human Hepatocellular Carcinoma. Oncotarget 2016, 7, 43475–43491. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ayoub, N.M.; Tajmim, A.; Meyer, S.A.; Hill, R.A.; El Sayed, K.A. (−)-Oleocanthal Prevents Breast Cancer Locoregional Recurrence After Primary Tumor Surgical Excision and Neoadjuvant Targeted Therapy in Orthotopic Nude Mouse Models. Cancers 2019, 11, 637. [Google Scholar] [CrossRef]
- John, D.N.S.; Mamat, T.H.T.; Surien, O.; Taib, I.S.; Masre, S.F. Pre-Initiation Effect of Oleuropein towards Apoptotic and Oxidative Stress Levels on the Early Development of Two-Stage Skin Carcinogenesis. J. Krishna Inst. Med. Sci. Univ. 2019, 8, 43–51. [Google Scholar]
- LeGendre, O.; Breslin, P.A.; Foster, D.A. (−)-Oleocanthal Rapidly and Selectively Induces Cancer Cell Death via Lysosomal Membrane Permeabilization. Mol. Cell. Oncol. 2015, 2, e1006077. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; El Sayed, K.A. Olive Oil-Derived Oleocanthal as Potent Inhibitor of Mammalian Target of Rapamycin: Biological Evaluation and Molecular Modeling Studies. Phytother. Res. 2015, 29, 1776–1782. [Google Scholar] [CrossRef]
- Siddique, A.B.; Kilgore, P.; Tajmim, A.; Singh, S.S.; Meyer, S.A.; Jois, S.D.; Cvek, U.; Trutschl, M.; El Sayed, K.A. (−)-Oleocanthal as a Dual c-MET-COX2 Inhibitor for the Control of Lung Cancer. Nutrients 2020, 12, 1749. [Google Scholar] [CrossRef] [PubMed]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.Y.; Sayed, K.A.E. Olive Phenolics as C-Met Inhibitors: (−)-Oleocanthal Attenuates Cell Proliferation, Invasiveness, and Tumor Growth in Breast Cancer Models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef]
- Elnagar, A.Y.; Sylvester, P.W.; El Sayed, K.A. (−)-Oleocanthal as a c-Met Inhibitor for the Control of Metastatic Breast and Prostate Cancers. Planta Med. 2011, 77, 1013–1019. [Google Scholar] [CrossRef]
- Qusa, M.H.; Abdelwahed, K.S.; Siddique, A.B.; El Sayed, K.A. Comparative Gene Signature of (−)-Oleocanthal Formulation Treatments in Heterogeneous Triple Negative Breast Tumor Models: Oncological Therapeutic Target Insights. Nutrients 2021, 13, 1706. [Google Scholar] [CrossRef]
- Tajmim, A.; Siddique, A.B.; El Sayed, K. Optimization of Taste-Masked (−)-Oleocanthal Effervescent Formulation with Potent Breast Cancer Progression and Recurrence Suppressive Activities. Pharmaceutics 2019, 11, 515. [Google Scholar] [CrossRef]
- Ünsal, Ü.; Mete, M.; Aydemir, I.; Duransoy, Y.K.; Umur, A.; Tuglu, M.I. Inhibiting Effect of Oleocanthal on Neuroblastoma Cancer Cell Proliferation in Culture. Biotech. Histochem. 2020, 95, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, J.; Peng, L. (−)-Oleocanthal Exerts Anti-Melanoma Activities and Inhibits STAT3 Signalling Pathway. Oncol. Rep. 2017, 37, 483–491. [Google Scholar] [CrossRef]
- Karousi, P.; Kontos, C.K.; Papakotsi, P.; Kostakis, I.K.; Skaltsounis, A.L.; Scorilas, A. Next-Generation Sequencing Reveals Altered Gene Expression and Enriched Pathways in Triple-Negative Breast Cancer Cells Treated with Oleuropein and Oleocanthal. Funct. Integr. Genom. 2023, 23, 299. [Google Scholar] [CrossRef]
- Bossio, S.; Perri, A.; Malivindi, R.; Giordano, F.; Rago, V.; Mirabelli, M.; Salatino, A.; Brunetti, A.; Greco, E.A.; Aversa, A. Oleuropein Counteracts Both the Proliferation and Migration of Intra- and Extragonadal Seminoma Cells. Nutrients 2022, 14, 2323. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Bellosta, S.; Galli, C. Oleuropein, the Bitter Principle of Olives, Enhances Nitric Oxide Production by Mouse Macrophages. Life Sci. 1998, 62, 541–546. [Google Scholar] [CrossRef]
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Elobeid, M.A.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive Oil Oleuropein Has Anti-Breast Cancer Properties with Higher Efficiency on ER-Negative Cells. Food Chem. Toxicol. 2013, 53, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Przychodzen, P.; Wyszkowska, R.; Gorzynik-Debicka, M.; Kostrzewa, T.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Anticancer Potential of Oleuropein, the Polyphenol of Olive Oil, With 2-Methoxyestradiol, Separately or in Combination, in Human Osteosarcoma Cells. Anticancer Res. 2019, 39, 1243–1251. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea Europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef]
- Han, J.; Talorete, T.P.; Yamada, P.; Isoda, H. Anti-Proliferative and Apoptotic Effects of Oleuropein and Hydroxytyrosol on Human Breast Cancer MCF-7 Cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.K.; Castellon, R. Oleuropein, a Non-Toxic Olive Iridoid, Is an Anti-Tumor Agent and Cytoskeleton Disruptor. Biochem. Biophys. Res. Commun. 2005, 334, 769–778. [Google Scholar] [CrossRef]
- Gioti, K.; Papachristodoulou, A.; Benaki, D.; Aligiannis, N.; Skaltsounis, A.L.; Mikros, E.; Tenta, R. Assessment of the Nutraceutical Effects of Oleuropein and the Cytotoxic Effects of Adriamycin, When Administered Alone and in Combination, in MG-63 Human Osteosarcoma Cells. Nutrients 2021, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, R.; Chimento, A.; Luca, A.; Casaburi, I.; Rizza, P.; Onofrio, A.; Iacopetta, D.; Puoci, F.; Andò, S.; Maggiolini, M.; et al. Oleuropein and Hydroxytyrosol Inhibit MCF-7 Breast Cancer Cell Proliferation Interfering with ERK1/2 Activation. Mol. Nutr. Food Res. 2010, 54, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Ouanouki, A.; Béliveau, R.; Desrosiers, R.R. Olive Oil Compounds Inhibit Vascular Endothelial Growth Factor Receptor-2 Phosphorylation. Exp. Cell Res. 2014, 322, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M. Olive Leaf Extract and Its Main Component Oleuropein Prevent Chronic Ultraviolet B Radiation-Induced Skin Damage and Carcinogenesis in Hairless Mice. J. Nutr. 2009, 139, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein Induces Apoptosis via Abrogating NF-κB Activation Cascade in Estrogen Receptor-Negative Breast Cancer Cells. J. Cell Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Yan, C.M.; Chai, E.Q.; Cai, H.Y.; Miao, G.Y.; Ma, W. Oleuropein Induces Apoptosis via Activation of Caspases and Suppression of Phosphatidylinositol 3-Kinase/Protein Kinase B Pathway in HepG2 Human Hepatoma Cell Line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef]
- Tzekaki, E.E.; Geromichalos, G.; Lavrentiadou, S.N.; Tsantarliotou, M.P.; Pantazaki, A.A.; Papaspyropoulos, A. Oleuropein Is a Natural Inhibitor of PAI-1-Mediated Proliferation in Human ER-/PR- Breast Cancer Cells. Breast Cancer Res. Treat. 2021, 186, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Gene Expression Alterations Associated with Oleuropein-Induced Antiproliferative Effects and S-Phase Cell Cycle Arrest in Triple-Negative Breast Cancer Cells. Nutrients 2020, 12, 3755. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Galvano, F.; Santangelo, R.; Cardile, V.; Gangia, S.; D’Orazio, N.; Abraham, N.G. Antiproliferative Effect of Oleuropein in Prostate Cell Lines. Int. J. Oncol. 2012, 41, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefani, M. Oleuropein Aglycone Induces Autophagy via the AMPK/mTOR Signalling Pathway: A Mechanistic Insight. Oncotarget 2015, 6, 35344–35357. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Elamin, M.H.; Omer, S.A.; Daghestani, M.H.; Al-Olayan, E.S.; Elobeid, M.A.; Virk, P. Oleuropein Induces Apoptosis via the P53 Pathway in Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 14, 6739–6742. [Google Scholar] [CrossRef]
- Hassan, Z.K.; Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Al-Olayan, E.M.; Elobeid, M.A.; Virk, P.; Mohammed, O.B. Oleuropein Induces Anti-Metastatic Effects in Breast Cancer. Asian Pac. J. Cancer Prev. 2012, 13, 4555–4559. [Google Scholar] [CrossRef]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Chemopreventive Effect of Oleuropein in Colitis-Associated Colorectal Cancer in C57bl/6 Mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Expósito, M.J.; Carrera-González, M.P.; Mayas, M.D.; Martínez-Martos, J.M. Gender Differences in the Antioxidant Response of Oral Administration of Hydroxytyrosol and Oleuropein against N-Ethyl-N-Nitrosourea (ENU)-Induced Glioma. Food Res. Int. 2021, 140, 110023. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Zhu, J.S.; Zhang, Z.; Shen, W.J.; Jiang, S.; Long, Y.F.; Wu, B.; Ding, T.; Huan, F.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion of MDA-MB-231 Triple-Negative Breast Cancer Cell via Induction of Autophagy. Anticancer Agents Med. Chem. 2019, 19, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Junkins, K.; Rodgers, M.; Phelan, S.A. Oleuropein Induces Cytotoxicity and Peroxiredoxin Over-Expression in MCF-7 Human Breast Cancer Cells. Anticancer Res. 2023, 43, 4333–4339. [Google Scholar] [CrossRef] [PubMed]
- Aktas, H.G.; Ayan, H. Oleuropein: A Potential Inhibitor for Prostate Cancer Cell Motility by Blocking Voltage-Gated Sodium Channels. Nutr. Cancer 2021, 73, 1758–1767. [Google Scholar] [CrossRef]
- Asgharzade, S.; Sheikhshabani, S.H.; Ghasempour, E.; Heidari, R.; Rahmati, S.; Mohammadi, M.; Jazaeri, A.; Amini-Farsani, Z. The Effect of Oleuropein on Apoptotic Pathway Regulators in Breast Cancer Cells. Eur. J. Pharmacol. 2020, 886, 173509. [Google Scholar] [CrossRef]
- Tezcan, G.; Aksoy, S.A.; Tunca, B.; Bekar, A.; Mutlu, M.; Cecener, G.; Egeli, U.; Kocaeli, H.; Demirci, H.; Taskapilioglu, M. Oleuropein Modulates Glioblastoma miRNA Pattern Different from Olea Europaea Leaf Extract. Hum. Exp. Toxicol. 2019, 38, 1102–1110. [Google Scholar] [CrossRef]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; de la Lastra, C.A. Oleuropein, a Secoiridoid Derived from Olive Tree, Inhibits the Proliferation of Human Colorectal Cancer Cell through Downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef]
- Türkdoğan, M.K.; Koçyiğit, A.; Güler, E.M.; Özer, F.; Demir, K.; Uğur, H. Oleuropein Exhibits Anticarcinogen Effects against Gastric Cancer Cell Lines. Mol. Biol. Rep. 2023, 50, 9099–9105. [Google Scholar] [CrossRef]
- Hamed, M.M.; Handoussa, H.; Hussein, N.H.; Eissa, R.A.; Abdel-Aal, L.K.; El Tayebi, H.M. Oleuropin Controls miR-194/XIST/PD-L1 Loop in Triple Negative Breast Cancer: New Role of Nutri-Epigenetics in Immune-Oncology. Life Sci. 2021, 277, 119353. [Google Scholar] [CrossRef] [PubMed]
- Seçme, M.; Eroğlu, C.; Dodurga, Y.; Bağcı, G. Investigation of Anticancer Mechanism of Oleuropein via Cell Cycle and Apoptotic Pathways in SH-SY5Y Neuroblastoma Cells. Gene 2016, 585, 93–99. [Google Scholar] [CrossRef]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M.; Fabiani, R. Oleuropein Prevents Azoxymethane-Induced Colon Crypt Dysplasia and Leukocytes DNA Damage in A/J Mice. J. Med. Food 2016, 19, 983–989. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and Antigrowth Action of Peracetylated Oleuropein in Thyroid Cancer Cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Abtin, M.; Alivand, M.R.; Khaniani, M.S.; Bastami, M.; Zaeifizadeh, M.; Derakhshan, S.M. Simultaneous Downregulation of miR-21 and miR-155 through Oleuropein for Breast Cancer Prevention and Therapy. J. Cell Biochem. 2018, 119, 7151–7165. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Zhang, Q.; Li, X.; Zhu, X.; Wang, Q.; Cao, S.; Du, L. Mitochondria-Mediated Apoptosis Was Induced by Oleuropein in H1299 Cells Involving Activation of P38 MAP Kinase. J. Cell Biochem. 2019, 120, 5480–5494. [Google Scholar] [CrossRef] [PubMed]
- Przychodzen, P.; Kuban-Jankowska, A.; Wyszkowska, R.; Barone, G.; Bosco, G.L.; Celso, F.L.; Kamm, A.; Daca, A.; Kostrzewa, T.; Gorska-Ponikowska, M. PTP1B Phosphatase as a Novel Target of Oleuropein Activity in MCF-7 Breast Cancer Model. Toxicol. Vitr. 2019, 61, 104624. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, N.; Alivand, M.R.; Bayat, S.; Khaniani, M.S.; Derakhshan, S.M. The Hopeful Anticancer Role of Oleuropein in Breast Cancer through Histone Deacetylase Modulation. J. Cell. Biochem. 2019, 120, 17042–17049. [Google Scholar] [CrossRef]
- Sherif, I.O.; Al-Gayyar, M.M.H. Oleuropein Potentiates Anti-Tumor Activity of Cisplatin against HepG2 through Affecting proNGF/NGF Balance. Life Sci. 2018, 198, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Sheikhshabani, S.; Amini-Farsani, Z.P.; Rahmati, S.; Jazaeri, A.; Mohammadi-Samani, M.; Asgharzade, S. Oleuropein Reduces Cisplatin Resistance in Ovarian Cancer by Targeting Apoptotic Pathway Regulators. Life Sci. 2021, 278, 119525. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, X.; Du, L. P38 MAP Kinase Is Involved in Oleuropein-Induced Apoptosis in A549 Cells by a Mitochondrial Apoptotic Cascade. Biomed. Pharmacother. 2017, 95, 1425–1435. [Google Scholar] [CrossRef]
- Hong, Z.; Lu, Y.; Liu, B.; Ran, C.; Lei, X.; Wang, M.; Wu, S.; Yang, Y.; Wu, H. Glycolysis, a New Mechanism of Oleuropein against Liver Tumor. Phytomedicine 2023, 114, 154770. [Google Scholar] [CrossRef] [PubMed]
- Nassir, A.M.; Ibrahim, I.A.A.; Md, S.; Waris, M.; Tanuja; Ain, M.R.; Ahmad, I.; Shahzad, N. Surface Functionalized Folate Targeted Oleuropein Nano-Liposomes for Prostate Tumor Targeting: Invitro and Invivo Activity. Life Sci. 2019, 220, 136–146. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Huang, B.; Chen, A.; Li, X. Oleuropein Inhibits the Proliferation and Invasion of Glioma Cells via Suppression of the AKT Signalling Pathway. Oncol. Rep. 2016, 36, 2009–2016. [Google Scholar] [CrossRef]
- Antognelli, C.; Frosini, R.; Santolla, M.F.; Peirce, M.J.; Talesa, V.N. Oleuropein-Induced Apoptosis Is Mediated by Mitochondrial Glyoxalase 2 in NSCLC A549 Cells: A Mechanistic Inside and a Possible Novel Nonenzymatic Role for an Ancient Enzyme. Oxid. Med. Cell. Longev. 2019, 2019, 8576961. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, S.; Vecchio, E.; Battaglia, A.M.; Oliverio, M.; Nardi, M.; Procopio, A.; Costanzo, F.; Biamonte, F.; Faniello, M.C. The Double-Edged Sword of Oleuropein in Ovarian Cancer Cells: From Antioxidant Functions to Cytotoxic Effects. Int. J. Mol. Sci. 2023, 24, 842. [Google Scholar] [CrossRef]
- Amini-Farsani, Z.; Hashemi Sheikhshabani, S.; Shaygan, N.; Asgharzade, S. The Impact of Oleuropein on miRNAs Regulating Cell Death Signalling Pathway in Human Cervical Cancer Cells. Biotechnol. Appl. Biochem. 2023, 71, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Choupani, J.; Alivand, M.R.; Derakhshan, S.M.; Zaeifizadeh, M.; Khaniani, M.S. Oleuropein Inhibits Migration Ability through Suppression of Epithelial-Mesenchymal Transition and Synergistically Enhances Doxorubicin-Mediated Apoptosis in MCF-7 Cells. J. Cell. Physiol. 2019, 234, 9093–9104. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, F.; Zaefizadeh, M.; Yari, R.; Salehzadeh, A. Synthesis of Nano-Paramagnetic Oleuropein to Induce KRAS Over-Expression: A New Mechanism to Inhibit AGS Cancer Cells. Medicina 2019, 55, 388. [Google Scholar] [CrossRef]
- Yao, J.; Wu, J.; Yang, X.; Yang, J.; Zhang, Y.; Du, L. Oleuropein Induced Apoptosis in HeLa Cells via a Mitochondrial Apoptotic Cascade Associated with Activation of the C-Jun NH2-Terminal Kinase. J. Pharmacol. Sci. 2014, 125, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Zhu, J.S.; Xie, J.; Zhang, Z.; Zhu, J.; Jiang, S.; Shen, W.-J.; Wu, B.; Ding, T.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion via Induction of Autophagy in ER-Positive Breast Cancer Cell Lines (MCF7 and T47D. Nutr. Cancer 2021, 73, 350–360. [Google Scholar] [CrossRef]
- Xu, T.; Liu, X. Oleuropein Inhibits Invasion of Squamous Cell Carcinoma of the Head and Neck through TGF-Β1 Signalling Pathway. BMC Cancer 2022, 22, 942. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi Niyaki, Z.; Salehzadeh, A.; Peymani, M.; Zaefizadeh, M. Exploring the Therapeutic Potential of Fe3O4@Glu-Oleuropein Nanoparticles in Targeting KRAS Pathway-Regulating lncRNAs in Colorectal Cancer Cells. Biol. Trace Element Res. 2023, 202, 3073–3085. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, S.; Yun, J.; Jang, W.; Rethineswaran, V.K.; Van, L.T.H.; Giang, L.T.T.; Choi, J.; Lim, H.J.; Kwon, S.-M. Oleuropein Induces Apoptosis in Colorectal Tumor Spheres via Mitochondrial Fission. Mol. Cell. Toxicol. 2023, 19, 311–319. [Google Scholar] [CrossRef]
- Kucukgul, A.; Isgor, M.M.; Duzguner, V.; Atabay, M.N.; Kucukgul, A. Antioxidant Effects of Oleuropein on Hydrogen Peroxide-Induced Neuronal Stress-An In Vitro Study. Antiinflamm. Antiallergy Agents Med. Chem. 2020, 19, 74–84. [Google Scholar] [CrossRef]
- Masre, S.F.; Izzuddeen, A.; John, D.N.S.; Hamid, Z.A. The Effects of Oleuropein on Apoptotic Rate and Oxidative Stress Profiles during Tumour Promotion Stage in the Mouse Skin Carcinogenesis Model. Sains Malays. 2019, 48, 347–352. [Google Scholar] [CrossRef]
- Margheri, F.; Menicacci, B.; Laurenzana, A.; Del Rosso, M.; Fibbi, G.; Cipolleschi, M.G.; Ruzzolini, J.; Nediani, C.; Mocali, A.; Giovannelli, L. Oleuropein Aglycone Attenuates the Pro-Angiogenic Phenotype of Senescent Fibroblasts: A Functional Study in Endothelial Cells. J. Funct. Foods 2019, 53, 219–226. [Google Scholar] [CrossRef]
- Kamrani, S.; Tooba, K.; Zaefizadeh, M. Study of a Nano-Oleuropein’s Effect on the TCA Cycles Protein Expression in the Breast Cancer Cell Line Using Proteomics. J. Intellect. Disabil. Diagn. Treat. 2019, 7, 47–52. [Google Scholar]
- Song, H.; Lim, D.Y.; Jung, J.I.; Cho, H.J.; Park, S.Y.; Kwon, G.T.; Kang, Y.-H.; Lee, K.W.; Choi, M.-S.; Park, J.H.Y. Dietary Oleuropein Inhibits Tumor Angiogenesis and Lymphangiogenesis in the B16F10 Melanoma Allograft Model: A Mechanism for the Suppression of High-Fat Diet-Induced Solid Tumor Growth and Lymph Node Metastasis. Oncotarget 2017, 8, 32027–32042. [Google Scholar] [CrossRef]
- Katsoulieris, E.N. The Olive Leaf Extract Oleuropein Exerts Protective Effects against Oxidant-Induced Cell Death, Concurrently Displaying pro-Oxidant Activity in Human Hepatocarcinoma Cells. Redox Rep. 2016, 21, 90–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jamshed, F.; Ahmad, W.; Haque, A.; Saad, A.; Al-Jassabi, S. Ameliorative Role of Oleuropein Extracted from Olive Leaf on Tamoxifen-Induced Hepatic 8-Hydroxydeoxyguanosine in DNA of Balb/C Mice. World Appl. Sci. J. 2014, 30, 765–769. [Google Scholar]
- Martínez-Martos, J.M.; Mayas, M.D.; Carrera, P.; Saavedra, J.M.A.; Sánchez-Agesta, R.; Arrazola, M.; Ramírez-Expósito, M.J. Phenolic Compounds Oleuropein and Hydroxytyrosol Exert Differential Effects on Glioma Development via Antioxidant Defense Systems. J. Funct. Foods 2014, 11, 221–234. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; D’Agostino, M.; Maiuolo, J.; Oliverio, M.; Procopio, A.; Iannone, M.; Rotiroti, D.; Russo, D. Antiproliferative and Antioxidant Effects on Breast Cancer Cells of Oleuropein and Its Semisynthetic Peracetylated Derivatives. Food Chem. 2011, 127, 1609–1614. [Google Scholar] [CrossRef]
- Cheng, J.-S.; Chou, C.-T.; Liu, Y.-Y.; Sun, W.-C.; Shieh, P.; Kuo, D.-H.; Kuo, C.-C.; Jan, C.-R.; Liang, W.-Z. The Effect of Oleuropein from Olive Leaf (Olea Europaea) Extract on Ca2+ Homeostasis, Cytotoxicity, Cell Cycle Distribution and ROS Signalling in HepG2 Human Hepatoma Cells. Food Chem. Toxicol. 2016, 91, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Marrero, A.D.; Ortega-Vidal, J.; Salido, S.; Castilla, L.; Vidal, I.; Quesada, A.R.; Altarejos, J.; Martínez-Poveda, B.; Medina, M. Anti-Angiogenic Effects of Oleacein and Oleocanthal: New Bioactivities of Compounds from Extra Virgin Olive Oil. Biomed. Pharmacother. 2023, 165, 115234. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. miRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells. Front. Pharmacol. 2020, 11, 574317. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Celano, M.; Lombardo, G.E.; Maggisano, V.; Procopio, A.; Russo, D.; Navarra, M. Oleacein Inhibits STAT3, Activates the Apoptotic Machinery, and Exerts Anti-Metastatic Effects in the SH-SY5Y Human Neuroblastoma Cells. Food Funct. 2020, 11, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Juli, G.; Oliverio, M.; Bellizzi, D.; Gallo Cantafio, M.E.; Grillone, K.; Passarino, G.; Colica, C.; Nardi, M.; Rossi, M.; Procopio, A.; et al. Anti-Tumor Activity and Epigenetic Impact of the Polyphenol Oleacein in Multiple Myeloma. Cancers 2019, 11, 990. [Google Scholar] [CrossRef]
- Pruthi, S.; Heisey, R.E.; Bevers, T.B. Chemoprevention for Breast Cancer. Ann. Surg. Oncol. 2015, 22, 3230–3235. [Google Scholar] [CrossRef]
- Flora, S.; Ferguson, L. Overview of Mechanisms of Cancer Chemopreventive Agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gutschner, T.; Diederichs, S. The Hallmarks of Cancer: A Long Non-Coding RNA Point of View. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Braun, M.; Strmiska, V.; Sicinski, P. Targeting Cell-Cycle Machinery in Cancer. Cancer Cell 2021, 39, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Katiyar, S.K. Cell Cycle Control as a Basis for Cancer Chemoprevention through Dietary Agents. Front. Biosci. 2008, 13, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Blelloch, R. Cell Cycle Regulation by MicroRNAs in Embryonic Stem Cells. Cancer Res. 2009, 69, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.; Lang, C.; Devgan, G.; Azare, J.; Berishaj, M.; Gerald, W.; Kim, Y.B.; Paz, K.; Darnell, J.E.; Albanese, C.; et al. Cyclin D1 Is Transcriptionally Regulated by and Required for Transformation by Activated Signal Transducer and Activator of Transcription 3. Cancer Res. 2006, 66, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Calcium Signalling and Cell Proliferation. Bioessays 1995, 17, 491–500. [Google Scholar] [CrossRef]
- Tanaka, T. Role of Apoptosis in the Chemoprevention of Cancer. J. Exp. Clin. Med. 2013, 5, 89–91. [Google Scholar] [CrossRef]
- Lin, L.; Baehrecke, E.H. Autophagy, Cell Death, and Cancer. Mol. Cell Oncol. 2015, 2, e985913. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell Death: Critical Control Points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, N.; Gyrd-Hansen, M.; Poulsen, B.; Felbor, U.; Kallunki, T.; Boes, M.; Weber, E.; Leist, M.; Jäättelä, M. Sensitization to the Lysosomal Cell Death Pathway upon Immortalization and Transformation. Cancer Res. 2004, 64, 5301–5310. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal Membrane Permeabilization and Cell Death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Antognelli, C.; Talesa, V.N. Glyoxalases in Urological Malignancies. Int. J. Mol. Sci. 2018, 19, 415. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Harris, A.L.; Dalgleish, A.G.; Steward, W.P.; O’Byrne, K.J. Angiogenesis as a Biomarker and Target in Cancer Chemoprevention. Lancet Oncol. 2001, 2, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR Signalling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Menicacci, B.; Margheri, F.; Laurenzana, A.; Chillà, A.; Del Rosso, M.; Giovannelli, L.; Fibbi, G.; Mocali, A. Chronic Resveratrol Treatment Reduces the Pro-Angiogenic Effect of Human Fibroblast “Senescent-Associated Secretory Phenotype” on Endothelial Colony-Forming Cells: The Role of IL8. J. Gerontol. Ser. A 2019, 74, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Hapach, L.A.; Mosier, J.A.; Wang, W.; Reinhart-King, C.A. Engineered Models to Parse Apart the Metastatic Cascade. NPJ Precis. Oncol. 2019, 3, 20. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Rho, O.; Kim, D.J.; Kiguchi, K.; DiGiovanni, J. Growth Factor Signalling Pathways as Targets for Prevention of Epithelial Carcinogenesis. Mol. Carcinog. 2011, 50, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Abdelmawgoud, H.; El Awady, R.R. Effect of Sirtuin 1 Inhibition on Matrix Metalloproteinase 2 and Forkhead Box O3a Expression in Breast Cancer Cells. Genes Dis. 2017, 4, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Byles, V.; Zhu, L.; Lovaas, J.D.; Chmilewski, L.K.; Wang, J.; Faller, D.V.; Dai, Y. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene 2012, 31, 4619–4629. [Google Scholar] [CrossRef]
- Luccarini, I.; Pantano, D.; Nardiello, P.; Cavone, L.; Lapucci, A.; Miceli, C.; Nediani, C.; Berti, A.; Stefani, M.; Casamenti, F. The Polyphenol Oleuropein Aglycone Modulates the PARP1-SIRT1 Interplay: An in Vitro and in Vivo Study. J. Alzheimers Dis. 2016, 54, 737–750. [Google Scholar] [CrossRef]
- Deshotels, M.R.; Spinu, E.; Bailey, E.; Filipeanu, C. The Role of A-and Β-Estrogen Receptors on Neurite Outgrowth In Neuro-2A Cells; Wiley Online Library: Hoboken, NJ, USA, 2013. [Google Scholar]
- Pritchard, A. Pan-Cancer Analysis of Whole Genomes. Nature 2020, 578, 82–93. [Google Scholar]
- Kruk, I.; Aboul-Enein, H.Y.; Michalska, T.; Lichszteld, K.; Kładna, A. Scavenging of Reactive Oxygen Species by the Plant Phenols Genistein and Oleuropein. Luminescence 2005, 20, 81–89. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Luo, T.; Shi, H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK Pathways for Cancer Therapy. Mol. Biomed. 2022, 3, 47. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Mohammad, I.S.; Liu, Z. Overview of the STAT-3 Signalling Pathway in Cancer and the Development of Specific Inhibitors. Oncol. Lett. 2020, 19, 2585–2594. [Google Scholar]
- Tian, T.; Li, X.; Zhang, J. mTOR Signalling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef]
- Gao, X.N.; Lin, J.; Li, Y.H.; Gao, L.; Wang, X.R.; Wang, W.; Kang, H.-Y.; Yan, G.-T.; Wang, L.-L.; Yu, L. MicroRNA-193a Represses c-Kit Expression and Functions as a Methylation-Silenced Tumor Suppressor in Acute Myeloid Leukemia. Oncogene 2011, 30, 3416–3428. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, J.; Yan, M.; Liu, L.; Lin, H.; Zhao, F.; Sun, L.; Zhang, Y.; Cui, Y.; Zhang, F.; et al. MicroRNA-193a-3p and -5p Suppress the Metastasis of Human Non-Small-Cell Lung Cancer by Downregulating the ERBB4/PIK3R3/mTOR/S6K2 Signalling Pathway. Oncogene 2015, 34, 413–423. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Liang, H.; Sun, W.; Li, J.; Liu, Y.; Fan, Q.; Zhang, H.; Yue, X.; Li, J.; Chen, X.; et al. Deregulation of the miR-16-KRAS Axis Promotes Colorectal Cancer. Sci. Rep. 2016, 6, 37459. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Is NF-kappaB a Good Target for Cancer Therapy? Hopes and Pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Sun, P. The Pathways to Tumor Suppression via Route P38. Trends Biochem. Sci. 2007, 32, 364–371. [Google Scholar] [CrossRef]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox Regulation in Cancer: A Double-Edged Sword with Therapeutic Potential. Oxid. Med. Cell Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef]
- Xing, F.; Hu, Q.; Qin, Y.; Xu, J.; Zhang, B.; Yu, X.; Wang, W. The Relationship of Redox With Hallmarks of Cancer: The Importance of Homeostasis and Context. Front. Oncol. 2022, 12, 862743. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta BBA Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Lee, J.T.; Gu, W. SIRT1: Regulator of p53 deacetylation. Genes Cancer 2013, 4, 112–117. [Google Scholar] [CrossRef]
- Thakur, B.K.; Chandra, A.; Dittrich, T.; Welte, K.; Chandra, P. Inhibition of SIRT1 by HIV-1 Viral Protein Tat Results in Activation of P53 Pathway. Biochem. Biophys. Res. Commun. 2012, 424, 245–250. [Google Scholar] [CrossRef] [PubMed]
| Compound | Study Design | Tissue/Cell Type | Major Finding | Mechanism | Ref. |
|---|---|---|---|---|---|
| Oleocanthal | in vitro and in vivo (mouse, RIP-Tag) | Pancreatic tumor/PC3, MDA-MB-231, MCF7, HEK-293T, MCF10A, BJ-hTert and PNET N134 | Oleocanthal induces damage to cancer cell lysosomes, causing lysosomal membrane permeabilization and the release of cathepsin into the cytosol. This process leads to cellular toxicity, resulting in the inhibition of cancer cell viability, reduced tumor burden, and an extended lifespan observed in mice with pancreatic neuroendocrine tumors. | Inducing cell death (necrosis) | Goren et al. [26] |
| Oleocanthal | in vitro and in vivo (mouse, athymic nude, male) | Metastatic castration-resistant prostate cancer (mCRPC) tumor/DU-145, PC-3, CWR-R1ca and PC-3M | Oleocanthal suppresses the viability, migration, invasion, and colony formation of prostate cancer cell lines. It also reduces the progression and metastasis of mCRPC tumors in nude mice by suppressing the downstream substrates and signalling of SMYD2 (EZH2, p65, mTOR, MAPK), which are crucial for prostate cancer proliferation and relapse. | Inhibition of cell proliferation, metastasis, and modulation of cancer cell signalling pathways | Siddique et al. [27] |
| Oleocanthal | in vitro | A375, 501Mel and HDFa | Oleocanthal suppresses the viability of melanoma cells (A375 and 501Mel) by inhibiting the oncogenic pathways (ERK1/2 and AKT), resulting in the downregulation of the anti-apoptotic protein Bcl-2. | Induction of cell death (apoptosis) and modulation of cancer cell signalling pathways | Fogli et al. [28] |
| Oleocanthal | in vitro | MDA-MB-231, MCF7, G361, HT144, HCT 116, HeLa, MIA PaCa-2, A549, 293T, Jurkat, CEM, Raji, K-562, Caco-2, JEG-3 and NCI-H460 | Oleocanthal suppresses the viability of cancer cells and induces apoptotic cell death via activation of the intrinsic apoptotic pathway (ROS production and mitochondrial membrane depolarization). | Inducing cell death (apoptosis) | Pastorio et al. [29] |
| Oleocanthal | in vitro, in silico and in vivo (mouse, athymic nude, female) | BT-474 tumor xenografts/BT-474, MCF-7, and T-47D | Oleocanthal, both alone and in combination with tamoxifen, inhibits the growth of luminal breast cancer cells. Additionally, oleocanthal reduces the growth of the BT-474 tumor. These effects are partly attributed to the reduction in total Erα levels in both cell culture and animal studies. Based on in silico studies, oleocanthal is hypothesized to directly interact with the Erα receptor, thereby modulating the pharmacological signalling of this crucial nuclear hormone receptor. | Inhibition of cell proliferation | Ayoub et al. [30] |
| Oleocanthal | in vitro | MCF10A, MCF7 and MDA-MB-231 | Oleocanthal disrupts the ability of breast cancer cells to proliferate and migrate through the downregulation of TRPC6 channel expression. Additionally, selective activation of TRPC6-dependent Ca2+ influx leads to Ca2+ entry and mobilization. | Inhibition of cell proliferation and inhibition of cell migration | Diez-Bello et al. [31] |
| Oleocanthal | in vitro | HepG2, Hep3B, Huh7, PLC/PRF/5, SW480 and HT29 | Oleocanthal suppresses colony formation ability and induces apoptosis in colorectal and hepatocellular carcinoma cells through the upregulation of reactive oxygen species (ROS). | Induction of cell death (apoptosis) and disruption of redox hemostasis | Cusimano et al. [32] |
| Oleocanthal | in vitro and in vivo (mouse, athymic nude, male) | Orthotopic tumor model of hepatocellular carcinoma (HCC)/LO2, HCCLM3, HepG2 and Huh-7 | Oleocanthal inhibits proliferation, induces apoptosis, and suppresses cell migration in HCC cells. Moreover, it suppresses tumor growth and metastasis in an orthotopic HCC model. These effects are attributed to the reduction in STAT3 nuclear translocation and DNA binding activity, downregulating its downstream effectors (cyclin D1, Bcl-2 survivin, MMP-2). | Induction of cell death (apoptosis), inhibition of metastasis, inhibition of cell proliferation, and modulation of cancer cell signalling pathways | Pei et al. [33] |
| Oleocanthal | in vivo (mouse, athymic nude, female) | BT-474 and tumor xenografts | Oleocanthal reduces the growth of recurrent breast cancer tumors. It upregulates the expression of the epithelial marker E-cadherin, downregulates the levels of the mesenchymal marker vimentin, and also reduces the activation of MET and HER2 receptors. | Inhibition of cell proliferation and metastasis | Siddique et al. [34] |
| Oleocanthal | in vitro | BxPC3, PC3, MDA-MB-231, BJ, 3Y1, and IMR90 | Oleocanthal triggers both apoptotic and necrotic cell death by inducing lysosomal membrane permeabilization (LMP). This occurs through the inhibition of acid sphingomyelinase [35] activity, leading to the destabilization of the interaction between proteins crucial for maintaining lysosomal membrane stability. | Inducing cell death (apoptosis and necrosis) | LeGendre et al. [36] |
| Oleocanthal | in vitro and in silico | MCF-7, T47D, Caco-2, MDA-MB-231 and HeLa | Oleocanthal reduces the viability of cancer cells. Molecular modeling studies have indicated that oleocanthal exhibits nine out of ten critical binding interactions similar to those of a potent dual PIK3-γ/mTOR natural inhibitor. Notably, treatment with oleocanthal significantly decreases the level of phosphorylated mTOR in the metastatic breast cancer cell line (MDA-MB-231). | Inhibition of cell viability | Khanfar et al. [37] |
| Oleocanthal | in vitro and in vivo (athmyc nude mouse, Foxn1nu/Fox+, female) | A549-Luc LC Metastasis/A549, A549-Luc and NCI-H322M | Oleocanthal exerts inhibitory effects on HGF-mediated proliferation and migration in human LC cell lines A549 and NCI-H322M cells. These effects are achieved through the dual targeting of c-MET and COX-2. The study demonstrated a significant decrease in total and activated c-MET levels and the inhibition of COX1/2 activity in lung adenocarcinoma cells. Additionally, oleocanthal treatments led to a notable inhibition of the progression and metastasis of NSCLC A549-Luc cells in a nude mouse tail vein model. c-MET activation was reduced in tumor cell lysates. Moreover, microarray data from oleocanthal-treated lung tumors revealed a distinct gene signature confirming the dual targeting of COX2 and c-MET. | Inhibition of cell proliferation and metastasis | Siddique et al. [38] |
| Oleocanthal | in vitro and in vivo (athymic nude mouse, Foxn1nu/Fox+, female) | MDA-MB-231/GFP-breast tumor/MDA-MB-231, MDA-MB-231/GFP, MCF-7, MCF10A and BT-474 | Oleocanthal inhibits the viability of various human breast cancer cell lines, namely MDA-MB-231, MCF-7, and BT-474, with a pronounced impact on apoptosis, particularly in MDA-MB-231 cells. Moreover, oleocanthal exhibits a dose-dependent suppression of HGF-induced cell migration, invasion, and G1/S cell cycle progression in MDA-MB-231 cells. Notably, the effects of oleocanthal are determined to be mediated through the inhibition of Brk/paxillin/Rac1 signalling pathways and the suppression of HGF-induced c-Met activation, along with subsequent downstream mitogenic signalling pathways. Additionally, oleocanthal plays a role in stabilizing the epithelial phenotype, as evidenced by increased expression of E-cadherin and zona occludens 1, while concurrently reducing the mesenchymal phenotype, indicated by decreased vimentin expression in breast cancer cells. | Inhibition of cell proliferation, induction of cell death (apoptosis), inhibition of metastasis and modulation of cancer cell signalling pathways | Akl et al. [39] |
| Oleocanthal | in vitro and in silico | MCF7, MDA-MB-231 and prostate cancer cell line PC-3 | Oleocanthal inhibits the proliferation, migration, and invasion of epithelial human breast and prostate cancer cell lines. Additionally, it exhibits anti-angiogenic activity by suppressing the expression of the microvessel density marker CD31 in endothelial colony-forming cells. | Inhibition of cell proliferation, inhibition of cell migration, and inhibition of angiogenesis | Elnagar et al. [40] |
| Oleocanthal | in vivo (transgenic mouse, FVB/N-Tg (MMTV-P), female) | Lung of Transgenic MMTV-PyVT Mice and Breast Cancer Patient-Derived Xenograft (PDX) Model | Oleocanthal inhibits the growth and metastasis of breast cancer. According to the ingenuity pathway analysis, there is a notable overlap in the top-downregulated pathways, including the PI3K-AKT, PD-1/PD-L1, and protein kinase A signalling pathways, in PDX and MMTV-PyVT mouse tumors after oleocanthal treatment. This implies a potential suppression effect on non-melanoma, glioma, colorectal, lung, breast, and prostate cancers. | Inhibition of cell proliferation and metastasis | Qusa et al. [41] |
| Oleocanthal | in vitro and in vivo (mouse, Swiss albino, male and female) | BT-474 BC cell xenograft model/MDA-MB-231 and BT-474 | Oleocanthal formulation EF-2 inhibits locoregional recurrence in BT-474 tumor cells following surgical excision of the primary tumor. Moreover, it suppresses over 70% of both hormone and HER2-positive BT-474 breast cancer cell growth in a nude mouse xenograft model. | Inhibition of proliferation | Tajmim et al. [42] |
| Oleocanthal | in vitro | NB2a and BMSCs | Oleocanthal suppresses proliferation of neuroblastoma cells by inducing apoptosis and oxidative stress through the upregulation of i-NOS and e-NOS. | Inhibition of proliferation, inducing cell death (apoptosis) and disruption of redox hemostasis | Ünsal et al. [43] |
| Oleocanthal | in vitro | A375, A2058, HUVEC and HaCaT | Oleocanthal inhibits the proliferation, angiogenesis, and metastasis of cancer cells by inhibiting the phosphorylation and nuclear translocation of STAT3. This inhibition results in the downregulation of STAT3 target genes, such as Bcl-xL, Mcl-1, MMP-2, VEGF, and MMP-9. These genes play crucial roles in the processes of apoptosis, invasion, and angiogenesis in melanoma. | Inhibition of angiogenesis, inducing cell death (apoptosis), inhibition of metastasis and modulation of cancer cell signalling pathways | Gu et al. [44] |
| Oleuropein and oleocanthal | in vitro | MDA-MB-231 and MDA-MB-468 | Oleuropein and oleocanthal exhibit anti-proliferative effects in triple-negative breast cancer (TNBC) cell lines by impeding cell survival and modifying the gene expression of these cells. Oleocanthal demonstrates a more potent inhibition of cancer cells’ viability compared to oleuropein. Pathway analysis unveiled several pathways linked to TNBC, including apoptotic processes, cell death, cellular response to stress, inducing cell cycle arrest by p21, inhibiting cancer progression, and invasion through decreasing MMPs expression. | Inhibition of cell proliferation | Karousi et al. [45] |
| Oleuropein | in vitro | SEM-1, HepG2 and TCAM-2 | Oleuropein reduces the viability of seminoma cells by inducing apoptosis through the inhibition of NF-κB nuclear translocation, resulting in the inhibition of cyclin-D1 expression and the upregulation of p21Cip/WAF1. Additionally, oleuropein suppresses the cellular motility and migration of seminoma cells by downregulating TGFβ-1 expression. | Inducing apoptosis and inhibition of cell migration | Bossio et al. [46] |
| Oleuropein | in vitro | J774A.1 and mouse peritoneal macrophages | Oleuropein enhances the macrophage-mediated response, leading to increased production of nitric oxide (NO). | Antioxidative properties | Visioli et al. [47] |
| Oleuropein | in vitro | MCF-10A, MDA-MB-231 and MCF-7 | Oleuropein reduces the viability of breast cancer cells by inducing apoptosis through modulation of mitochondrial pathway (↑ Bax, ↓ Bcl-2, ↓ survivin). Oleuropein also inhibits breast cancer cell proliferation by downregulating NF-kB and cyclin D1 but activates p21, leading to cell cycle arrest at the S phase. | Induction of cell death (apoptosis), inhibition of cell proliferation, and modulation of cancer cell signalling pathways | Elamin et al. [48] |
| Oleuropein | in vitro | 143B OS | Oleuropein inhibits the proliferation and migration of highly metastatic osteosarcoma cells, and these effects are augmented when combined with 2-methoxyestradiol. Furthermore, oleuropein, whether administered alone or in conjunction with 2-methoxyestradiol, triggers autophagy in these highly metastatic osteosarcoma cells. | Inhibition of cell proliferation, autophagy, and inhibition of cell migration | Przychodzen et al. [49] |
| Oleuropein | in vitro | A375 | Oleuropein inhibits cell proliferation and induces apoptosis by suppressing the pAKT/pS6 pathway. It enhances the cytotoxic impact of Dacarbazine (DTIC). Oleuropein has been observed to synergize with Everolimus (RAD001) and exhibit efficacy against PLX4032-resistant BRAF melanoma cells, suggesting potential collaboration in inhibiting the pAKT/pS6 pathway. | Inhibition of cell proliferation and induction of cell death (apoptosis) | Ruzzolini et al. [50] |
| Oleuropein | in vitro | MCF-7 | Oleuropein induces apoptosis and suppresses cell proliferation by triggering G1 cell cycle arrest in MCF-7 cells. | Inhibition of cell proliferation and induction of cell death (apoptosis) | Han et al. [51] |
| Oleuropein | in vitro and in vivo (mouse, Swiss albino, male and female) | Soft tissue sarcomas/NL-Fib, LN-18, TF-1a, MCF-7, 786-O, T-47D, RPMI-7951, and LoVo | Oleuropein inhibits the growth and migration of various tumor cell lines. In a newly developed tube-disruption assay, oleuropein hampers angiogenesis by interrupting the division, motility, and invasiveness of cells through the disruption of their cytoskeleton, particularly the organization of actin filaments. When orally administered to mice with spontaneously developing tumors (soft tissue sarcomas), oleuropein effectively regresses tumor growth. | Inhibition of cell proliferation, inhibition of angiogenesis, and inhibition of cell migration | Hamdi et al. [52] |
| Oleuropein | in vitro | MG-63 | Oleuropein inhibits the proliferation of osteosarcoma cells on its own, and when combined with adriamycin, it further enhances this inhibitory effect. Notably, oleuropein also increases the expression of autophagy-related genes (AMBRA1, ULK1, and BNiP3L). Additionally, the levels of LC3B are significantly reduced after treatment with oleuropein alone and in combination with adriamycin. Consistent with these findings, oleuropein markedly amplifies the expression of p62, an autophagy substrate. | Inhibition of cell proliferation and induction of autophagy | Gioti et al. [53] |
| Oleuropein | in vitro | MCF-7 | Oleuropein inhibits the growth of estradiol-induced MCF-7 cells by modulating ERα transactivation, resulting in the inhibition of estradiol-dependent ERK1/2 activation. | Inhibition of cell proliferation and modulation of cancer cell signalling pathways | Sirianni et al. [54] |
| Oleuropein | in vitro | HUVECs and HMVECs-d-Ad | Oleuropein abrogates the tube formation of HUVECs cells. | Inhibition of angiogenesis | Lamy et al. [55] |
| Oleuropein | in vivo (mouse, albino hairless, male) | UVB irradiation induced skin tumor | Oleuropein demonstrates potent inhibition of skin carcinogenesis as well as the growth of blood vessels and tumors induced by UVB irradiation. Furthermore, it enhances the expression of MMP-2, MMP-9, and MMP-13, along with elevated levels of VEGF and COX-2 in the skin. Additionally, oleuropein impedes the increase in the expression of Ki-67 and CD31 in the skin of mice following UVB irradiation. | Inhibition of cell proliferation | Kimura et al. [56] |
| Oleuropein | in vitro | MCF-7 and MDA-MB-231 | Oleuropein inhibits the viability, migration, and invasion of breast cancer cells while promoting apoptosis through the increased production of reactive oxygen species (ROS) and the inhibition of NF-κB activation in these cells. | Inhibition of cell proliferation, inhibition of cell migration, and modulation of cancer cell signalling pathways | Liu et al. [57] |
| Oleuropein | in vitro | HepG2, RKO and Huh7 | Oleuropein inhibits the growth of HepG2 cells by triggering apoptosis, characterized by the upregulation of BAX and the downregulation of Bcl-2. This effect is achieved through the induced generation of reactive oxygen species (ROS), resulting in the inactivation of PI3K/AKT signalling. | Induction of cell death (apoptosis) and modulation of cancer cell signalling pathways | Yan et al. [58] |
| Oleuropein | in vitro and in silico | MDA-MB-231 and MCF-7 cells | Based on molecular docking analysis, oleuropein has been recognized as a potent molecule that forms a binding interaction with PAI-1. Additionally, oleuropein exhibits the capacity to act as a natural inhibitor of PAI-1 by progressively destabilizing PAI-1 levels, particularly in ER-/PR- breast cancer cells. This impact is accompanied by the suppression of cell growth and a downstream caspase activation. | Inhibition of cell proliferation | Tzekaki et al. [59] |
| Oleuropein | in vitro | MDA-MB-231 and MDA-MB-468 | Oleuropein reduces cell viability by inducing cell cycle arrest and stimulating apoptosis in triple-negative breast cancer cells. In MDA-MB-468 cells, a significant increase in the expression of apoptosis-related genes is observed, particularly in two members of the caspase family (CASP1 and CASP14), proapoptotic genes (BNIP2, GADD45A, BID, BNIP3, and BCL10), and the TNF receptors TNFRSF21 and FADD. In MDA-MB-231 cells, RIPK2 and BIRC3 were upregulated and PYCARD and CASP6 were downregulated. Additionally, the antiapoptotic gene TNFRSF11B and the survivin BIRC5 were also downregulated. | Inhibition of cell proliferation and induction of cell death (apoptosis) | Messeha et al. [60] |
| Oleuropein | in vitro | BPH-1, LNCaP and DU145 | Oleuropein reduces cell viability and induces changes in thiol groups, reactive oxygen species, γ-glutamylcysteine synthetase, and hemeoxygenase-1 in prostate cancer cell lines. Moreover, it suppresses Akt signalling by inhibiting pAkt(Ser473) and Akt(Thr308). | Inhibition of cell proliferation and modulation of cancer cell signalling pathways | Acquaviva et al. [61] |
| Oleuropein | in vitro and in vivo (mouse, TgCRND8) | TgCRND8 mice/SH-SY5Y and RIN-5F | Oleuropein induces autophagy by triggering the Ca2+-CAMKKβ–AMPK pathway. Additionally, Oleuropein inhibits mTOR through the activation of AMPK in the cortex of TgCRND8 mice. This suggests that the induction of autophagy by oleuropein develops through the AMPK/mTOR signalling pathway. | Induction of autophagy and modulation of cancer cell signalling pathways | Rigacci et al. [62] |
| Oleuropein | in vitro | MCF-7 | Oleuropein induces apoptosis by upregulating the expression levels of both p53 and Bax genes while concurrently downregulating the expression of Bcl2. | Inducing cell death (apoptosis) | Hassan et al. [63] |
| Oleuropein | in vitro | MDA | Oleuropein prevents cancer metastasis by upregulating the TIMPs (TIMP1,-3, and -4) and downregulating the MMPs (MMP2 and MMP9) gene expressions. | Inhibition of metastasis | Hassan et al. [64] |
| Oleuropein | in vivo (mouse, C57BL/6, female) | AOM/DSS-induced CRC in C57BL/6 mice | Oleuropein inhibits the onset of colonic neoplasia in AOM/DSS-induced colorectal cancer (CRC) in mice by inhibiting inflammation in the colon and restricting the activation of STAT3, NF-κB, PI3K/Akt, and β-catenin. | Inhibition of cell proliferation | Giner et al. [65] |
| Oleuropein | in vivo (rat, Wistar, female) | N-ethyl-N-nitrosourea (ENU)-induced brain tumors | Oleuropein exhibits a restricted yet beneficial impact as an anticancer compound. These effects manifest through redox control mechanisms that engage both endogenous enzymatic and non-enzymatic antioxidant defense systems. Notably, the observed effects are closely tied to the gender of the animals. | Disruption of redox hemostasis | Ramírez-Expósito et al. [66] |
| Oleuropein | in vitro | MIA PaCa2, BxPC-3, CFPAC-1, HPDE and ASPC-1 | Oleuropein arrests the cell cycle and induces apoptosis in pancreatic cancer cells by raising the Bax/Bcl-2 ratio. Moreover, oleuropein enhances the expression of c-Jun and c-Fos and thereby induces apoptosis. | Inducing cell death (apoptosis) | Goldsmith et al. [67] |
| Oleuropein | in vitro | MDA-MB-231 | Oleuropein inhibits the viability of breast cancer cells and decreases cell migration and invasion through HGF or 3-MA induction causing autophagy. This is achieved by reversing the downregulation of LC3-II/LC3-I and Beclin-1, as well as the upregulation of p62. | Induction of authophagy | Lu et al. [68] |
| Oleuropein | in vitro | MCF-7 and MCF-10A | Oleuropein triggers apoptosis and significantly enhances the expression of Prdx genes in breast cancer cells. | Inducing cell death (apoptosis) | Junkins et al. [69] |
| Oleuropein | in vitro | MAT-LyLu | Oleuropein inhibits cell migration and suppress the invasiveness of MAT-LyLu cells. This effect was associated with the inhibition of VGSCs, primarily resulting from the direct reduction in mRNA expression of SCN9A. | Inhibition of cell migration | Aktas et al. [70] |
| Oleuropein | in vitro | MCF7 and MDA-MB-231 | Oleuropein shows a substantial reduction in cell viability and induces apoptosis in breast cancer cells. Additionally, it elevates the expression levels of miR-125b, miR-16, miR-34a, p53, p21, and TNFRS10B while concurrently diminishing the expression of bcl-2, mcl1, miR-221, and miR-29a. | Inducing cell death (apoptosis) | Asgharzade et al. [71] |
| Oleuropein | in vitro | T98G | Oleuropein reduces cell viability and enhances the therapeutic effect of temozolomide in glioblastoma cells while also upregulating the expression of miR-181b, miR-137, and Let-7d in these cells. | Inhibition of proliferation | Tezcan et al. [72] |
| Oleuropein | in vitro | HT29 | Oleuropein reduces cell viability, alters the distribution of the cell cycle, and triggers apoptosis, concurrently decreasing the expression of HIF-1α protein and elevating p53 protein levels in human colon cancer cells. | Inhibition of proliferation | Cárdeno et al. [73] |
| Oleuropein | in vitro | AGS | OLE reduces cell viability, causes DNA damage, and initiates apoptosis followed by necrosis in AGS cells. Furthermore, there is a significant decline in cell viability associated with elevated intracellular levels of reactive oxygen species (ROS). | Inhibition of proliferation | Türkdoğan et al. [74] |
| Oleuropein | in vitro | MDA-MB-231 | Oleuropein inhibits the viability and migration capacity of breast cancer cells by decreasing the expression of miR194 and PD-L1 while concurrently upregulating the level of XIST. | Inhibition of proliferation and migration | Hamed et al. [75] |
| Oleuropein | in vitro | SH-SY5Y | Oleuropein induces apoptosis by activating the Bax gene and inhibiting the expression of Bcl-2. Additionally, it leads to cell cycle arrest by upregulating p53 and CDKN2A while simultaneously downregulating the expressions of CyclinD1, CyclinD2, CyclinD3, CDK4, and CDK6 genes. | Inhibition of proliferation and induction of cell death (apoptosis) | Seçme et al. [76] |
| Oleuropein | in vivo (A/J mouse, female) | AOM-induced colon tumorigenesis in A/J mice | Oleuropein inhibits the process of AOM-induced carcinogenesis in mice by diminishing the formation of dysplastic crypts in various segments of the colon. Additionally, it inhibits the progression from low dysplasia to high dysplasia. Moreover, OL has a capacity to decrease DNA damage in peripheral leukocytes. | Inhibition of proliferation | Sepporta et al. [77] |
| Oleuropein | in vitro | TPC-1 and BCPAP | Oleuropein inhibits the proliferation of thyroid cancer cells by suppressing the phosphorylation of ERK and Akt and decreasing levels of H2O2-induced reactive oxygen species (ROS). | Inhibition of proliferation, modulation of cancer cell signalling pathways, and disruption of redox hemostasis | Bulotta et al. [78] |
| Oleuropein | in vitro | MCF-7 | Oleuropein reduces the proliferation of MCF-7 cells, enhances apoptosis, inhibits migration and invasion, and decreases the expression of miR-21 and miR-155. | Inhibition of proliferation and migration | Abtin et al. [79] |
| Oleuropein | in vitro | H1299 | Oleuropein induces apoptosis and arrests the cell cycle at the G2/M phase. The mitochondrial pathway plays a crucial role in this process, involving an increase in the Bax/Bcl-2 ratio, release of cytochrome c, and activation of caspase-3. Additionally, the p38 MAPK signalling pathways are essential in the oleuropein-induced apoptosis. | Inhibition of proliferation, induction of cell death (apoptosis), and modulation of cancer cell signalling pathways | Wang et al. [80] |
| Oleuropein | in vitro and in silico | MCF-7 | Oleuropein reduces the viability of breast cancer cells and triggers cell cycle arrest by reducing the activity of the phosphatase PTP1B. | Inhibition of proliferation | Przychodzen et al. [81] |
| Oleuropein | in vitro | MCF-7 | Oleuropein inhibits the proliferation and invasion of cells by inducing apoptosis via the modulation of a crucial epigenetic factor, HDAC4. | Inhibition of proliferation and inhibition of cell invasion | Mansouri et al. [82] |
| Oleuropein | in vitro | HepG2 | Oleuropein enhances the antitumor efficacy of cisplatin by modulating the regular proNGF/NGF equilibrium in HepG2 cells. This modulation is accomplished by modulating MMP-7 activity while leaving the gene expression of NGF unaffected. | Inhibition of proliferation | Sherif et al. [83] |
| Oleuropein | in vitro | A2780 S and A278/CP | Oleuropein inhibits cisplatin resistance in ovarian cancer by suppressing the expression of Bcl-2 and Mcl1 while concurrently enhancing the levels of P53, P21, and TNFRSF10B. Furthermore, there is a substantial decrease in the expression of miR-21 and an increase in the expression of miR-125b, miR-34a, and miR-16. These changes are potentially implicated in the reduction in cisplatin resistance. | Inhibition of proliferation | Sheikhshabani et al. [84] |
| Oleuropein | in vitro | A549 and BEAS–2 B | Oleuropein plays a role in causing G2/M phase cell cycle arrest and inducing apoptotic cell death in lung cancer cells. The activation of p38MAPK is crucial for oleuropein’s induction of apoptosis. Oleuropein triggers apoptosis by leading to a decrease in mitochondrial membrane potential, a decrease in Bcl-2 expression, and an increase in Bax expression. | Inhibition of proliferation, induction of cell death (apoptosis), and modulation of cancer cell signalling pathways | Cao et al. [85] |
| Oleuropein | in vitro and in vivo (mouse, Balb/c, male) | H22 hepatoma-mouse tumor-bearing model/HepG2 and HuH7 | Oleuropein inhibits glycolysis by binding to GPI and may exhibit inhibitor-like effects on PYGM and PFKFB4. Consequently, it inhibits glycolytic metabolism, leading to an anti-tumor effect. | Disruption of redox homeostasis | Hong et al. [86] |
| Oleuropein | in vitro and in vivo (mouse, Balb/c nude, male) | 22Rv1 xenograft tumor model/22Rv1 | Surface-functionalized folate-targeted PEG liposomes loaded with oleuropein (OL-FML) exhibit anti-proliferative and apoptotic effects on prostate cancer cells. Additionally, enhanced tumor suppression and improved survival probability are observed in mice bearing 22Rv1-induced tumors when compared to the effects of oleuropein alone. | Inhibition of proliferation | Nassir et al. [87] |
| Oleuropein | in vitro | U251 and A172 | Oleuropein reduces cell viability, triggers apoptosis, inhibits metastasis, and suppresses invasion in glioma cells by attenuating AKT signalling, concomitant with the elevation of Bax and the reduction in Bcl-2. Furthermore, it diminishes the expression of matrix metalloproteinase-9 (MMP-9) and MMP-2. | Inhibition of proliferation, metastasis, and modulation of cancer cell signalling pathways | Liu et al. [88] |
| Oleuropein | in vitro | A549, BEAS-2B | Oleuropein triggers apoptosis in lung cancer cells via the SOD2/O2⋅- /Akt/mGlo2 axis. This process involves the upregulation of mitochondrial Glo2 facilitated by the superoxide anion and the Akt signalling pathway. | Inducing cell death (apoptosis) | Antognelli et al. [89] |
| Oleuropein | in vitro | HEY and MCF-7 | At a high dose, oleuropein inhibits the viability and growth of ovarian cancer cells by elevating reactive oxygen species (ROS) production and LIP levels, leading to the disruption of the cell cycle S-phase and the initiation of apoptosis. Conversely, at a low dose, oleuropein reduces ROS and LIP contents in ovarian cancer cells. Interestingly, this downregulation contradicts cell death induced by erastin-mediated oxidative stress. | Induction of cell death (apoptosis) and disruption of redox hemostasis | Scicchitano et al. [90] |
| Oleuropein | in vitro | Hela | Oleuropein reduces cell viability by inducing apoptosis, accomplished by suppressing anti-apoptotic genes such as Mcl1 and Bcl-2. Concurrently, it increases the expression of pro-apoptotic genes, such as Fas, Bid, TNFRSF10B, and the p53 tumor suppressor. Additionally, oleuropein increases the expression of miR-34a, miR-125b, and miR-29a while exhibiting decreased levels of miR-181b, miR-221, and miR-16. | Inducing cell death (apoptosis) | Amini-Farsani et al. [91] |
| Oleuropein | in vitro | MCF-7 | Oleuropein reduces the migratory ability of breast cancer cells by inhibiting the epithelial-to-mesenchymal transition through the downregulation of sirtuin1 (SIRT1). Additionally, the simultaneous administration of oleuropein and doxorubicin amplifies their apoptotic and cytotoxic effects on these cell lines. | Inhibition of proliferation and migration | Choupani et al. [92] |
| Oleuropein | in vitro | AGS | Magnetic nano-oleuropein leads to the inhibition of gastric adenocarcinoma cell proliferation through the upregulation of KRAS and miR-200 gene expression. | Inhibition of proliferation | Barzegar et al. [93] |
| Oleuropein | in vitro | MCF-7, H1299, and HeLa | Oleuropein inhibits the growth of HeLa cells by inducing cell cycle arrest at the G2/M phase and promoting apoptosis. Its impact includes the upregulation of Bax and the concurrent downregulation of Bcl-2, potentially leading to the release of cytochrome c, which is associated with the mitochondrial apoptotic pathway. Additionally, oleuropein activates the JNK signalling pathway. | Inducing cell death (apoptosis) | Yao et al. [94] |
| Oleuropein | in vitro | MCF-7 and T47D | Oleuropein inhibits migration and invasion in ER-positive breast cancer by inducing autophagy through the upregulation of LC3II/LC3I and Beclin1 expression, along with a simultaneous downregulation of p62 expression. | Inhibition of migration and induction of autophagy | Lu et al. [95] |
| Oleuropein | in vitro and in vivo (mouse, BALB/c, male) | squamous cell carcinoma of the head and neck xenograft tumor mice/Tu686, 686LN-M2 and CAL-27 | Oleuropein triggers apoptosis, reverses epithelial to mesenchymal transition (increase E-cadherin while reducing vimentin, snail, and MMP9), suppresses migration and invasion by suppressing the TGF-β1 signalling pathway. This inhibition affects the classical TGF-β1-Smad2 pathway and HIF-1α-related signalling in squamous cell carcinoma of the head and neck cells. | Inhibition of proliferation and inhibition of migration | Xu et al. [96] |
| Oleuropein | in vitro | SW480 and HEK293 | Nanoparticles of iron oxide, coated with glucose and conjugated with oleuropein (Fe3O4@Glu-Oleuropein NPs), enhance the induction of necrotic and apoptotic cell death in colorectal cancer. Simultaneously, these nanoparticles lead to the down-regulation of GAS6-AS1, LINC00920, and FEZF1-AS1 long non-coding RNAs (lncRNAs). These genes are correlated with mutated KRAS and exhibit strong associations with hypoxia, KRAS signalling, DNA repair, and the IL-2/STAT5 signalling pathways. | Inhibition of proliferation | Niyaki et al. [97] |
| Oleuropein | in vitro | Tumor spheres/DLD-1 and 5-FU-resistant cells | Oleuropein inhibits the capacity for colorectal tumor sphere formation and triggers apoptosis. It also induces mitochondrial fragmentation and enhances mitochondrial superoxide production. Furthermore, the combination of oleuropein with 5-FU exhibits a synergistic effect in reducing colorectal cancer cells proliferation. | Inhibition of proliferation | Kim et al. [98] |
| Oleuropein | in vitro | U87 | Oleuropein enhances cell viability, total oxidant capacity, and glutathione levels in glioblastoma cells following H2O2 administration. This suggests that oleuropein possesses strong antioxidative properties. | Antioxidative properties | Kucukgul et al. [99] |
| Oleuropein | in vivo (mouse. ICR female) | Mouse Skin Carcinogenesis | Oleuropein demonstrates a reduction in the thickness of epidermal hyperplasia when compared to the thick hyperplasia and epidermal disorganization observed in the DMBA/TPA control group. It also enhances apoptotic rates, as indicated by the activation of caspase-3, while concurrently decreasing the levels of MDA and GSH. Additionally, there is an increase in SOD levels. Oleuropein may function as a potential chemopreventive agent by exerting apoptotic and antioxidant defense activities during tumor development in skin carcinogenesis. | Inhibition of proliferation and antioxidative properties | Masre et al. [100] |
| Oleuropein | in vitro | MRC5, hMVECs and ECFCs | Oleuropein reduces migration of fibroblast SASP-dependent cells and tube formation of endothelial cells via the modulation of pro-angiogenic factor secretion surrounding of cell microenvironment. | Inhibition of migration and angiogenesis | Margheri et al. [101] |
| Oleuropein | in vitro | MCF-7 | Nano-oleuropein enhances the Krebs cycle via upregulation of fumarylacetoacetase, succinate-CoA ligase, and isocitrate dehydrogenase1. Oleuropein functions by inhibiting glycolysis in cancer cells, prompting a shift towards the TCA cycle. This transformation may indicate inhibition of carcinogenesis in breast cancer cells. | Inhibition of proliferation | Kamrani et al. [102] |
| Oleuropein | in vivo (mouse, ICR mouse, female) | Mouse Skin Carcinogenesis | Oleuropein inhibits the progression of skin carcinogenesis through its antioxidant properties, reducing MDA levels while increasing GSH and SOD levels, and exerting an antiapoptotic effect on precancerous cells. | Inhibition of proliferation and antioxidative properties | John et al. [35] |
| Oleuropein | in vivo and in vitro (mouse, C57BL/6N, male) | Skin melanoma model/B16F10, Raw264.7, HUVECs and 3T3-L1 | Oleuropein has inhibitory effects on the development of solid tumors and lymph node metastasis in C57BL/6 mice injected with B16F10 melanoma cells. Moreover, it directly impedes the differentiation of adipocytes. Additionally, dietary oleuropein restricts the accumulation of adipocytes and M2-MΦs induced by a high-fat diet (HFD). It also reduces the expression of VEGF-A, VEGF-D, and HIF-1α in tumor tissues. This suppression results in the inhibition of tumor angiogenesis and lymphangiogenesis in melanoma obese mice. | Inhibition of proliferation, inhibition of angiogenesis, and inhibition of metastasis | Song et al. [103] |
| Oleuropein | in vitro | HepG2 | Oleuropein exhibits suppressive effects on paraquat (PQ)-induced oxidative stress in hepatocarcinoma cells by enhancing cell viability, normalizing β-tubulin expression levels, and reducing Casp-3. Furthermore, these protective effects mediated by oleuropein are linked to elevated malondialdehyde levels and increased superoxide generation. | Inhibition of oxidative stress | Katsoulieris et al. [104] |
| Oleuropein | in vivo (mouse, Balb/c male) | Balb/C | Oleuropein prevents tamoxifen from inducing oxidative DNA damage by decreasing the formation of 8-hydroxydeoxyguanosine. | Disruption of redox hemostasis | Jamshed et al. [105] |
| Oleuropein | in vitro and in vivo (mouse, Wistar, male) | Glioma cell implantation/C6 | Oleuropein demonstrates the inhibition of glioma cell proliferation in vitro; however, it does not exhibit an anti-tumor effect on glioma tumors in vivo. | Inhibition of proliferation | Martínez-Martos et al. [106] |
| Oleuropein | in vitro | T-47D and MCF-7 | Peracetylated derivatives of oleuropein demonstrate in vitro inhibitory effects on the proliferation of breast cancer cells and exhibit stronger antioxidant properties compared to free oleuropein. | Inhibition of proliferation | Bulotta et al. [107] |
| Oleuropein | in vitro | HepG2, HA22T, AML12 and HA59T | Oleuropein triggers cell cycle arrest, a process linked to the modulation of p53, p21, CDK1, and cyclin B1 levels. Additionally, oleuropein increases intracellular ROS levels while decreasing GSH levels. In HepG2 cells, oleuropein induces a rise of Ca2+ by releasing Ca2+ from the endoplasmic reticulum (ER) and facilitating Ca2+ influx through store-operated Ca2+ channels. Furthermore, oleuropein triggers Ca2+-associated cytotoxicity through ROS signalling and leads to cell cycle arrest. | Inhibition of proliferation | Cheng et al. [108] |
| Oleacein and oleacanthal | in vitro and in vivo (chicken chorioallantoic membrane (CAM)) | Chicken chorioallantoic membrane and zebrafish caudal fin/BAEC, HeLa and HGF-1 | Oleocanthal and oleacein inhibit the invasion, proliferation, and tube formation of endothelial cells, demonstrating an antiangiogenic effect. Furthermore, oleacein suppresses migration and induces apoptosis. Mechanistically, these compounds modulate signalling pathways associated with proliferation and survival, such as AKT and ERK1/2. | Induction of cell death (apoptosis), inhibition of angiogenesis, and modulation of cancer cell signalling pathways | Marrero et al. [109] |
| Oleacein | in vitro | 501Mel | Oleacein induces cell growth inhibition in 501Mel melanoma cells through the induction of G1/S phase arrest, DNA fragmentation, and downregulation of antiapoptotic genes (BCL2 and MCL1) and proproliferative proteins (c-KIT, K-RAS, PIK3R3, mTOR). Moreover, oleacein increases the levels of miR-193a-5p (targeting PIK3R3 and mTOR), miR-193a-3p (targeting c-KIT, MCL1, and K-RAS), miR-34a-5p (targeting c-KIT and BCL2), and miR-16-5p (targeting K-RAS, BCL2, and mTOR) while decreasing miR-214-3p (targeting BAX). | Induction of cell death (apoptosis), inhibition of cell proliferation, and modulation of cancer cell signalling pathways | Carpi et al. [110] |
| Oleacein | in vitro | SH-SY5Y and WI-38 | Oleacein inhibits the proliferation of neuroblastoma cells by inhibiting the cell cycle at the S phase and triggering apoptotic cell death. Apoptotic effect occurs markedly through upregulation of both p53 and Bax levels, coupled with a downregulation of Bcl-2 expression and STAT3 phosphorylation. Additionally, oleacein induces a decrease in migration and cell adhesion in these cells. | Induction of cell death (apoptosis), inhibition of migration, inhibition of cell proliferation, and modulation of cancer cell signalling pathways | Cirmi et al. [111] |
| Oleacein | in vitro | RPMI-8226, NCI-H929, MM1s, U266, and JJN3 | Oleacein induces cell cycle arrest and apoptosis in multiple myeloma. It enhances the accumulation of α-tubulin and acetylated histones while concurrently reducing the expression of several class I/II histone deacetylases (HDACs) at both the protein and mRNA levels. The inhibition of HDACs by oleacein is linked to the down-regulation of Sp1, the primary transactivator of the HDACs promoter, through the activation of Caspase 8. | Inhibition of proliferation | Juli et al. [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusuma, I.Y.; Habibie, H.; Bahar, M.A.; Budán, F.; Csupor, D. Anticancer Effects of Secoiridoids—A Scoping Review of the Molecular Mechanisms behind the Chemopreventive Effects of the Olive Tree Components Oleocanthal, Oleacein, and Oleuropein. Nutrients 2024, 16, 2755. https://doi.org/10.3390/nu16162755
Kusuma IY, Habibie H, Bahar MA, Budán F, Csupor D. Anticancer Effects of Secoiridoids—A Scoping Review of the Molecular Mechanisms behind the Chemopreventive Effects of the Olive Tree Components Oleocanthal, Oleacein, and Oleuropein. Nutrients. 2024; 16(16):2755. https://doi.org/10.3390/nu16162755
Chicago/Turabian StyleKusuma, Ikhwan Yuda, Habibie Habibie, Muh. Akbar Bahar, Ferenc Budán, and Dezső Csupor. 2024. "Anticancer Effects of Secoiridoids—A Scoping Review of the Molecular Mechanisms behind the Chemopreventive Effects of the Olive Tree Components Oleocanthal, Oleacein, and Oleuropein" Nutrients 16, no. 16: 2755. https://doi.org/10.3390/nu16162755
APA StyleKusuma, I. Y., Habibie, H., Bahar, M. A., Budán, F., & Csupor, D. (2024). Anticancer Effects of Secoiridoids—A Scoping Review of the Molecular Mechanisms behind the Chemopreventive Effects of the Olive Tree Components Oleocanthal, Oleacein, and Oleuropein. Nutrients, 16(16), 2755. https://doi.org/10.3390/nu16162755






