Is Oral Iron and Folate Supplementation during Pregnancy Protective against Low Birth Weight and Preterm Birth in Africa? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
- To synthesise available evidence on the relationship between oral iron and/or folate supplementation on low birth weight and preterm birth;
- To evaluate the dose–effect relationship and effect of duration of oral iron and/or folate supplementation on low birth weight and preterm birth;
- If the data allow, to conduct a subgroup analysis based on common sociodemographic characteristics
2. Materials and Methods
2.1. Selection Criteria
2.1.1. Types of Studies
2.1.2. Population of Interest
2.1.3. Exposure and Comparators
2.2. Outcomes
Birth Outcomes
2.3. Context and Language
2.4. Information Sources and Search Strategy
2.5. Study Selection
2.6. Data Extraction
2.7. Quality Appraisal
2.8. Data Synthesis and Meta-Analysis
3. Results
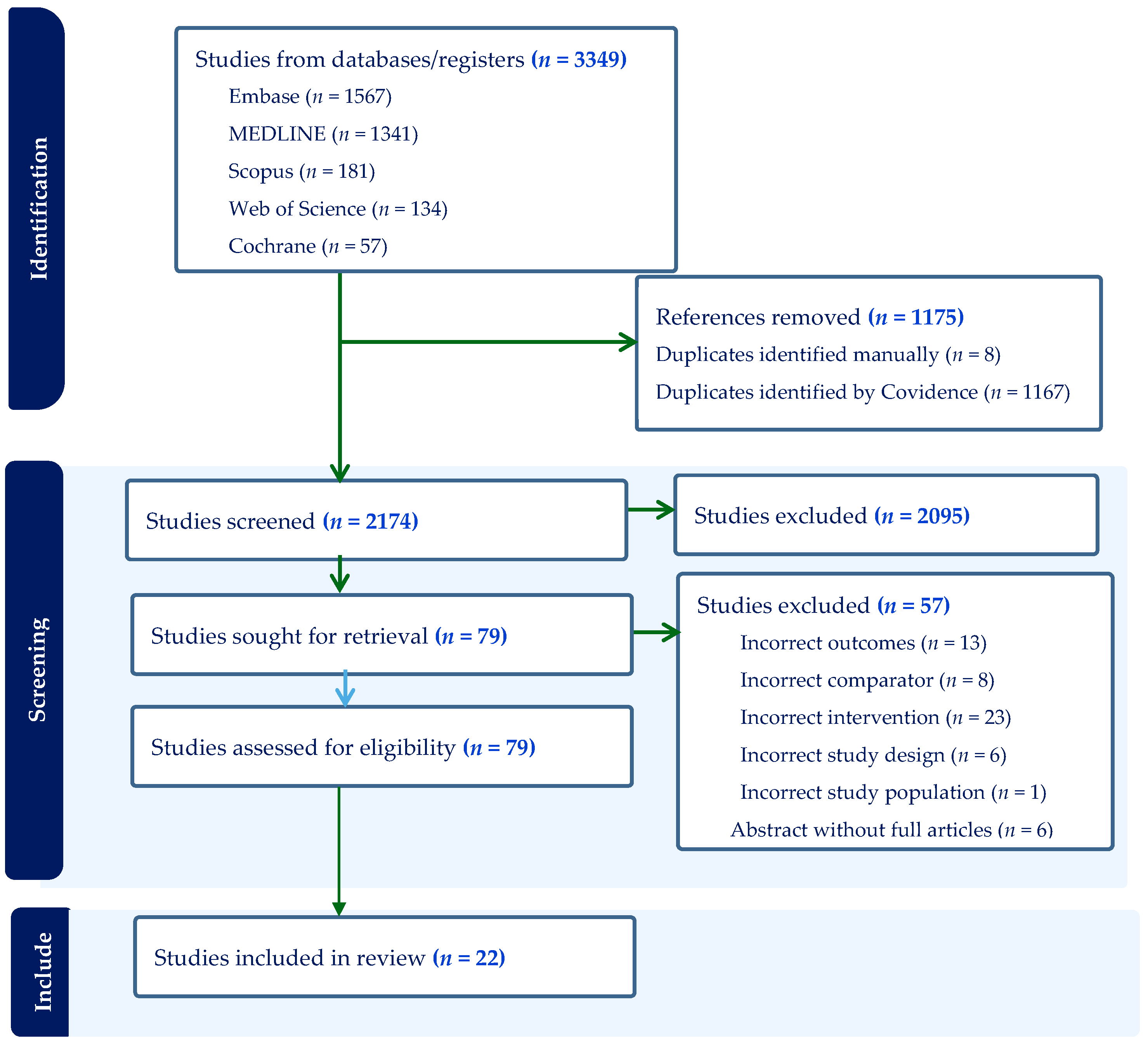
3.1. Search Results and Characteristics of Included Studies
3.2. Participants
3.3. Interventions/Exposures
3.4. Quality Assessment
3.5. Outcomes (n = 22)
3.5.1. Low Birth Weight (n = 19)
3.5.2. Preterm Birth (n = 4)
3.6. Meta-Analysis
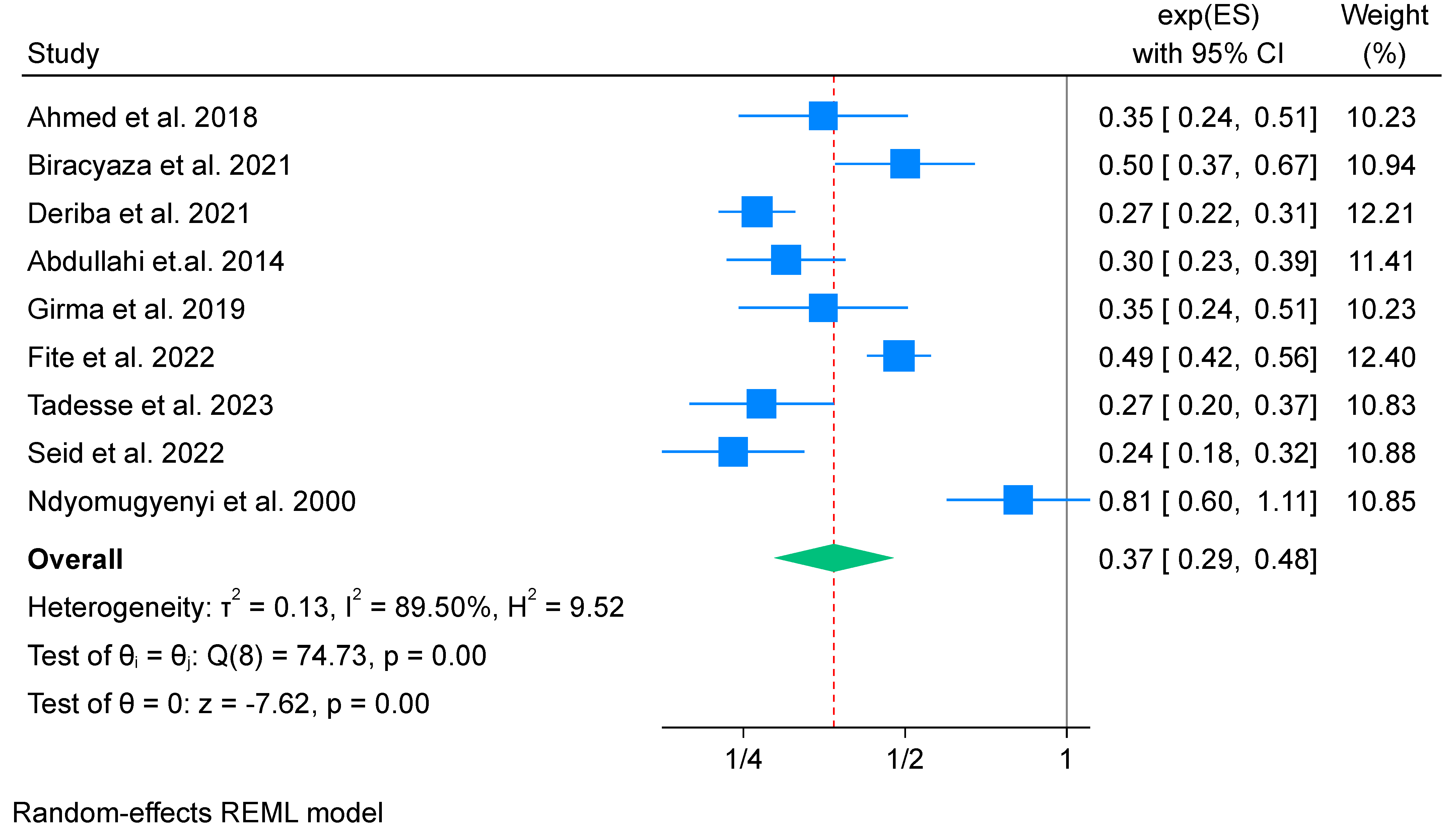
3.6.1. IFA Supplementation on Low Birth Weight (Meta-Analysis) (n = 9)
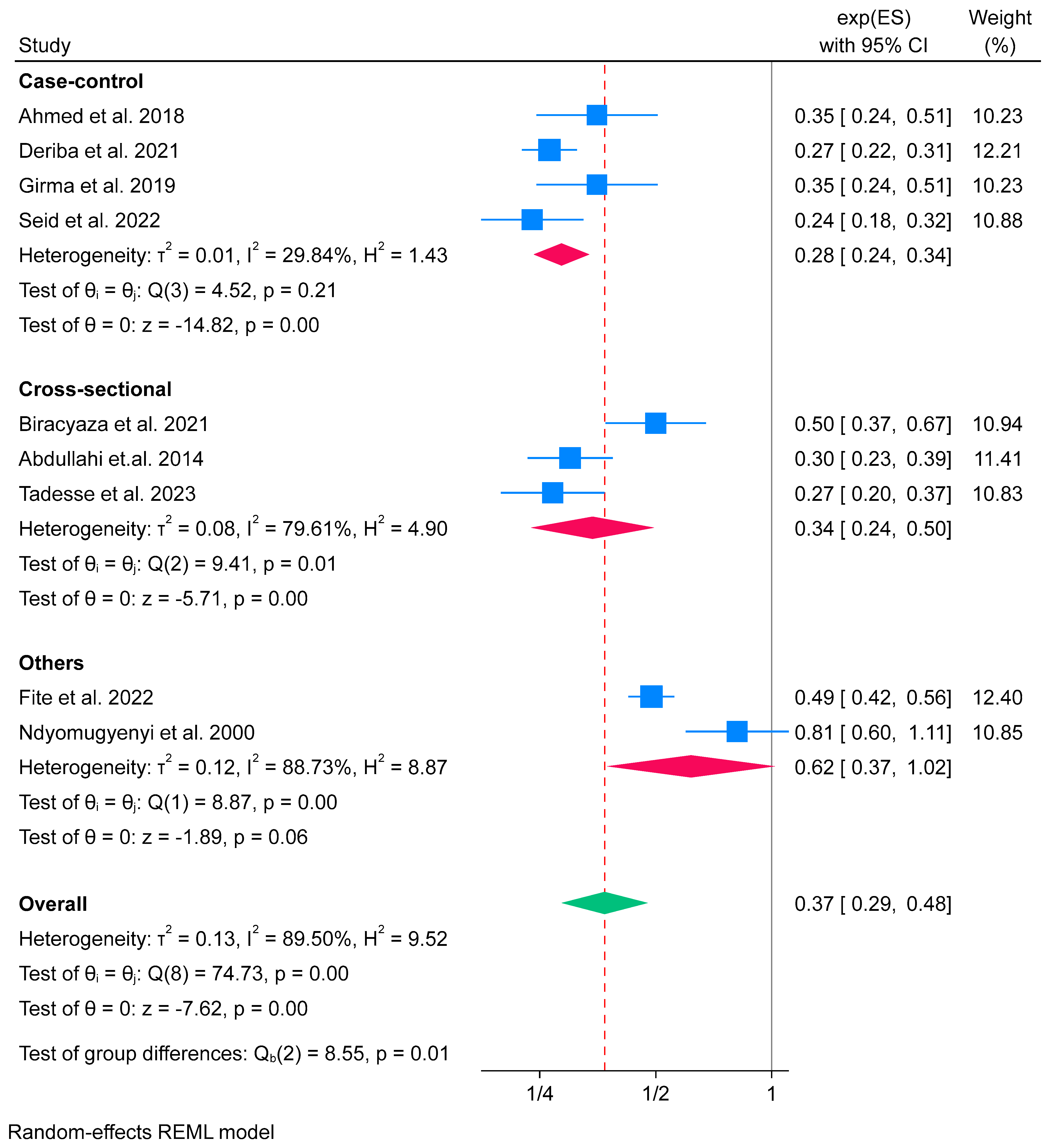
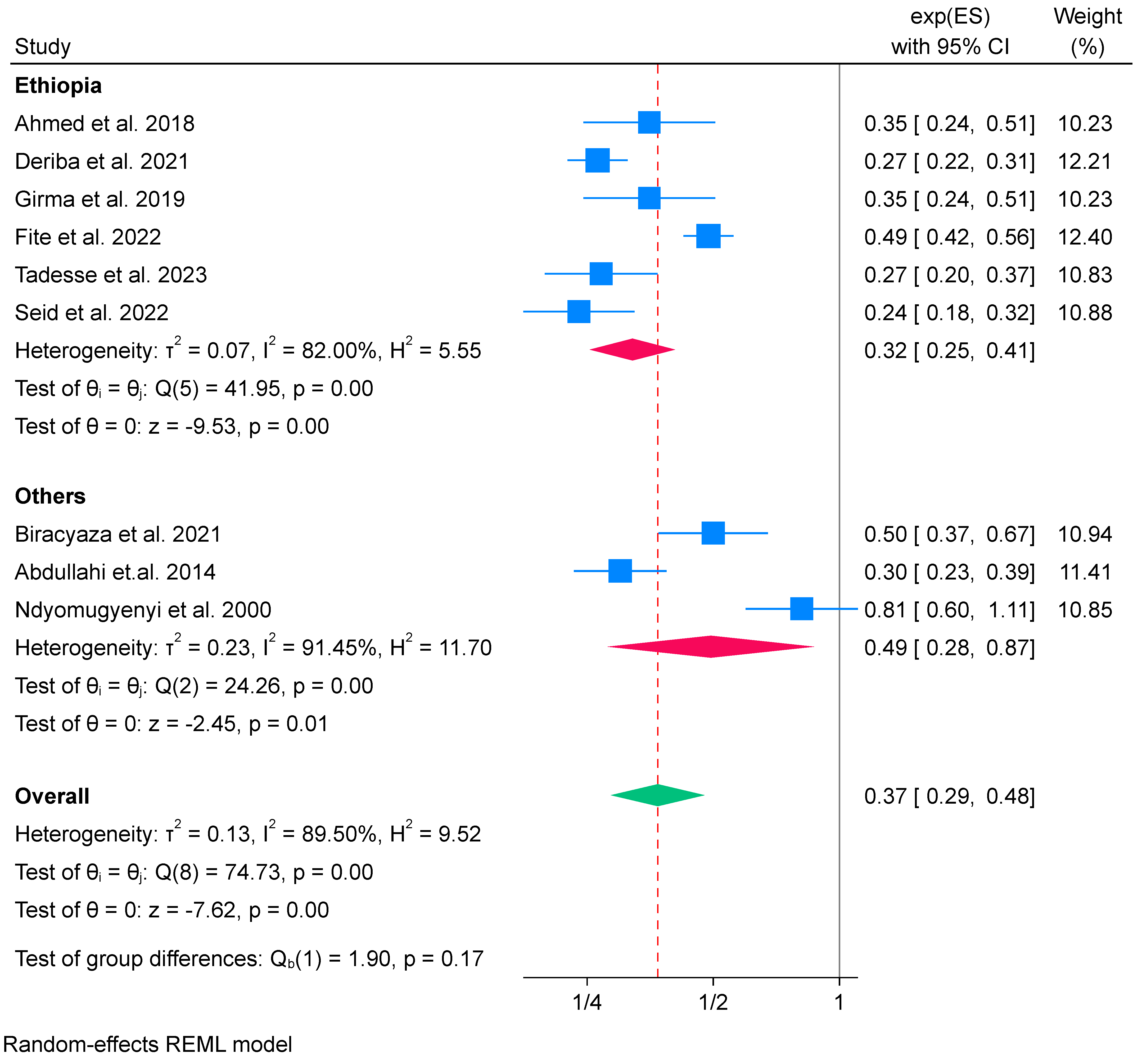
Subgroup Analysis of Iron Folate Supplementation and Low Birth Weight
Sensitivity Test
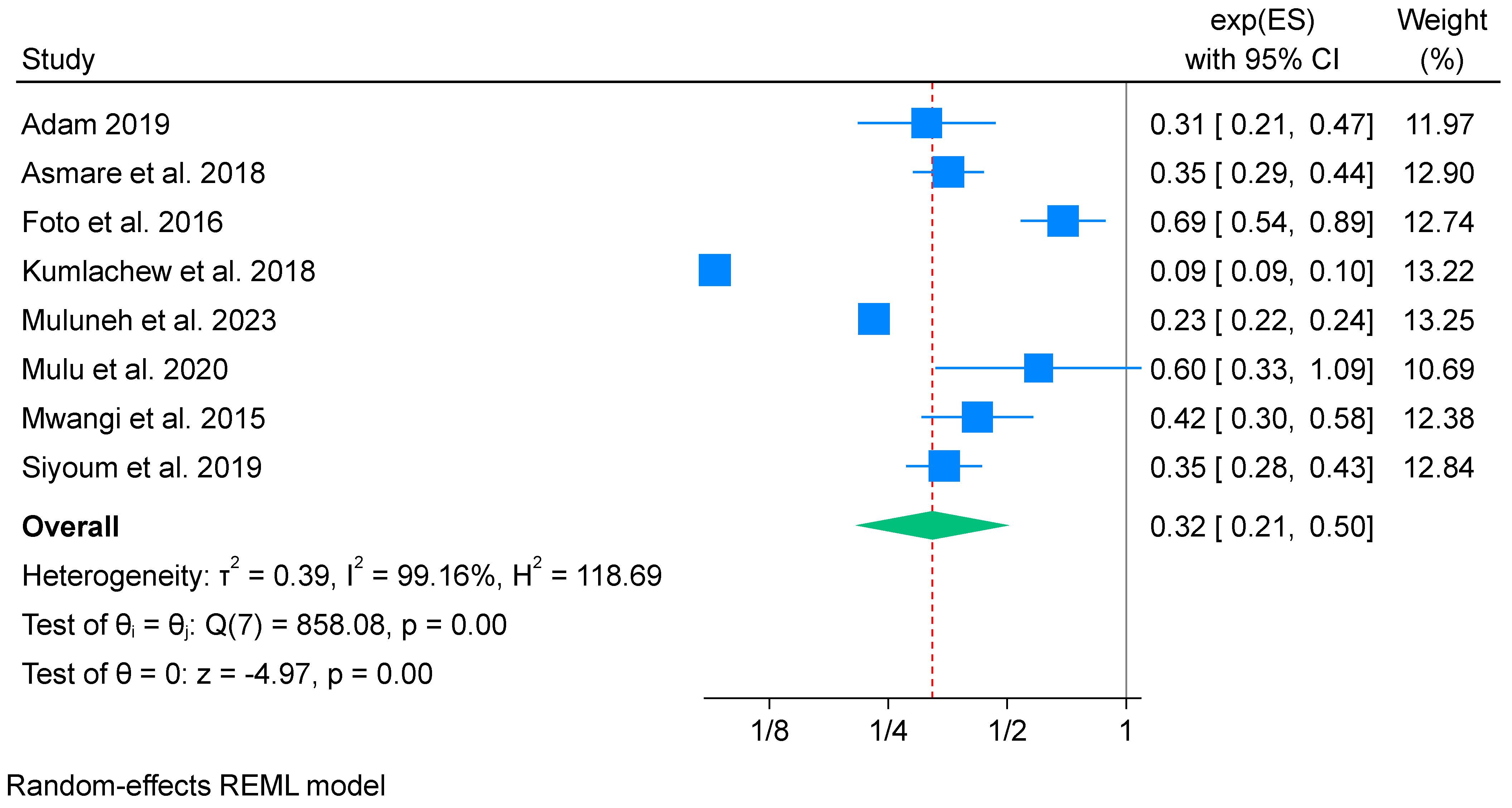
3.6.2. Effects of Iron-Only Supplementation on Low Birth Weight (Meta-Analysis) (n = 8)
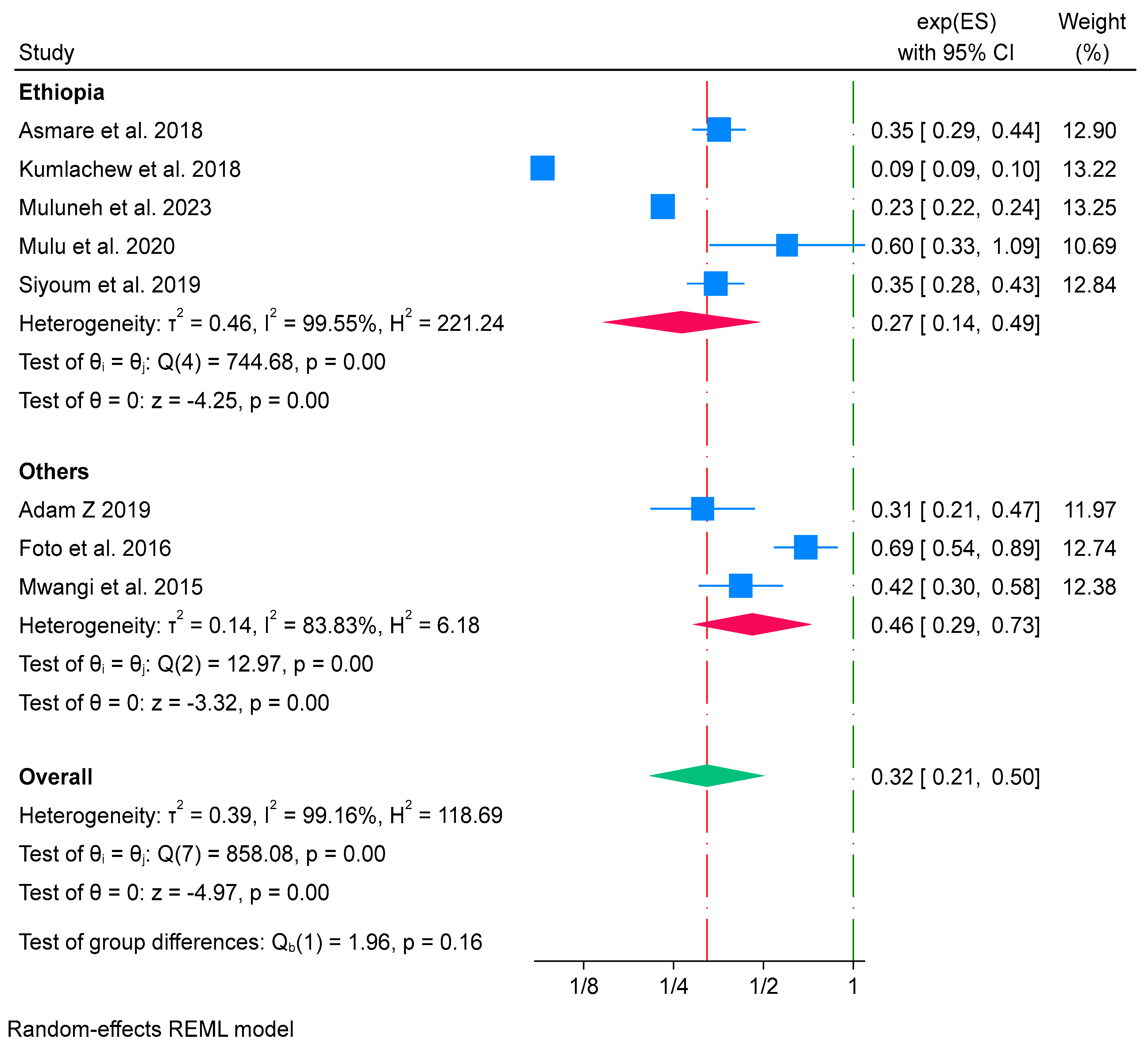
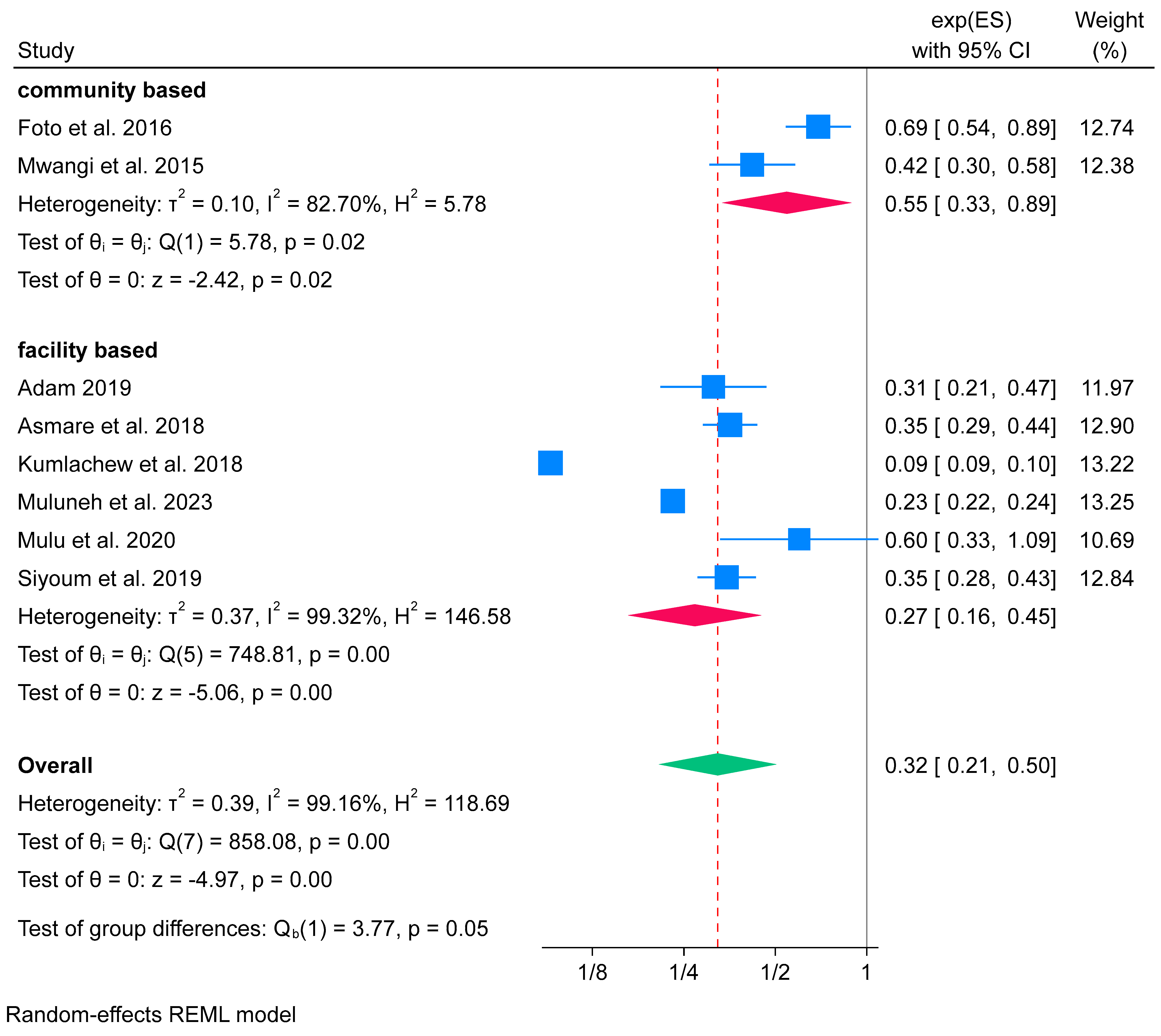
Subgroup Analysis of Iron-Only Supplementation and Low Birth Weight
Sensitivity Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marchant, T.; Willey, B.; Katz, J.; Clarke, S.; Kariuki, S.; ter Kuile, F.; Llusingu, J.; Ndyomugyenyi, R.; Schmiegelow, C.; Watson-Jones, D.; et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: Individual participant level meta-analysis. PLoS Med. 2012, 9, e1001292. [Google Scholar] [CrossRef]
- World Health Organization. UNICEF-WHO Low Birthweight Estimates: Levels and Trends 2000–2015; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Blencowe, H.; Krasevec, J.; de Onis, M.; E Black, R.; An, X.; A Stevens, G.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef] [PubMed]
- O Ohuma, E.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Hug, L.; You, D.; Blencowe, H.; Mishra, A.; Wang, Z.; Fix, M.J.; Wakefield, J.; Moran, A.C.; Gaigbe-Togbe, V.; Suzuki, E.; et al. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: A systematic assessment. Lancet 2021, 398, 772–785. [Google Scholar] [CrossRef]
- Hug, L.; Alexander, M.; You, D.; Alkema, L. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: A systematic analysis. Lancet Glob. Health 2019, 7, e710–e720. [Google Scholar] [CrossRef] [PubMed]
- Sema, A.; Tesfaye, F.; Belay, Y.; Amsalu, B.; Bekele, D.; Desalew, A. Associated Factors with Low Birth Weight in Dire Dawa City, Eastern Ethiopia: A Cross-Sectional Study. BioMed Res. Int. 2019, 2019, 2965094. [Google Scholar] [CrossRef]
- Anto, E.O.; Ofori Boadu, W.I.; Opoku, S.; Senu, E.; Tamakloe, V.C.K.T.; Tawiah, A.; Ankobea, F.; Acheampong, E.; Anto, A.O.; Appiah, M.; et al. Prevalence and risk factors of preterm birth among pregnant women admitted at the labor ward of the Komfo Anokye Teaching Hospital, Ghana. Front. Glob. Women’s Health 2022, 3, 801092. [Google Scholar] [CrossRef] [PubMed]
- United Nations (UN), General Assembly. Transforming our World: The 2030 Agenda for Sustainable Development; United Nations General Assembly: New York, NY, USA, 2016. [Google Scholar]
- Petrou, S.; Sach, T.; Davidson, L. The long-term costs of preterm birth and low birth weight: Results of a systematic review. Child Care Health Dev. 2001, 27, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Petrou, S.; Eddama, O.; Mangham, L. A structured review of the recent literature on the economic consequences of preterm birth. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 96, F225–F232. [Google Scholar] [CrossRef]
- Bilgin, A.; Mendonca, M.; Wolke, D. Preterm birth/low birth weight and markers reflective of wealth in adulthood: A meta-analysis. Pediatrics 2018, 142, e20173625. [Google Scholar] [CrossRef]
- Moster, D.; Lie, R.T.; Markestad, T. Long-term medical and social consequences of preterm birth. N. Engl. J. Med. 2008, 359, 262–273. [Google Scholar] [CrossRef]
- Alderman, H.; Behrman, J.R. Reducing the incidence of low birth weight in low-income countries has substantial economic benefits. World Bank Res. Obs. 2006, 21, 25–48. [Google Scholar] [CrossRef]
- Kc, A.; Wrammert, J.; Nelin, V.; Ewald, U.; Clark, R.; Målqvist, M. Level of mortality risk for babies born preterm or with a small weight for gestation in a tertiary hospital of Nepal. BMC Public Health 2015, 15, 877. [Google Scholar] [CrossRef]
- Haider, B.A.; Olofin, I.; Wang, M.; Spiegelman, D.; Ezzati, M.; Fawzi, W.W. Anaemia, prenatal iron use, and risk of adverse preg-nancy outcomes: Systematic review and meta-analysis. BMJ 2013, 346, f3443. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.T.; Binyaruka, P.; Hangoma, P.; Robberstad, B.; Sandoy, I. Patient and health system costs of managing pregnancy and birth-related complications in sub-Saharan Africa: A systematic review. Health Econ. Rev. 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Tongo, O.O.; Orimadegun, A.E.; Ajayi, S.O.; Akinyinka, O.O. The Economic Burden of Preterm/Very Low Birth Weight Care in Nigeria. J. Trop. Pediatr. 2009, 55, 262–264. [Google Scholar] [CrossRef]
- Ngabonzima, A.; Asingizwe, D.; Cechetto, D.; Mukunde, G.; Nyalihama, A.; Gakwerere, M.; Epstein, D.M. Evaluating the medical direct costs associated with prematurity during the initial hospitalization in Rwanda: A prevalence based cost of illness study. BMC Health Serv. Res. 2022, 22, 953. [Google Scholar] [CrossRef]
- Scholl, T.O. Maternal iron status: Relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr. Rev. 2011, 69 (Suppl. S1), S23–S29. [Google Scholar] [CrossRef]
- Rahmati, S.; Azami, M.; Badfar, G.; Parizad, N.; Sayehmiri, K. The relationship between maternal anemia during pregnancy with preterm birth: A systematic review and meta-analysis. J. Matern. Neonatal Med. 2020, 33, 2679–2689. [Google Scholar] [CrossRef]
- Most, J.; Marlatt, K.L.; Altazan, A.D.; Redman, L.M. Advances in assessing body composition during pregnancy. Eur. J. Clin. Nutr. 2018, 72, 645–656. [Google Scholar] [CrossRef]
- Georgieff, M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Purvarshi, G.; Wang, S.; Zhong, L.; Tang, H. The Influence of Iron-deficiency Anemia during the Pregnancy on Preterm Birth and Birth Weight in South China. J. Food Nutr. Res. 2015, 3, 570–574. [Google Scholar] [CrossRef][Green Version]
- Kemppinen, L.; Mattila, M.; Ekholm, E.; Pallasmaa, N.; Törmä, A.; Varakas, L.; Mäkikallio, K. Gestational iron deficiency anemia is associated with preterm birth, fetal growth restriction, and postpartum infections. J. Perinat. Med. 2021, 49, 431–438. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Sanghvi, T.G.; Nguyen, P.H.; Forissier, T.; Ghosh, S.; Zafimanjaka, M.; Walissa, T.; Mahmud, Z.; Kim, S. Comprehensive Approach for Improving Adherence to Prenatal Iron and Folic Acid Supplements Based on Intervention Studies in Bangladesh, Burkina Faso, Ethiopia, and India. Food Nutr. Bull. 2023, 44, 183–194. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience: Summary: Highlights and Key Messages from the World Health Organization’s 2016 Global Recommendations for Routine Antenatal Care; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Fite, M.B.; Roba, K.T.; Oljira, L.; Tura, A.K.; Yadeta, T.A. Compliance with Iron and Folic Acid Supplementation (IFAS) and associated factors among pregnant women in Sub-Saharan Africa: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0249789. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Drakesmith, H.; Black, J.; Hipgrave, D.; Biggs, B.-A. Control of iron deficiency anemia in low- and middle-income countries. Blood 2013, 121, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Siekmans, K.; Roche, M.; Kung’U, J.K.; Desrochers, R.E.; De-Regil, L.M. Barriers and enablers for iron folic acid (IFA) supplementation in pregnant women. Matern. Child Nutr. 2018, 14, e12532. [Google Scholar] [CrossRef] [PubMed]
- Mahundi, P.; Pillay, K.; Wiles, N. Barriers to Optimal Iron Supplementation by Pregnant Women Attending the Mutare City Clinic, Manicaland, Zimbabwe. Afr. J. Nurs. Midwifery 2021, 23, 17. [Google Scholar] [CrossRef]
- Ghana Statistical Service (GSS); Ghana Health Service (GHSG); ICF International. Ghana Demographic and Health Survey 2014; GSS: Accra, Ghana; GHS: Accra, Ghana; ICF International: Rockville, MD, USA, 2015.
- ICF. CSACEa. Ethiopia Demographic and Health Survey; Central Statistical Agency: Addis Ababa, Etiopia, 2016.
- National Population Commission (NPC); ICF International. Nigerian Demographic Health Survey; NPC: Abuja, Nigeria; ICF International: Rockville, MD, USA, 2019.
- Breymann, C. Iron deficiency anemia in pregnancy. Semin. Hematol. 2015, 52, 339–347. [Google Scholar] [CrossRef]
- Headey, D.D. The global landscape of poverty, food insecurity, and malnutrition and implications for agricultural development strategies. SSRN Electron. J. 2013. [Google Scholar] [CrossRef]
- Alam, N.; Hajizadeh, M.; Dumont, A.; Fournier, P. Inequalities in Maternal Health Care Utilization in Sub-Saharan African Countries: A Multiyear and Multi-Country Analysis. PLoS ONE 2015, 10, e0120922. [Google Scholar] [CrossRef]
- Fite, M.B.; Assefa, N.; Mengiste, B. Prevalence and determinants of Anemia among pregnant women in sub-Saharan Africa: A systematic review and Meta-analysis. Arch. Public Health 2021, 79, 219. [Google Scholar] [CrossRef]
- Imdad, A.; Bhutta, Z.A. Routine Iron/Folate Supplementation during Pregnancy: Effect on Maternal Anaemia and Birth Outcomes. Paediatr. Périnat. Epidemiology 2012, 26, 168–177. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 12, CD004736. [Google Scholar] [CrossRef] [PubMed]
- Keats, E.C.; Oh, C.; Chau, T.; Khalifa, D.S.; Imdad, A.; Bhutta, Z.A. Effects of vitamin and mineral supplementation during pregnancy on maternal, birth, child health and development outcomes in low-and middle-income countries: A systematic review. Campbell Syst. Rev. 2021, 17, e1127. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Villar, J.; Ismail, L.C.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Effective Practice and Organisation of Care (EPOC). Cochrane Effective Practice and Organisation of Care (EPOC), Risk of Bias Assessment Tool for Randomized Control Trial; Cochrane Effective Practice and Organisation of Care: Oxford, UK, 2017. [Google Scholar]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, Canada, 2011; Volume 2, pp. 1–12. [Google Scholar]
- Ortiz, G. New meta-analysis features in Stata 18. In Proceedings of the 2023 Spanish Stata Conference, Madrid, Spain, 19 October 2023. [Google Scholar]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
- Toe, L.C.; Bouckaert, K.P.; De Beuf, K.; Roberfroid, D.; Meda, N.; Thas, O.; Van Camp, J.; Kolsteren, P.W.; Huybregts, L.F. Seasonality modifies the effect of a lipid-based nu-trient supplement for pregnant rural women on birth length. J. Nutr. 2015, 145, 634–639. [Google Scholar] [CrossRef]
- Roberfroid, D.; Huybregts, L.; Lanou, H.; Habicht, J.-P.; Henry, M.-C.; Meda, N.; Kolsteren, P. Prenatal micronutrient supplements cumu-latively increase fetal growth. J. Nutr. 2012, 142, 548–554. [Google Scholar] [CrossRef]
- Caniglia, E.C.; Zash, R.; Swanson, S.A.; Smith, E.; Sudfeld, C.; Finkelstein, J.L.; Diseko, M.; Mayondi, G.; Mmalane, M.; Makhema, J.; et al. Iron, folic acid, and multiple micronutrient sup-plementation strategies during pregnancy and adverse birth outcomes in Botswana. Lancet Glob. Health 2022, 10, e850–e861. [Google Scholar] [CrossRef]
- Ahmed, S.; Hassen, K.; Wakayo, T. A health facility based case-control study on determinants of low birth weight in Dassie town, Northeast Ethiopia: The role of nutritional factors. Nutr. J. 2018, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Adam, Z.; Ameme, D.K.; Nortey, P.; Afari, E.A.; Kenu, E. Determinants of low birth weight in neonates born in three hospitals in Brong Ahafo region, Ghana, 2016—An unmatched case-control study. BMC Pregnancy Childbirth 2019, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Asmare, G.; Berhan, N.; Berhanu, M.; Alebel, A. Determinants of low birth weight among neonates born in Amhara Regional State Referral Hospitals of Ethiopia: Unmatched case control study. BMC Res. Notes 2018, 11, 447. [Google Scholar] [CrossRef]
- Deriba, B.S. Nutritional-Related Predictors of Preterm Birth in North Shewa Hospitals, Central Ethiopia: A Case-Control Study. Pediatr. Health Med. Ther. 2021, 12, 315–324. [Google Scholar] [CrossRef]
- Deriba, B.S.; Jemal, K. Determinants of Low Birth Weight Among Women Who Gave Birth at Public Health Facilities in North Shewa Zone: Unmatched Case-Control Study. Inq. J. Health Care Organ. Provis. Financ. 2021, 58, 1–11. [Google Scholar] [CrossRef]
- Girma, S.; Fikadu, T.; Agdew, E.; Haftu, D.; Gedamu, G.; Dewana, Z.; Getachew, B. Factors associated with low birthweight among newborns delivered at public health facilities of Nekemte town, West Ethiopia: A case control study. BMC Pregnancy Childbirth 2019, 19, 220. [Google Scholar] [CrossRef] [PubMed]
- Mulu, G.B.; Gebremichael, B.; Desta, K.W.; Kebede, M.A.; Aynalem, Y.A.; Getahun, M.B. Determinants of Low Birth Weight Among Newborns Delivered in Public Hospitals in Addis Ababa, Ethiopia: Case-Control Study. Pediatr. Health Med. Ther. 2020, 11, 119–126. [Google Scholar] [CrossRef]
- Seid, S.; Wondafrash, B.; Gali, N.; Ali, A.; Mohammed, B.; Kedir, S. Determinants of Low Birth Weight Among Newborns Delivered in Silte Zone Public Health Facilities, Southern Ethiopia: A Case-Control Study. Res. Rep. Neonatol. 2022, 12, 19–29. [Google Scholar] [CrossRef]
- Siyoum, M.; Melese, T. Factors associated with low birth weight among babies born at Hawassa University Comprehensive Specialized Hospital, Hawassa, Ethiopia. Ital. J. Pediatr. 2019, 45, 48. [Google Scholar] [CrossRef]
- Wudie, F.T.; A Tesfamicheal, F.; Fisseha, H.Z.; Weldehawaria, N.B.; Misgena, K.H.; Alema, H.B.; Gebregziabher, Y.S.; Fisseha, G.K.; Woldu, M.G. Determinants of preterm delivery in the central zone of Tigray, northern Ethiopia: A case-control study. South Afr. J. Child Health 2019, 13, 108–114. [Google Scholar] [CrossRef]
- Omar, A.I.; Mohamed, A.D.; Farah, M.G.; Mahad, I.A.; Mohamed, S.A.; Dimbil, A.H.; Mohamud, N.S.; Abshir, F.A.; Abdulkadir, U.A. Maternal Risk Factors Associated with Preterm Births among Pregnant Women in Mogadishu, Somalia. Children 2022, 9, 1518. [Google Scholar] [CrossRef] [PubMed]
- Mehare, T.; Sharew, Y. Prevalence and Associated Factors of Low Birth Weight among Term Newborns in Dilla Town, Southern Ethiopia. Int. J. Pediatr. 2020, 2020, 8394578. [Google Scholar] [CrossRef]
- Biracyaza, E.; Habimana, S.; Rusengamihigo, D.; Evans, H. Regular antenatal care visits were associated with low risk of low birth weight among newborns in Rwanda: Evidence from the 2014/2015 Rwanda Demographic Health Survey (RDHS) Data. F1000Research 2022, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Foto, T.G.; Chapman, R.S.; Lashari, A.G. Risk Factors for Low Birth Weight: Bivariate Analysis of Findings From The Zimbabwe 2014 Multiple Indicator Cluster Survey. J. Health Res. 2016, 30, S1–S8. [Google Scholar]
- Kumlachew, W.; Tezera, N.; Endalamaw, A. Below normal birth weight in the Northwest part of Ethiopia. BMC Res. Notes 2018, 11, 611. [Google Scholar] [CrossRef]
- Muluneh, M.W.; Mulugeta, S.S.; Belay, A.T.; Moyehodie, Y.A. Determinants of Low Birth Weight Among Newborns at Debre Tabor Referral Hospital, Northwest Ethiopia: A Cross-Sectional Study. SAGE Open Nurs. 2023, 9. [Google Scholar] [CrossRef]
- Abdullahi, H.; I Gasim, G.; Saeed, A.; Imam, A.M.; Adam, I. Antenatal iron and folic acid supplementation use by pregnant women in Khartoum, Sudan. BMC Res. Notes 2014, 7, 498. [Google Scholar] [CrossRef]
- Traore, S.S.; Bo, Y.; Kou, G.; Lyu, Q. Iron supplementation and deworming during pregnancy reduces the risk of anemia and stunting in infants less than 2 years of age: A study from Sub-Saharan Africa. BMC Pregnancy Childbirth 2023, 23, 63. [Google Scholar] [CrossRef]
- Tadesse, T.; Abebe, M.; Molla, W.; Mahamed, A.A.; Mebratu, A. Magnitude and associated factors of low birth weight among term newborns delivered in Addis Ababa public hospitals, Ethiopia, 2021. J. Obstet. Gynaecol. 2022, 43, 2114332. [Google Scholar] [CrossRef]
- Fite, M.B.; Tura, A.K.; Yadeta, T.A.; Oljira, L.; Roba, K.T. Prevalence, predictors of low birth weight and its association with maternal iron status using serum ferritin concentration in rural Eastern Ethiopia: A prospective cohort study. BMC Nutr. 2022, 8, 70. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Roth, J.M.; Smit, M.R.; Trijsburg, L.; Mwangi, A.M.; Demir, A.Y.; Wielders, J.P.; Mens, P.F.; Verweij, J.J.; Cox, S.E.; et al. Effect of Daily Antenatal Iron Supplementation on Plasmodium Infection in Kenyan Women: A Randomized Clinical Trial. J. Am. Med. Assoc. 2015, 314, 1009–1020. [Google Scholar] [CrossRef]
- Ndyomugyenyi, R.; Magnussen, P. Chloroquine prophylaxis, iron-folic acid supplementation or case management of malaria attacks in primigravidae in western Uganda: Effects on maternal parasitaemia and haemoglobin levels and on birthweight. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health—Ethiopia. National Antenatal Care Guideline; Ministry of Health—Ethiopia: Addis Ababa, Ethiopia, 2022.
- Klemm, R.; Sommerfelt, A.; Boyo, A.; Barba, C.; Kotecha, P.; Steffen, M.; Franklin, N. Are We Making Progress on Reducing Anemia in Women. Cross-Country Comparison of Anemia Prevalence, Reach, and Use of Antenatal Care and Anemia Reduction Interventions; A2Z: The USAID Micronutriend and Child Blindness Project: Washington, DC, USA, 2011. [Google Scholar]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Mallen, C.; McDOUGALL, N.; Lind, T. Effect of iron supplementation on serum ferritin levels during and after pregnancy. BJOG: Int. J. Obstet. Gynaecol. 1982, 89, 1011–1017. [Google Scholar] [CrossRef]
- Cavill, I. Erythropoiesis and iron. Best Pract. Res. Clin. Haematol. 2002, 15, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C. Iron nutriture of the fetus, neonate, infant, and child. Ann. Nutr. Metab. 2017, 71 (Suppl. S3), 8–14. [Google Scholar] [CrossRef]
- Ali, S.A.; Razzaq, S.; Aziz, S.; Allana, A.; Ali, A.A.; Naeem, S.; Khowaja, N.; Ur Rehman, F. Role of iron in the reduction of anemia among women of re-productive age in low-middle income countries: Insights from systematic review and meta-analysis. BMC Women’s Health 2023, 23, 1–22. [Google Scholar] [CrossRef]
- Low, M.S.Y.; Speedy, J.; E Styles, C.; De-Regil, L.M.; Pasricha, S.-R. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst. Rev. 2016, 2016, CD009747. [Google Scholar] [CrossRef]
- Singh, P.K.; Dubey, R.; Singh, L.; Kumar, C.; Rai, R.K.; Singh, S. Public health interventions to improve maternal nutrition during pregnancy: A nationally representative study of iron and folic acid consumption and food supplements in India. Public Health Nutr. 2020, 23, 2671–2686. [Google Scholar] [CrossRef]
- Hunter, P.J.; Muthiani, Y.; Näsänen-Gilmore, P.K.; Koivu, A.M.; Pörtfors, P.; Bastola, K.; Vimpeli, R.; Luoma, J.; Ashorn, U.; Ashorn, P. A modular systematic review of an-tenatal interventions to address undernutrition during pregnancy in the prevention of low birth weight. Am. J. Clin. Nutr. 2023, 117, S134–S147. [Google Scholar] [CrossRef] [PubMed]
- Kassa, G.M.; Muche, A.A.; Berhe, A.K.; Fekadu, G.A. Prevalence and determinants of anemia among pregnant women in Ethiopia; a systematic review and meta-analysis. BMC Hematol. 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Okube, O.T.; Mirie, W.; Odhiambo, E.; Sabina, W.; Habtu, M. Prevalence and Factors Associated with Anaemia among Pregnant Women Attending Antenatal Clinic in the Second and Third Trimesters at Pumwani Maternity Hospital, Kenya. Open J. Obstet. Gynecol. 2016, 6, 16–27. [Google Scholar] [CrossRef]
- Rono, B.C.; Kombe, Y.; Makokha, A. Multiple Micronutrients Versus Iron Folic Acid on Pica and Hemoglobin Levels Among Pregnant Women in Kenya. Central Afr. J. Public Health 2018, 4, 95. [Google Scholar] [CrossRef]
- Zegeye, B.; Adjei, N.K.; Olorunsaiye, C.Z.; Ahinkorah, B.O.; Ameyaw, E.K.; Seidu, A.-A.; Yaya, S. Pregnant women’s decision-making capacity and adherence to iron supplementation in sub-Saharan Africa: A multi-country analysis of 25 countries. BMC Pregnancy Childbirth 2021, 21, 822. [Google Scholar] [CrossRef] [PubMed]
- Sendeku, F.W.; Azeze, G.G.; Fenta, S.L. Adherence to iron-folic acid supplementation among pregnant women in Ethiopia: A sys-tematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 138. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; A Serdar, M. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Medica 2021, 31, 27–53. [Google Scholar] [CrossRef]
- Delie, A.M.; Gezie, L.D.; Gebeyehu, A.A.; Tarekegn, G.E.; Muche, A.A. Trend of adherence to iron supplementation during pregnancy among Ethiopian women based on Ethiopian demographic and health surveys: A Multivariable decomposition analysis. Front. Nutr. 2022, 9, 955819. [Google Scholar] [CrossRef]
- Ba, D.M.; Ssentongo, P.; Kjerulff, K.H.; Na, M.; Liu, G.; Gao, X.; Du, P. Adherence to Iron Supplementation in 22 Sub-Saharan African Countries and Associated Factors among Pregnant Women: A Large Population-Based Study. Curr. Dev. Nutr. 2019, 3, nzz120. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Krull, J.L.; Lockwood, C.M. Equivalence of the Mediation, Confounding and Suppression Effect. Prev. Sci. 2000, 1, 173–181. [Google Scholar] [CrossRef]



| # | First Author, Pub. Year (Ref) | Country (Setting) | Design/(Case to Control Ratio) | Study Population | Year Study | Sample Size (% Rural) | Exposure Assessment | Outcome Assessment |
|---|---|---|---|---|---|---|---|---|
| 1. | Ahmed 2018 [52] | Ethiopia (Facility-based) | Unmatched Case-control (1 to 2) | Mother–newborn pairs. | 2017 | 286 (40.9%) | Self-reported | Birth weight was assessed within one hour using a digital Seca scale, and then categorized as low birth weight (less than 2.5 kg) or normal birth weight (2.5 kg or greater). |
| 2. | Biracyaza 2021 [64] | Rwanda (Community-based) | Cross-sectional | Mother–newborn pairs. | 2014/2015 | 7381 (82.3%) | Self-reported | Birth weight was measured using metric units (in kilograms) and subsequently classified as low birth weight (less than 2.5 kg) or normal birth weight (2.5 kg or greater). |
| 3. | Deriba 2021 [56] | Ethiopia (Facility-based) | Unmatched Case-control (1 to 2) | Mother–newborn pairs. | 2020 | 555 (56%) | Self-reported | Birth weight was assessed within one hour using a digital Seca scale and categorized as either low birth weight (less than 2.5 kg) or normal birth weight (2.5 kg or greater). |
| 4. | Abdullahi 2014 [68] | Sudan (Facility-based) | Cross-sectional | Mother–newborn pairs. | 2012 | 856 (38.7%) | Self-reported | Birth weight was extracted from the client’s card and categorized as either low birth weight (less than 2.5 kg) or normal birth weight (2.5 kg or greater). |
| 5. | Girma 2019 [57] | Ethiopia (Facility-based) | Unmatched Case-control (1 to 2) | Mother–newborn pairs. | 2017 | 279 (41.6%) | Self-reported | Birth weight was determined using a balanced Seca scale with precision to the nearest 0.01 g and categorized as low birth weight (less than 2.5 kg) or normal birth weight (2.5 kg or greater). |
| 6. | Fite 2022 [71] | Ethiopia (Community-based) | Prospective Cohort | Mother–newborn pairs. | 2021 | 427 (100%) | Self-reported | Birth weight was assessed using a calibrated Docbel BRAUNH scale, rounded to the nearest 100 g, and subsequently categorized as either low birth weight (less than 2500 g) or normal (2500 g or greater). |
| 7. | Tadesse 2023 [70] | Ethiopia study (Facility-based) | Cross-sectional | Mother–newborn pairs | 2021 | 337 (8.6%) | Self-reported | A birth weight below 2500 g was classified as low birth weight. |
| 8. | Seid 2022 [59] | Ethiopia (Facility-based) | Unmatched Case-control (1 to 2) | Mother–newborn pairs | 2020 | 255 (70.2%) | Self-reported | Birth weight was recorded within one hour using a Salter weight measurement scale and then categorized as low birth weight (less than 2.5 kg) or normal birth weight (2.5 kg or greater). |
| 9. | Ndyomugyenyi 2000 [73] | Uganda (Facility-based) | Double-blind, randomized trial | Mother–newborn pairs | 1996 to 1998 | 860 (100%) | By researchers | For babies born in health facilities, birth weight was measured immediately after delivery, while for those born in the community, only birth weights measured within 7 days after delivery were included. Birth weight of less than 2.5 kg was classified as low birth weight, and normal birth weight was defined as greater than or equal to 2.5 kg. |
| 10. | Deriba 2021 [55] | Ethiopia (Facility-based) | Unmatched Case-control (1 to 2) | Mother–newborn pairs | 2020 | 483 (54.55%) | Self-reported | Preterm birth was determined by examining the last menstrual period from an ANC card or an early period ultrasound. It was then categorized as preterm (birth occurring before 37 completed weeks) or normal (birth occurring after 37 completed weeks and within 42 weeks of gestation). |
| 11. | Omar 2022 [62] | Somalia (Facility-based) | Case-control (approximately 1 to 6) | Mother–newborn pairs | 2021 | 499 (3.4%) | Self-reported | Preterm birth, defined as birth occurring before 37 weeks of gestation, was assessed by a physician or midwife. |
| 12. | Adam 2019 [53] | Ghana (Facility-based) | Case-control (1 to 2) | Mother–newborn pairs | 2015 to 2016 | 360 (33.1%) | Self-reported | Birth weight was assessed within 24 h after delivery using standard weight measurement techniques and categorized as low birth weight (birth weight less than 2500 g) or normal birth weight (birth weight between 2500 g and 3400 g). |
| 13. | Asmare 2018 [54] | Ethiopia (Facility-based) | Unmatched Case-control (1 to 2) | Mother–newborn pairs | 2017 | 453 (40.8%) | Self-reported | The outcome was determined within 15 min using a standard scale. A newborn weighing less than 2500 g was classified as having a low birth weight, while a weight greater than 2500 g was considered a normal birth weight. |
| 14. | Foto 2016 [65] | Zimbabwe (Community-based) | Cross-sectional | Mother–newborn pairs | 2014 | 2950 (not reported) | Self-reported | Birth weight was evaluated by reviewing the client card or self-report, and categorized as low birth weight if it was less than 2500 g; otherwise, it was considered normal. |
| 15. | Kumlachew 2018 [66] | Ethiopia (Facility-based) | Cross-sectional | Mother–newborn pairs | 2018 | 375 (33.3%) | Self-reported | Birth weight was measured after 30 min using a balanced weight scale and categorized as low birth weight if it was less than 2.5 kg; otherwise, it was considered normal. |
| 16. | Muluneh 2023 [67] | Ethiopia (Facility-based) | Cross-sectional | Mother–newborn pairs | 2021 | 422 (63.27%) | Self-reported | A standard weight scale was utilised, and a newborn weighing less than 2500 g was classified as having a low birth weight, while those weighing between 2500 g and 4000 g were considered normal. |
| 17. | Mulu 2020 [58] | Ethiopia (Facility-based) | Unmatched Case-control (1 to 2) | Mother–newborn pairs | 2019 | 279 (all from urban area) | Self-reported | Birth weight was measured within 1–2 h after birth using a balanced Seca Scale and categorized as low birth weight if it was less than 2.5 kg; otherwise, it was considered normal. |
| 18. | Siyoum 2019 [60] | Ethiopia (Facility-based) | Unmatched Case-control (1 to 2) | Mother–newborn pairs | 2018 | 330 (33.9%) | Self-reported | Birth weight was assessed using a calibrated Seca scale and rounded to the nearest 100 g. It was then categorized into low birth weight (less than 2500 g) and normal (between 2500 g and 4000 g). |
| 19. | Mwangi 2015 [72] | Kenya (Community-based) | Double-blind randomized controlled trial | Mother–newborn pairs | 2011 to 2013 | 430 (all from rural area) | By researcher | Birth weight was measured immediately after delivery or upon presentation at a research clinic within 7 days for those who delivered at home. Women whose newborns had a birth weight of less than 2500 g were classified as having a low birth weight. |
| Preterm birth, defined as birth occurring before 37 weeks of gestation, was determined by a physician or midwife. | ||||||||
| 20. | Traore 2023 [69] | Sub-Saharan Africa (Community-based) | Cross-sectional | Mother–infant pairs | 2014 to 2020 | 36,879 (68%) | Self-reported | Birth weight was evaluated through a record review (card) and classified as low birth weight (birth weight less than 2500 g) or normal (birth weight greater than or equal to 2500 g). |
| 21. | Mehare 2020 [63] | Ethiopia (Facility-based) | Cross-sectional | Mother–newborn pairs | 2018 to 2019 | 472 ((21.8%) | Self-reported | Birth weight was measured within 24 h using a standard weight scale with precision to the nearest 1 g and categorized as low birth weight (less than 2500 g) or normal birth weight (2500 g or greater). |
| 22. | Wudie 2019 [61] | Ethiopia (Facility-based) | Unmatched Case-control (1 to 2) | Mother–newborn pairs | 2016 | 288 (34%) | Self-reported | Preterm birth, assessed by gestational age (birth of the newborn before 28 weeks of gestation), was documented by referring to the client’s records. |
| Low Birth Weight | |||||||
|---|---|---|---|---|---|---|---|
| # | Author (Year) | Exposure | Outcome | Statistical Method | Effect Estimates (OR, RR, HR) (95% CI) | Confounder Adjustment | |
| 1. | Ahmed 2018 [52] | IFA supplementation | Low birth weight | Binary logistic regression | IFA | Ref | Maternal age, educational status of the mother, occupation of mother, height, MUAC, any multivitamin, anaemia, ANC follow-up, nutritional counselling, additional food intake during pregnancy, MDD-W, khat chewing, history of abortion, history of preterm delivery, HIV status, type of pregnancy, and infant’s sex. |
| No | aOR 2.84 (1.15 to 7.03) | ||||||
| 2. | Biracyaza 2021 [64] | IFA supplementation | Low birth weight | Binary logistic regression | No | Ref | Residence, sex of newborn, maternal education, maternal age, TT vaccine, BMI, gestational age, smoking during pregnancy, ANC utilisation, family size, household wealth index, anaemia, marital status, counselling about nutrition, provided information about complications, and head of the household. |
| IFA | aOR 0.50 (0.30 to 0.90) | ||||||
| 3. | Deriba 2021 [56] | IFA supplementation | Low birth weight | Binary logistic regression | IFA | Ref | Educational status, nutrition counselling, additional meals, restriction of foods during pregnancy, MUAC of the mother, height of the mother, frequency of eating, ANC for recent pregnancy, anaemia status of the mother, medical illness on recent pregnancy, pregnancy-related complications, drinking alcohol, and gestational age in weeks. |
| No | aOR 3.78 (2.10 to 6.85) | ||||||
| 4. | Abdullahi 2014 [68] | IFA supplementation | Low birth weight | Binary logistic regression | No | Ref | None of the variables were controlled. |
| IFA | cOR 0.30 (0.17 to 0.68) | ||||||
| 5. | Girma 2019 [57] | IFA supplementation | Low birth weight | Binary logistic regression | IFA | Ref | Nutritional counselling, taking snacks during pregnancy, maternal MUAC, anaemia, and minimum dietary diversity score of women. |
| No | aOR 2.84 (1.15 to 7.03) | ||||||
| 6. | Fite 2022 [71] | IFA supplementation | Low birth weight | Poisson regression | No | Ref | Occupation of the women, sex of the neonate, maternal nutritional status, fertility desire, maternal height, and time to health facilities. |
| IFA | aPR 0.55 (0.36 to 0.84) | ||||||
| 7. | Tadesse 2023 [70] | IFA supplementation | Low birth weight | Binary logistic regression | No | Ref | Respondents’ age, history of stillbirth, previous history of LBW, history of chronic medical illness, haemoglobin level, history of ANC, follow-up, sex of newborn, and extra meals during their pregnancy. |
| IFA | aOR 0.27 (0.10 to 0.72) | ||||||
| 8. | Seid 2022 [59] | IFA supplementation | Low birth weight | Binary logistic regression | IFA | Ref | Additional food, haemoglobin, food security, and MDD-W score. |
| No | aOR 4.17 (1.44 to 12.30) | ||||||
| 9. | Ndyomugyenyi 2000 [73] | IFA supplementation | Low birth weight | Linear regression | IFA | Ref | Timing of ANC. |
| No | aRR 1.23 (0.85 to 1.78) | ||||||
| 10. | Adam 2019 [53] | Iron-only supplementation | Low birth weight | Binary logistic regression | Iron-only | Ref | Planned pregnancy, mode of delivery, parity, and previous low birth weight. |
| No | aOR 3.20 (1.10 to 9.50) | ||||||
| Always | Ref | ||||||
| Irregular intake | aOR 2.19 (0.99 to 4.63) | ||||||
| No intake | aOR 3.19 (0.99 to 4.63) | ||||||
| 11. | Asmare 2018 [54] | Iron-only supplementation | Low birth weight | Binary logistic regression | Iron-only | Ref | Sex of the newborn, residence, educational status, MUAC category, history of abortion, ANC visit, complications during pregnancy, parity, gestational age, and history of LBW. |
| No | aOR 2.82 (1.62 to 4.91) | ||||||
| 12. | Foto 2016 [65] | Iron-only supplementation | Low birth weight | Bivariable analysis | No | Ref | None of the variables were controlled. |
| Iron-only | cOR 0.69 (0.49 to 0.98) | ||||||
| Low birth weight | Bivariable analysis | Duration of iron-only supplementation | cOR 0.89 (0.82 to 0.97) | ||||
| Folate-only supplementation | Low birth weight | Bivariable analysis | No | Ref | |||
| Folate | cOR 0.95 (0.71 to 1.27) | ||||||
| 13. | Kumlachew 2018 [66] | Iron-only supplementation | Low birth weight | Binary logistic regression | Iron more than 2 months | Ref | Marital status, residence, educational status, occupation, ethnicity, parity, maternal MUAC, history of abortion, number of ANC, malaria, PIH, anaemia, previous LBW, confirmed DM, maternal weight, maternal height, substance use, maternal abuse, gestational age (weeks), and sex of neonate. |
| Up to 1 month | aOR 2.43 (0.83, 7.17) | ||||||
| Not taken | aOR 4.00 (1.30 to12.60) | ||||||
| 14. | Muluneh 2023 [67] | Iron-only supplementation | Low birth weight | Binary logistic regression | No | Ref | Mother’s age, residence, marital status education, occupation, alcohol consumption during pregnancy, smoking during pregnancy, history of spontaneous abortion, ANC visit, DM during pregnancy, history of hypertension, and history of anaemia. |
| Iron-only | aOR 0.23 (0.20 to 0.25) | ||||||
| 15. | Mulu 2020 [58] | Iron-only supplementation | Low birth weight | Binary logistic regression | No | Ref | Mother’s education, parity, pregnancy complications, gestational hypertension, nutritional counselling, height, age, anaemia, additional meal intake, BMI, incomplete ANC visit, maternal MUAC, pre-pregnancy weight, and gravidity. |
| Iron-only | aOR 0.60 (0.30 to 1.50) | ||||||
| 16. | Siyoum 2019 [60] | Iron-only supplementation | Low birth weight | Binary logistic regression | Iron-only | Ref | Age of the mother, MUAC, GA, occupation, presence of complications during pregnancy, nutritional counselling, residence of the mother, level of education, ethnicity of the mother, age at first birth, age of the mother, and diseases. |
| No | aOR 2.89 (1.58 to 5.29) | ||||||
| 17. | Mwangi 2015 [72] | Iron supplementation | Low birth weight | Multiple logistic regression | No | Ref | Gravidity, maternal age, HIV infection, plasmodium infection status, haemoglobin concentration, and gestational age. |
| Iron-only | aOR 0.42 (0.13 to 0.78) | ||||||
| 18. | Traore 2023 [69] | Iron-only supplementation | Low birth weight | Binary logistic regression | No | Ref. | None of the variables were controlled. |
| Iron < 60 days | NS (results not reported) | ||||||
| Iron 60 to 89 days | NS (Results not reported) | ||||||
| Iron 90 or more days | NS (Results not reported) | ||||||
| 19. | Mehare 2020 [63] | Folate-only supplementation | Low birth weight | Binary logistic regression | Folate-only | Ref | Maternal age, residency, educational status, occupation, marital status, birth interval, pregnancy type, ANC follow-up, dietary counsel, alcohol drinking, and cigarette smoking. |
| No | aOR 5.48 (2.93 to 10.25) | ||||||
| Preterm birth | |||||||
| 20. | Deriba 2021 [55] | IFA supplementation | Preterm birth | Binary logistic regression | IFA | Ref | Family size, education of mother, education of husband, occupation of mother, occupation of husband, nutritional counselling, taking additional meals, restriction of food, frequency of taking DGLV, meal frequency, and MUAC of mother. |
| No | aOR 2.26 (1.22 to 4.18) | ||||||
| 21. | Omar 2022 [62] | IFA supplementation | Preterm birth | Bivariable logistic regression | IFA | Ref | None of the variables were controlled. |
| No | cOR 2.80 (1.67 to 4.68) | ||||||
| No | Ref | ||||||
| 1st trimester | cOR 0.51 (0.32 to 0.82) | ||||||
| 2nd trimester | cOR 0.35 (0.18 to 0.69) | ||||||
| 3rd trimester | cOR 0.25 (0.12 to 0.52) | ||||||
| 22. | Mwangi 2015 [72] | Iron-only supplementation | Preterm birth | Binary logistic regression | No | Ref | Gravidity, maternal age, HIV infection, plasmodium infection status, haemoglobin concentration, iron deficiency, and gestational age. |
| Iron-only | ARD −7.00 (−13.20 to −1.10) | ||||||
| 23. | Wudie 2019 [61] | Folate-only supplementation | Premature delivery | Binary logistic regression | No | Ref | Residence, monthly income, occupation, level of education, gynaecological problems, number of antenatal clinic visits, age at first delivery (years), number of pregnancies, history of preterm birth, history of multiple pregnancies, history of stillbirth, nutritional counselling during pregnancy, additional nutrition during pregnancy, activity during pregnancy, and support during pregnancy. |
| Folate-only | aOR 0.26 (0.008 to 0.084) | ||||||
| Omitted Study | Exp (Theta) | 95% CI | p Value |
|---|---|---|---|
| Ahmed et al., 2018 [52] | 0.371 | 0.278, 0.494 | 0.000 |
| Biracyaza et al., 2021 [64] | 0.355 | 0.269, 0.469 | 0.000 |
| Deriba et al., 2021 [56] | 0.386 | 0.293,0.509 | 0.000 |
| Abdullahi et al., 2014 [68] | 0.378 | 0.284, 0.509 | 0.000 |
| Girma et al., 2019 [57] | 0.371 | 0.278, 0.494 | 0.000 |
| Fite et al., 2022 [71] | 0.354 | 0.267, 0.470 | 0.000 |
| Taddess et al., 2023 [70] | 0.383 | 0.290, 0.505 | 0.000 |
| Seid et al., 2022 [59] | 0.388 | 0.298, 0.507 | 0.000 |
| Ndyomugyenyi et al., 2000 [73] | 0.335 | 0.274, 0.410 | 0.000 |
| Exp (theta) | 0.37 | 0.29, 0.48 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekele, Y.; Gallagher, C.; Batra, M.; Vicendese, D.; Buultjens, M.; Erbas, B. Is Oral Iron and Folate Supplementation during Pregnancy Protective against Low Birth Weight and Preterm Birth in Africa? A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2801. https://doi.org/10.3390/nu16162801
Bekele Y, Gallagher C, Batra M, Vicendese D, Buultjens M, Erbas B. Is Oral Iron and Folate Supplementation during Pregnancy Protective against Low Birth Weight and Preterm Birth in Africa? A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(16):2801. https://doi.org/10.3390/nu16162801
Chicago/Turabian StyleBekele, Yibeltal, Claire Gallagher, Mehak Batra, Don Vicendese, Melissa Buultjens, and Bircan Erbas. 2024. "Is Oral Iron and Folate Supplementation during Pregnancy Protective against Low Birth Weight and Preterm Birth in Africa? A Systematic Review and Meta-Analysis" Nutrients 16, no. 16: 2801. https://doi.org/10.3390/nu16162801
APA StyleBekele, Y., Gallagher, C., Batra, M., Vicendese, D., Buultjens, M., & Erbas, B. (2024). Is Oral Iron and Folate Supplementation during Pregnancy Protective against Low Birth Weight and Preterm Birth in Africa? A Systematic Review and Meta-Analysis. Nutrients, 16(16), 2801. https://doi.org/10.3390/nu16162801





