The Role of Inflammatory Mediators in the Pathogenesis of Obesity
Abstract
:1. Introduction
2. Brief Overview of the Inflammatory Landscape of Obesity
3. How Is Obesity Linked to Inflammation?
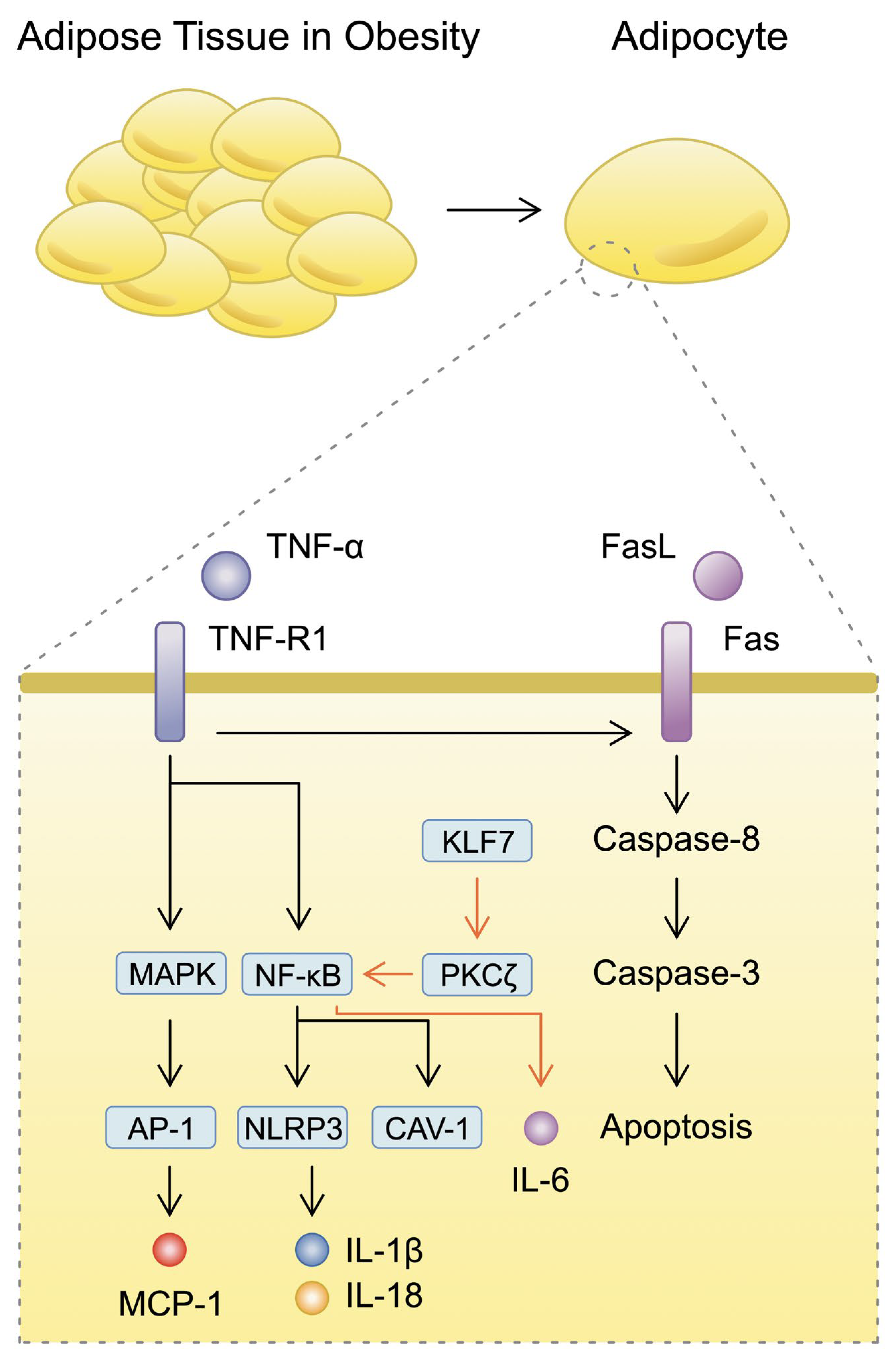
3.1. Inflammatory Mechanisms Surrounding Adipocytes
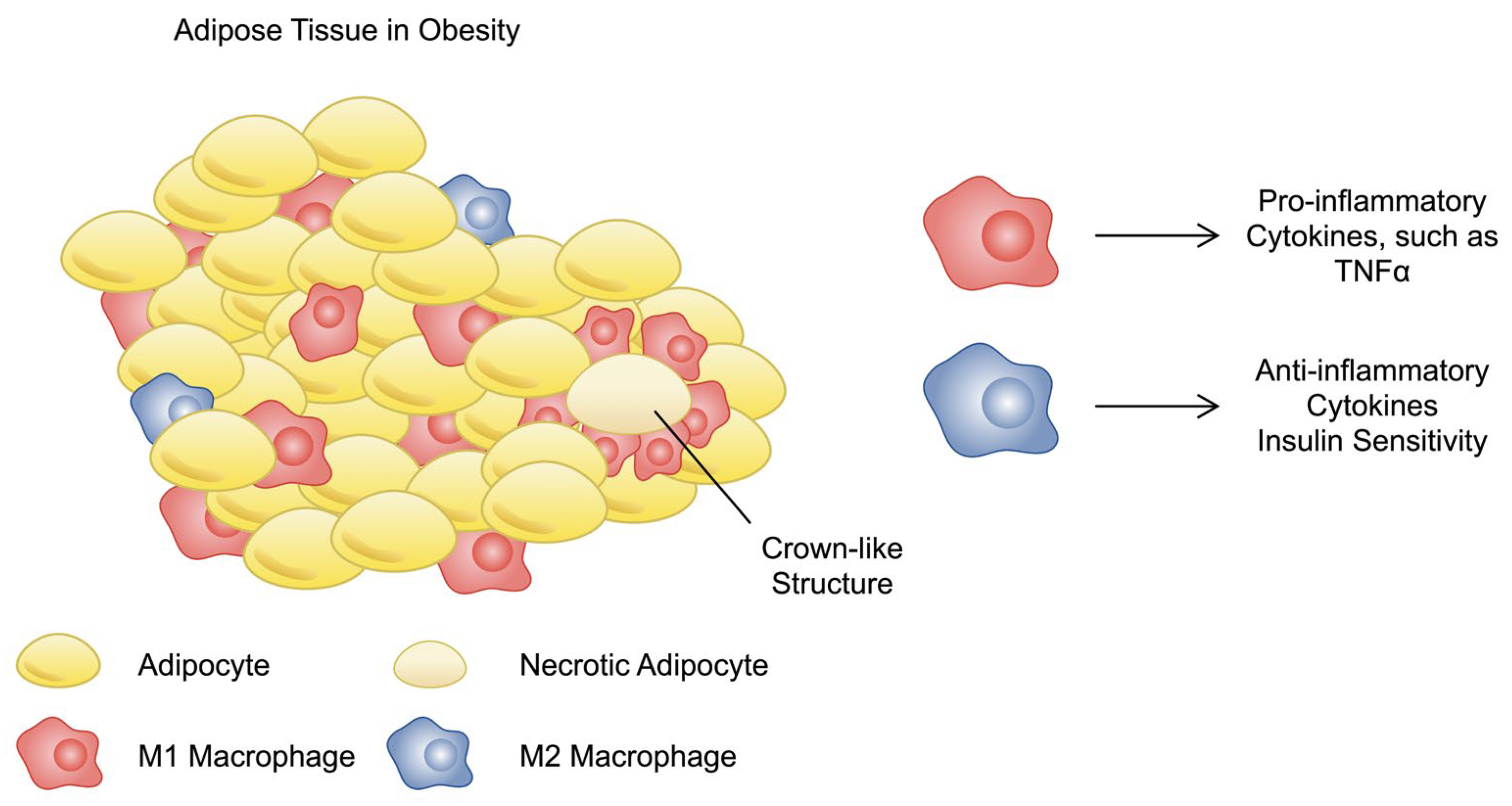
3.2. Immune Cells in Obesity
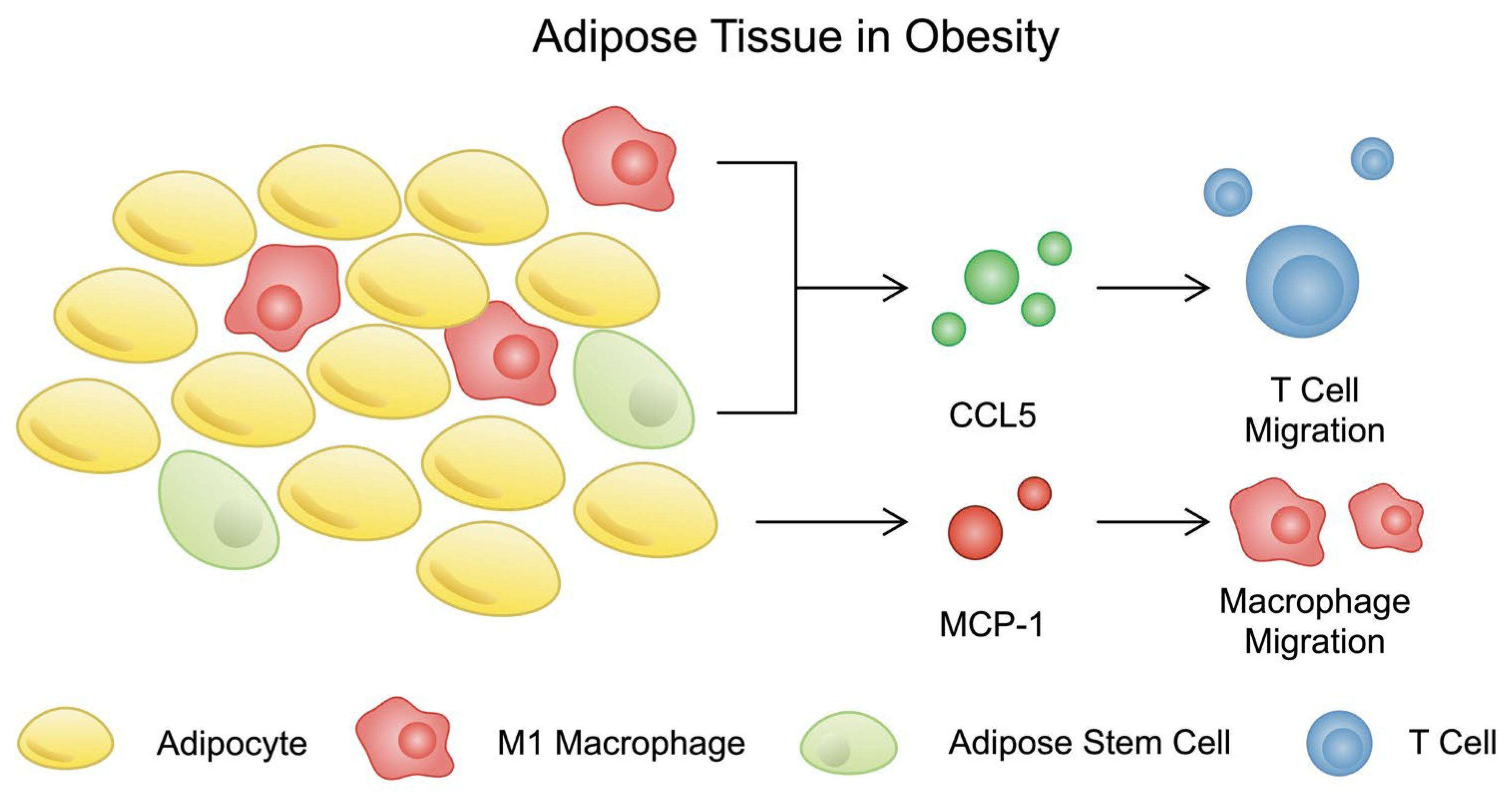
3.3. Chemokines
3.4. Inflammasome
4. The Omics Studies
5. Obesity and Cancer—A Focus on Immune Modulation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. One in Eight People Are Now Living with Obesity. Available online: https://www.who.int/news/item/01-03-2024-one-in-eight-people-are-now-living-with-obesity (accessed on 29 June 2024).
- NCD Risk Factor Collaboration. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Muniesa, P.; Martinez-Gonzalez, M.A.; Hu, F.B.; Despres, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Kwiat, V.R.; Reis, G.; Valera, I.C.; Parvatiyar, K.; Parvatiyar, M.S. Autoimmunity as a sequela to obesity and systemic inflammation. Front. Physiol. 2022, 13, 887702. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Nimitphong, H.; Park, E.; Lee, M.J. Vitamin D regulation of adipogenesis and adipose tissue functions. Nutr. Res. Pr. 2020, 14, 553–567. [Google Scholar] [CrossRef]
- Laforest, S.; Pelletier, M.; Denver, N.; Poirier, B.; Nguyen, S.; Walker, B.R.; Durocher, F.; Homer, N.Z.M.; Diorio, C.; Tchernof, A.; et al. Simultaneous quantification of estrogens and glucocorticoids in human adipose tissue by liquid-chromatography-tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 2019, 195, 105476. [Google Scholar] [CrossRef]
- Song, J.; Deng, T. The Adipocyte and Adaptive Immunity. Front. Immunol. 2020, 11, 593058. [Google Scholar] [CrossRef]
- Bradley, D.; Xu, A.; Hsueh, W.A. Editorial: The Immunomodulatory Roles of Adipocytes. Front. Immunol. 2021, 12, 827281. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The colors of adipose tissue. Gac. Med. Mex. 2020, 156, 142–149. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Kielbowski, K.; Bakinowska, E.; Ostrowski, P.; Pala, B.; Gromowska, E.; Gurazda, K.; Dec, P.; Modrzejewski, A.; Pawlik, A. The Role of Adipokines in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2023, 24, 6390. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.; Ji, X.; Zhang, W.; Luan, J.; Zahr, T.; Qiang, L. Adipokines, adiposity, and atherosclerosis. Cell Mol. Life Sci. 2022, 79, 272. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Variya, B.; Langlois, M.F.; Ramanathan, S. Chemokines in human obesity. Cytokine 2020, 127, 154953. [Google Scholar] [CrossRef]
- Liang, W.; Qi, Y.; Yi, H.; Mao, C.; Meng, Q.; Wang, H.; Zheng, C. The Roles of Adipose Tissue Macrophages in Human Disease. Front. Immunol. 2022, 13, 908749. [Google Scholar] [CrossRef] [PubMed]
- Ribot, J.C.; Lopes, N.; Silva-Santos, B. gammadelta T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 2021, 21, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Bai, H.; Wu, F.; Chen, J.; Li, B.; Li, Y. Tissue adaptation of regulatory T cells in adipose tissue. Eur. J. Immunol. 2022, 52, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-alpha and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15 (Suppl. 2), 120–122. [Google Scholar] [CrossRef]
- Rodriguez, A.; Gomez-Ambrosi, J.; Catalan, V.; Rotellar, F.; Valenti, V.; Silva, C.; Mugueta, C.; Pulido, M.R.; Vazquez, R.; Salvador, J.; et al. The ghrelin O-acyltransferase-ghrelin system reduces TNF-alpha-induced apoptosis and autophagy in human visceral adipocytes. Diabetologia 2012, 55, 3038–3050. [Google Scholar] [CrossRef]
- Hasan Azeez, S. Influence of IL-10, IL-6 and TNF-alpha gene polymorphism on obesity. Cell Mol. Biol. 2023, 69, 277–282. [Google Scholar] [CrossRef]
- Yu, G.I.; Ha, E.; Park, S.H.; Park, J.H.; Jang, H.S.; Bae, J.H.; Chung, I.S.; Shin, D.H.; Song, D.K. Association of tumor necrosis factor-alpha (TNF-alpha) promoter polymorphisms with overweight/obesity in a Korean population. Inflamm. Res. 2011, 60, 1099–1105. [Google Scholar] [CrossRef]
- de Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef]
- Xu, S.; Xi, J.; Wu, T.; Wang, Z. The Role of Adipocyte Endoplasmic Reticulum Stress in Obese Adipose Tissue Dysfunction: A Review. Int. J. Gen. Med. 2023, 16, 4405–4418. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Gornicka, A.; Berk, M.P.; Thapaliya, S.; Dixon, L.J.; Kashyap, S.; Schauer, P.R.; Feldstein, A.E. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J. Biol. Chem. 2010, 285, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Luk, C.T.; Chan, C.K.; Chiu, F.; Shi, S.Y.; Misra, P.S.; Li, Y.Z.; Pollock-Tahiri, E.; Schroer, S.A.; Desai, H.R.; Sivasubramaniyam, T.; et al. Dual Role of Caspase 8 in Adipocyte Apoptosis and Metabolic Inflammation. Diabetes 2023, 72, 1751–1765. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Goeddel, D.V. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Li, W.; Kang, Y.; Xu, Z.; Li, X.; Gao, Y.; Qi, Y. MAPKs/AP-1, not NF-kappaB, is responsible for MCP-1 production in TNF-alpha-activated adipocytes. Adipocyte 2022, 11, 477–486. [Google Scholar] [CrossRef]
- Bluher, M.; Kloting, N.; Wueest, S.; Schoenle, E.J.; Schon, M.R.; Dietrich, A.; Fasshauer, M.; Stumvoll, M.; Konrad, D. Fas and FasL expression in human adipose tissue is related to obesity, insulin resistance, and type 2 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E36–E44. [Google Scholar] [CrossRef]
- Krinninger, P.; Brunner, C.; Ruiz, P.A.; Schneider, E.; Marx, N.; Foryst-Ludwig, A.; Kintscher, U.; Haller, D.; Laumen, H.; Hauner, H. Role of the adipocyte-specific NF-kappaB activity in the regulation of IP-10 and T cell migration. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E304–E311. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Kochumon, S.; Haddad, D.; Thomas, R.; Nizam, R.; Miranda, L.; Sindhu, S.; Bitar, M.S.; Ahmad, R.; Al-Mulla, F. Adipose Tissue Caveolin-1 Upregulation in Obesity Involves TNF-alpha/NF-kappaB Mediated Signaling. Cells 2023, 12, 1019. [Google Scholar] [CrossRef]
- Razani, B.; Combs, T.P.; Wang, X.B.; Frank, P.G.; Park, D.S.; Russell, R.G.; Li, M.; Tang, B.; Jelicks, L.A.; Scherer, P.E.; et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 2002, 277, 8635–8647. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liang, M.; Tang, Y.; Ma, D.; Li, M.; Yuan, C.; Hou, Y.; Sun, C.; Liu, J.; Wei, Q.; et al. KLF7 promotes adipocyte inflammation and glucose metabolism disorder by activating the PKCzeta/NF-kappaB pathway. FASEB J. 2023, 37, e23033. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Liu, Z.; Sui, Y.; Helsley, R.N.; Zhu, B.; Powell, D.K.; Kern, P.A.; Zhou, C. IKKbeta Is Essential for Adipocyte Survival and Adaptive Adipose Remodeling in Obesity. Diabetes 2016, 65, 1616–1629. [Google Scholar] [CrossRef]
- Renovato-Martins, M.; Moreira-Nunes, C.; Atella, G.C.; Barja-Fidalgo, C.; Moraes, J.A. Obese Adipose Tissue Secretion Induces Inflammation in Preadipocytes: Role of Toll-Like Receptor-4. Nutrients 2020, 12, 2828. [Google Scholar] [CrossRef]
- Eslick, S.; Williams, E.J.; Berthon, B.S.; Wright, T.; Karihaloo, C.; Gately, M.; Wood, L.G. Weight Loss and Short-Chain Fatty Acids Reduce Systemic Inflammation in Monocytes and Adipose Tissue Macrophages from Obese Subjects. Nutrients 2022, 14, 765. [Google Scholar] [CrossRef]
- Hepprich, M.; Mudry, J.M.; Gregoriano, C.; Jornayvaz, F.R.; Carballo, S.; Wojtusciszyn, A.; Bart, P.A.; Chiche, J.D.; Fischli, S.; Baumgartner, T.; et al. Canakinumab in patients with COVID-19 and type 2 diabetes—A multicentre, randomised, double-blind, placebo-controlled trial. EClinicalMedicine 2022, 53, 101649. [Google Scholar] [CrossRef]
- Carvalho, F.M.C.; Lima, V.C.O.; Costa, I.S.; Luz, A.B.S.; Ladd, F.V.L.; Serquiz, A.C.; Bortolin, R.H.; Silbiger, V.N.; Maciel, B.L.L.; Santos, E.A.; et al. Anti-TNF-alpha Agent Tamarind Kunitz Trypsin Inhibitor Improves Lipid Profile of Wistar Rats Presenting Dyslipidemia and Diet-induced Obesity Regardless of PPAR-gamma Induction. Nutrients 2019, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.F.; Costa, I.S.; Carvalho, F.M.C.; Kiyota, S.; Souza, B.B.P.; Sifuentes, D.N.; Serquiz, R.P.; Maciel, B.L.L.; Uchoa, A.F.; Santos, E.A.D.; et al. Biochemical characterisation of a Kunitz-type inhibitor from Tamarindus indica L. seeds and its efficacy in reducing plasma leptin in an experimental model of obesity. J. Enzym. Inhib. Med. Chem. 2018, 33, 334–348. [Google Scholar] [CrossRef]
- Vilarino-Garcia, T.; Polonio-Gonzalez, M.L.; Perez-Perez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.C.; Gimeno-Orna, J.A.; Iglesias, P.; Navarro, J.; et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 2338. [Google Scholar] [CrossRef]
- Khan, S.; Chan, Y.T.; Revelo, X.S.; Winer, D.A. The Immune Landscape of Visceral Adipose Tissue During Obesity and Aging. Front. Endocrinol. 2020, 11, 267. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef] [PubMed]
- Cottam, M.A.; Caslin, H.L.; Winn, N.C.; Hasty, A.H. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat. Commun. 2022, 13, 2950. [Google Scholar] [CrossRef]
- Gordon, S. The role of the macrophage in immune regulation. Res. Immunol. 1998, 149, 685–688. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Li, P.; Ma, C.; Li, J.; You, S.; Dang, L.; Wu, J.; Hao, Z.; Zhi, Y.; Chen, L.; Sun, S. Proteomic characterization of four subtypes of M2 macrophages derived from human THP-1 cells. J. Zhejiang Univ. Sci. B 2022, 23, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef]
- Kratz, M.; Coats, B.R.; Hisert, K.B.; Hagman, D.; Mutskov, V.; Peris, E.; Schoenfelt, K.Q.; Kuzma, J.N.; Larson, I.; Billing, P.S.; et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014, 20, 614–625. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Gao, H.; Dos Reis, F.C.G.; Bandyopadhyay, G.; Ofrecio, J.M.; Luo, Z.; Ji, Y.; Jin, Z.; Ly, C.; Olefsky, J.M. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 2021, 33, 781–790.e785. [Google Scholar] [CrossRef] [PubMed]
- Phu, T.A.; Ng, M.; Vu, N.K.; Bouchareychas, L.; Raffai, R.L. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity. Mol. Ther. 2022, 30, 2274–2297. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, A.; Yuan, L.; Wang, Y.; Zhang, C.; Jiang, J.; Xu, H.; Yuan, H.; Yao, H.; Zhang, Q.; et al. Targeting parvalbumin promotes M2 macrophage polarization and energy expenditure in mice. Nat. Commun. 2022, 13, 3301. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, J.; Kong, D.; Guo, X.; Li, J.; Long, Z.; Peng, J.; Wang, Z.; Wu, H.; Liu, P.; et al. Irisin drives macrophage anti-inflammatory differentiation via JAK2-STAT6-dependent activation of PPARgamma and Nrf2 signaling. Free Radic. Biol. Med. 2023, 201, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Ai, L.; Wang, B.; Wang, L.; Gan, Y.; Liu, C.; Jensen, J.; Zhou, Y. Eccentric exercise and dietary restriction inhibits M1 macrophage polarization activated by high-fat diet-induced obesity. Life Sci. 2020, 243, 117246. [Google Scholar] [CrossRef]
- da Costa Fernandes, C.J.; da Cruz Rodrigues, K.C.; de Melo, D.G.; de Campos, T.D.P.; Dos Santos Canciglieri, R.; Simabuco, F.M.; da Silva, A.S.R.; Cintra, D.E.; Ropelle, E.R.; Pauli, J.R.; et al. Short-term strength exercise reduces the macrophage M1/M2 ratio in white adipose tissue of obese animals. Life Sci. 2023, 329, 121916. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cordero, L.; Galvez, I.; Hinchado, M.D.; Ortega, E. Influence of Obesity and Exercise on beta2-Adrenergic-Mediated Anti-Inflammatory Effects in Peritoneal Murine Macrophages. Biomedicines 2020, 8, 556. [Google Scholar] [CrossRef]
- Allawadhi, P.; Beyer, G.; Mahajan, U.M.; Mayerle, J. Novel Insights Into Macrophage Diversity During the Course of Pancreatitis. Gastroenterology 2021, 161, 1802–1805. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Raman, D.; Sobolik-Delmaire, T.; Richmond, A. Chemokines in health and disease. Exp. Cell Res. 2011, 317, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Zhou, Y.; Iribarren, P.; Wang, J. Chemokines and chemokine receptors: Their manifold roles in homeostasis and disease. Cell Mol. Immunol. 2004, 1, 95–104. [Google Scholar] [PubMed]
- Youn, B.S.; Mantel, C.; Broxmeyer, H.E. Chemokines, chemokine receptors and hematopoiesis. Immunol. Rev. 2000, 177, 150–174. [Google Scholar] [CrossRef]
- Van Coillie, E.; Van Damme, J.; Opdenakker, G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor. Rev. 1999, 10, 61–86. [Google Scholar] [CrossRef]
- Monteclaro, F.S.; Charo, I.F. The amino-terminal domain of CCR2 is both necessary and sufficient for high affinity binding of monocyte chemoattractant protein 1. Receptor activation by a pseudo-tethered ligand. J. Biol. Chem. 1997, 272, 23186–23190. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Huber, J.; Kiefer, F.W.; Zeyda, M.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; Zlabinger, G.J.; Stulnig, T.M. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J. Clin. Endocrinol. Metab. 2008, 93, 3215–3221. [Google Scholar] [CrossRef]
- Harman-Boehm, I.; Blüher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Klöting, N.; Stumvoll, M.; Bashan, N.; et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: Effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 2007, 92, 2240–2247. [Google Scholar] [CrossRef]
- Takahashi, K.; Mizuarai, S.; Araki, H.; Mashiko, S.; Ishihara, A.; Kanatani, A.; Itadani, H.; Kotani, H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J. Biol. Chem. 2003, 278, 46654–46660. [Google Scholar] [CrossRef]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Mumick, S.; Zhang, C.; Lamb, J.; Dai, H.; Weingarth, D.; Mudgett, J.; Chen, H.; MacNeil, D.J.; Reitman, M.L.; et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes. Res. 2005, 13, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Park, H.S.; Kawada, T.; Kim, J.H.; Lim, D.; Hubbard, N.E.; Kwon, B.S.; Erickson, K.L.; Yu, R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef]
- Buyuk, E.; Asemota, O.A.; Merhi, Z.; Charron, M.J.; Berger, D.S.; Zapantis, A.; Jindal, S.K. Serum and follicular fluid monocyte chemotactic protein-1 levels are elevated in obese women and are associated with poorer clinical pregnancy rate after in vitro fertilization: A pilot study. Fertil. Steril. 2017, 107, 632–640.e633. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Ramirez, B.; Rotellar, F.; Pastor, C.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Proinflammatory cytokines in obesity: Impact of type 2 diabetes mellitus and gastric bypass. Obes. Surg. 2007, 17, 1464–1474. [Google Scholar] [CrossRef]
- Breslin, W.L.; Johnston, C.A.; Strohacker, K.; Carpenter, K.C.; Davidson, T.R.; Moreno, J.P.; Foreyt, J.P.; McFarlin, B.K. Obese Mexican American children have elevated MCP-1, TNF-α, monocyte concentration, and dyslipidemia. Pediatrics 2012, 129, e1180–e1186. [Google Scholar] [CrossRef]
- Ismail, N.A.; Abd El Baky, A.N.; Ragab, S.; Hamed, M.; Hashish, M.A.; Shehata, A. Monocyte chemoattractant protein 1 and macrophage migration inhibitory factor in children with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2016, 29, 641–645. [Google Scholar] [CrossRef]
- Kostopoulou, E.; Kalavrizioti, D.; Davoulou, P.; Papachristou, E.; Sinopidis, X.; Fouzas, S.; Dassios, T.; Gkentzi, D.; Kyriakou, S.I.; Karatza, A.; et al. Monocyte Chemoattractant Protein-1 (MCP-1), Activin-A and Clusterin in Children and Adolescents with Obesity or Type-1 Diabetes Mellitus. Diagnostics 2024, 14, 450. [Google Scholar] [CrossRef]
- Castoldi, A.; Naffah de Souza, C.; Câmara, N.O.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2015, 6, 637. [Google Scholar] [CrossRef]
- Chylikova, J.; Dvorackova, J.; Tauber, Z.; Kamarad, V. M1/M2 macrophage polarization in human obese adipose tissue. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2018, 162, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef]
- Coll, B.; Alonso-Villaverde, C.; Joven, J. Monocyte chemoattractant protein-1 and atherosclerosis: Is there room for an additional biomarker? Clin. Chim. Acta 2007, 383, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, D.; Partridge, N.C.; Wang, X.; Shapses, S.A. The high serum monocyte chemoattractant protein-1 in obesity is influenced by high parathyroid hormone and not adiposity. J. Clin. Endocrinol. Metab. 2011, 96, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.J.; Hoffman, J.R.; Jajtner, A.R.; Varanoske, A.N.; Church, D.D.; Gonzalez, A.M.; Townsend, J.R.; Boone, C.H.; Baker, K.M.; Beyer, K.S.; et al. The Effect of Post-Resistance Exercise Amino Acids on Plasma MCP-1 and CCR2 Expression. Nutrients 2016, 8, 409. [Google Scholar] [CrossRef]
- Schild, M.; Eichner, G.; Beiter, T.; Zügel, M.; Krumholz-Wagner, I.; Hudemann, J.; Pilat, C.; Krüger, K.; Niess, A.M.; Steinacker, J.M.; et al. Effects of Acute Endurance Exercise on Plasma Protein Profiles of Endurance-Trained and Untrained Individuals over Time. Mediat. Inflamm. 2016, 2016, 4851935. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Parissis, J.; Kroupis, C.; Georgiadis, M.; Karatzas, D.; Karavolias, G.; Koniavitou, K.; Coats, A.J.; Kremastinos, D.T. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur. Heart J. 2001, 22, 791–797. [Google Scholar] [CrossRef]
- Kaspar, F.; Jelinek, H.F.; Perkins, S.; Al-Aubaidy, H.A.; deJong, B.; Butkowski, E. Acute-Phase Inflammatory Response to Single-Bout HIIT and Endurance Training: A Comparative Study. Mediat. Inflamm. 2016, 2016, 5474837. [Google Scholar] [CrossRef]
- Trøseid, M.; Lappegård, K.T.; Claudi, T.; Damås, J.K.; Mørkrid, L.; Brendberg, R.; Mollnes, T.E. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur. Heart J. 2004, 25, 349–355. [Google Scholar] [CrossRef]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): Implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar] [CrossRef]
- Liang, D.; Song, Z.; Liang, W.; Li, Y.; Liu, S. Metformin inhibits TGF-beta 1-induced MCP-1 expression through BAMBI-mediated suppression of MEK/ERK1/2 signalling. Nephrology 2019, 24, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Kher, M.; Beri, S.; Rehan, H.S.; Prakash, A.; Gupta, L.K. Effect of metformin and insulin combination on monocyte chemoattractant protein-1 and cathepsin-D in type 2 diabetes mellitus. Diabetes Metab. Syndr. 2020, 14, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, T.; Li, J.; Yang, R.; Qi, S.; Zhao, Y.; Li, L.; Zhang, X.; Yang, K.; Xu, Y.; et al. Metformin prevents nephrolithiasis formation by inhibiting the expression of OPN and MCP-1 in vitro and in vivo. Int. J. Mol. Med. 2019, 43, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, X.; Ye, S. Effects of metformin on blood and urine pro-inflammatory mediators in patients with type 2 diabetes. J. Inflamm. 2016, 13, 34. [Google Scholar] [CrossRef]
- Amann, B.; Tinzmann, R.; Angelkort, B. ACE Inhibitors Improve Diabetic Nephropathy Through Suppression of Renal MCP-1. Diabetes Care 2003, 26, 2421–2425. [Google Scholar] [CrossRef]
- Hachuła, M.; Basiak, M.; Kosowski, M.; Okopień, B. Effect of GLP-1RA Treatment on Adhesion Molecules and Monocyte Chemoattractant Protein-1 in Diabetic Patients with Atherosclerosis. Life 2024, 14, 690. [Google Scholar] [CrossRef]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.L.; et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef]
- Liang, Y.L.; Lin, C.N.; Tsai, H.F.; Wu, P.Y.; Lin, S.H.; Hong, T.M.; Hsu, K.F. Omental Macrophagic “Crown-like Structures” Are Associated with Poor Prognosis in Advanced-Stage Serous Ovarian Cancer. Curr. Oncol. 2021, 28, 4234–4246. [Google Scholar] [CrossRef] [PubMed]
- Malavazos, A.E.; Di Vincenzo, A.; Iacobellis, G.; Basilico, S.; Dubini, C.; Morricone, L.; Menicanti, L.; Luca, T.; Giordano, A.; Castorina, S.; et al. The density of crown-like structures in epicardial adipose tissue could play a role in cardiovascular diseases. Eat. Weight. Disord. 2022, 27, 2905–2910. [Google Scholar] [CrossRef]
- Chang, M.C.; Eslami, Z.; Ennis, M.; Goodwin, P.J. Crown-like structures in breast adipose tissue of breast cancer patients: Associations with CD68 expression, obesity, metabolic factors and prognosis. NPJ Breast Cancer 2021, 7, 97. [Google Scholar] [CrossRef]
- Galvez, I.; Hinchado, M.D.; Martin-Cordero, L.; Moran-Plata, F.J.; Graham, G.; Francisco-Morcillo, J.; Ortega, E. The anti-inflammatory and bioregulatory effects of habitual exercise in high-fat diet-induced obesity involve crown-like structures and MCP-1 in white adipose tissue. Exerc. Immunol. Rev. 2023, 29, 111–120. [Google Scholar]
- Fan, M.; Song, E.; Zhang, Y.; Zhang, P.; Huang, B.; Yan, K.; Yang, W.; Chakrabarti, S.; Mahajan, H.; Yan, S.; et al. Metabolic Dysfunction-Associated Steatohepatitis Detected by Neutrophilic Crown-Like Structures in Morbidly Obese Patients: A Multicenter and Clinicopathological Study. Research 2024, 7, 0382. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Shouzu, A.; Omoto, S.; Nishikawa, M.; Fukuhara, S. Significance of chemokines and activated platelets in patients with diabetes. Clin. Exp. Immunol. 2000, 121, 437–443. [Google Scholar] [CrossRef]
- Herder, C.; Haastert, B.; Müller-Scholze, S.; Koenig, W.; Thorand, B.; Holle, R.; Wichmann, H.E.; Scherbaum, W.A.; Martin, S.; Kolb, H. Association of systemic chemokine concentrations with impaired glucose tolerance and type 2 diabetes: Results from the Cooperative Health Research in the Region of Augsburg Survey S4 (KORA S4). Diabetes 2005, 54 (Suppl. 2), S11–S17. [Google Scholar] [CrossRef]
- Nasser, M.W.; Elbaz, M.; Ahirwar, D.K.; Ganju, R.K. Conditioning solid tumor microenvironment through inflammatory chemokines and S100 family proteins. Cancer Lett. 2015, 365, 11–22. [Google Scholar] [CrossRef]
- Soria, G.; Ben-Baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Madan, A.K. Regulation of monocyte chemoattractant protein 1 (MCP-1) release by explants of human visceral adipose tissue. Int. J. Obes. 2005, 29, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Madani, R.; Karastergiou, K.; Ogston, N.C.; Miheisi, N.; Bhome, R.; Haloob, N.; Tan, G.D.; Karpe, F.; Malone-Lee, J.; Hashemi, M.; et al. RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1262–E1268. [Google Scholar] [CrossRef]
- Skurk, T.; Mack, I.; Kempf, K.; Kolb, H.; Hauner, H.; Herder, C. Expression and secretion of RANTES (CCL5) in human adipocytes in response to immunological stimuli and hypoxia. Horm. Metab. Res. 2009, 41, 183–189. [Google Scholar] [CrossRef]
- Zhou, H.; Liao, X.; Zeng, Q.; Zhang, H.; Song, J.; Hu, W.; Sun, X.; Ding, Y.; Wang, D.; Xiao, Y.; et al. Metabolic effects of CCL5 deficiency in lean and obese mice. Front. Immunol. 2022, 13, 1059687. [Google Scholar] [CrossRef]
- Baturcam, E.; Abubaker, J.; Tiss, A.; Abu-Farha, M.; Khadir, A.; Al-Ghimlas, F.; Al-Khairi, I.; Cherian, P.; Elkum, N.; Hammad, M.; et al. Physical exercise reduces the expression of RANTES and its CCR5 receptor in the adipose tissue of obese humans. Mediat. Inflamm. 2014, 2014, 627150. [Google Scholar] [CrossRef]
- Liao, X.; Zeng, Q.; Xie, L.; Zhang, H.; Hu, W.; Xiao, L.; Zhou, H.; Wang, F.; Xie, W.; Song, J.; et al. Adipose stem cells control obesity-induced T cell infiltration into adipose tissue. Cell Rep. 2024, 43, 113963. [Google Scholar] [CrossRef]
- Keophiphath, M.; Rouault, C.; Divoux, A.; Clement, K.; Lacasa, D. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arter. Thromb. Vasc. Biol. 2010, 30, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Madhoun, A.A.; Al-Rashed, F.; Azim, R.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Adipose tissue gene expression of CXCL10 and CXCL11 modulates inflammatory markers in obesity: Implications for metabolic inflammation and insulin resistance. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820930902. [Google Scholar] [CrossRef]
- Ullah, A.; Ud Din, A.; Ding, W.; Shi, Z.; Pervaz, S.; Shen, B. A narrative review: CXC chemokines influence immune surveillance in obesity and obesity-related diseases: Type 2 diabetes and nonalcoholic fatty liver disease. Rev. Endocr. Metab. Disord. 2023, 24, 611–631. [Google Scholar] [CrossRef] [PubMed]
- Cereijo, R.; Quesada-López, T.; Gavaldà-Navarro, A.; Tarascó, J.; Pellitero, S.; Reyes, M.; Puig-Domingo, M.; Giralt, M.; Sánchez-Infantes, D.; Villarroya, F. The chemokine CXCL14 is negatively associated with obesity and concomitant type-2 diabetes in humans. Int. J. Obes. 2021, 45, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gomez-Ambrosi, J.; Ramirez, B.; Rodriguez, A.; Becerril, S.; Valenti, V.; Moncada, R.; Silva, C.; Salvador, J.; Fruhbeck, G.; et al. NLRP3 inflammasome blockade reduces adipose tissue inflammation and extracellular matrix remodeling. Cell Mol. Immunol. 2021, 18, 1045–1057. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Song, Y.; Xie, H.; Dong, M. An update on brown adipose tissue and obesity intervention: Function, regulation and therapeutic implications. Front. Endocrinol. 2022, 13, 1065263. [Google Scholar] [CrossRef]
- Okla, M.; Zaher, W.; Alfayez, M.; Chung, S. Inhibitory Effects of Toll-Like Receptor 4, NLRP3 Inflammasome, and Interleukin-1beta on White Adipocyte Browning. Inflammation 2018, 41, 626–642. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Chimin, P.; Andrade, M.L.; Belchior, T.; Paschoal, V.A.; Magdalon, J.; Yamashita, A.S.; Castro, E.; Castoldi, A.; Chaves-Filho, A.B.; Yoshinaga, M.Y.; et al. Adipocyte mTORC1 deficiency promotes adipose tissue inflammation and NLRP3 inflammasome activation via oxidative stress and de novo ceramide synthesis. J. Lipid Res. 2017, 58, 1797–1807. [Google Scholar] [CrossRef]
- ZhuGe, D.L.; Javaid, H.M.A.; Sahar, N.E.; Zhao, Y.Z.; Huh, J.Y. Fibroblast growth factor 2 exacerbates inflammation in adipocytes through NLRP3 inflammasome activation. Arch. Pharm. Res. 2020, 43, 1311–1324. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-kappaB/GSDMD signal in mice adipose tissue. J. Pineal Res. 2017, 63, e12414. [Google Scholar] [CrossRef]
- Wang, X.; He, G.; Peng, Y.; Zhong, W.; Wang, Y.; Zhang, B. Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway. Sci. Rep. 2015, 5, 12676. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Midgette, Y.; Shah, R. Fish Oil Derived Omega 3 Fatty Acids Suppress Adipose NLRP3 Inflammasome Signaling in Human Obesity. J. Endocr. Soc. 2019, 3, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.; Reader, V.; Digby, Z.; Smolak, P.; Lindsay, N.; Harrison, D.; Clarke, N.; Watt, A.P. Reversal of High Fat Diet-Induced Obesity, Systemic Inflammation, and Astrogliosis by the NLRP3 Inflammasome Inhibitors NT-0249 and NT-0796. J. Pharmacol. Exp. Ther. 2024, 388, 813–826. [Google Scholar] [CrossRef]
- Henriksbo, B.D.; Lau, T.C.; Cavallari, J.F.; Denou, E.; Chi, W.; Lally, J.S.; Crane, J.D.; Duggan, B.M.; Foley, K.P.; Fullerton, M.D.; et al. Fluvastatin causes NLRP3 inflammasome-mediated adipose insulin resistance. Diabetes 2014, 63, 3742–3747. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-18. Methods 1999, 19, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Landy, E.; Carol, H.; Ring, A.; Canna, S. Biological and clinical roles of IL-18 in inflammatory diseases. Nat. Rev. Rheumatol. 2024, 20, 33–47. [Google Scholar] [CrossRef]
- Krumm, B.; Meng, X.; Xiang, Y.; Deng, J. Identification of small molecule inhibitors of Interleukin-18. Sci. Rep. 2017, 7, 483. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Botella-Carretero, J.I.; Villuendas, G.; Sancho, J.; San Millán, J.L. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: Relationship to insulin resistance and to obesity. J. Clin. Endocrinol. Metab. 2004, 89, 806–811. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- Hung, J.; McQuillan, B.M.; Chapman, C.M.; Thompson, P.L.; Beilby, J.P. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arter. Thromb. Vasc. Biol. 2005, 25, 1268–1273. [Google Scholar] [CrossRef]
- Aso, Y.; Okumura, K.; Takebayashi, K.; Wakabayashi, S.; Inukai, T. Relationships of plasma interleukin-18 concentrations to hyperhomocysteinemia and carotid intimal-media wall thickness in patients with type 2 diabetes. Diabetes Care 2003, 26, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.; Kamenov, Z.; Tsakova, A.; El-Darawish, Y.; Okamura, H. Interleukin-18 and testosterone levels in men with metabolic syndrome. Aging Male 2018, 21, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Nedeva, I.; Gateva, A.; Assyov, Y.; Karamfilova, V.; Hristova, J.; Yamanishi, K.; Kamenov, Z.; Okamura, H. IL-18 Serum Levels in Patients with Obesity, Prediabetes and Newly Diagnosed Type 2 Diabetes. Iran. J. Immunol. 2022, 19, 193–200. [Google Scholar] [CrossRef]
- Ahmad, R.; Thomas, R.; Kochumon, S.; Sindhu, S. Increased adipose tissue expression of IL-18R and its ligand IL-18 associates with inflammation and insulin resistance in obesity. Immun. Inflamm. Dis. 2017, 5, 318–335. [Google Scholar] [CrossRef]
- Fatima, S.S.; Jamil, Z.; Abidi, S.H.; Nadeem, D.; Bashir, Z.; Ansari, A. Interleukin-18 polymorphism as an inflammatory index in metabolic syndrome: A preliminary study. World J. Diabetes 2017, 8, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Cho, S.O.; Kim, S.Y.; Kim, S.H.; Chung, W.S.; Chung, S.H.; Kim, S.S.; Ko, S.G.; Jeong, C.H.; Kim, S.J.; et al. Association of interleukin-18 gene polymorphism with body mass index in women. Reprod. Biol. Endocrinol. 2012, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Otziomek, E.; Adamska, A.; Karolczuk-Zarachowicz, M.; Gorska, M. Increased serum interleukin-18 concentration is associated with hypoadiponectinemia in obesity, independently of insulin resistance. Int. J. Obes. 2007, 31, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Chan, K.L.; Zhang, S.; Mejdani, M.; Jacobson, M.R.; Ducos, A.; Bilan, P.J.; Niu, W.; Klip, A. Saturated fatty acids activate caspase-4/5 in human monocytes, triggering IL-1β and IL-18 release. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E825–E835. [Google Scholar] [CrossRef]
- Flisiak-Jackiewicz, M.; Bobrus-Chociej, A.; Tarasów, E.; Wojtkowska, M.; Białokoz-Kalinowska, I.; Lebensztejn, D.M. Predictive Role of Interleukin-18 in Liver Steatosis in Obese Children. Can. J. Gastroenterol. Hepatol. 2018, 2018, 3870454. [Google Scholar] [CrossRef]
- Botella-Carretero, J.I.; Alvarez-Blasco, F.; Martinez-García, M.A.; Luque-Ramírez, M.; San Millán, J.L.; Escobar-Morreale, H.F. The decrease in serum IL-18 levels after bariatric surgery in morbidly obese women is a time-dependent event. Obes. Surg. 2007, 17, 1199–1208. [Google Scholar] [CrossRef]
- Esposito, K.; Pontillo, A.; Ciotola, M.; Di Palo, C.; Grella, E.; Nicoletti, G.; Giugliano, D. Weight loss reduces interleukin-18 levels in obese women. J. Clin. Endocrinol. Metab. 2002, 87, 3864–3866. [Google Scholar] [CrossRef]
- Zorrilla, E.P.; Conti, B. Interleukin-18 null mutation increases weight and food intake and reduces energy expenditure and lipid substrate utilization in high-fat diet fed mice. Brain Behav. Immun. 2014, 37, 45–53. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Lewis, E.; Jensen, D.R.; Voshol, P.J.; Kullberg, B.J.; Tack, C.J.; van Krieken, H.; Kim, S.H.; Stalenhoef, A.F.; et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat. Med. 2006, 12, 650–656. [Google Scholar] [CrossRef]
- Lana, J.P.; de Oliveira, M.C.; Silveira, A.L.M.; Yamada, L.T.P.; Costa, K.A.; da Silva, S.V.; de Assis-Ferreira, A.; Gautier, E.L.; Dussaud, S.; Pinho, V.; et al. Role of IL-18 in adipose tissue remodeling and metabolic dysfunction. Int. J. Obes. 2024, 48, 964–972. [Google Scholar] [CrossRef]
- Wang, H.; Capell, W.; Yoon, J.H.; Faubel, S.; Eckel, R.H. Obesity development in caspase-1-deficient mice. Int. J. Obes. 2014, 38, 152–155. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, G. Depletion of microglia mitigates cerebrovascular dysfunction in diet-induced obesity mice. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E367–E375. [Google Scholar] [CrossRef]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, W.; Sánchez-Alavez, M.; Berton, F.; Alboni, S.; Benatti, C.; Mori, S.; Nguyen, W.; Zorrilla, E.; Moroncini, G.; Tascedda, F.; et al. The Proinflammatory Cytokine Interleukin 18 Regulates Feeding by Acting on the Bed Nucleus of the Stria Terminalis. J. Neurosci. 2016, 36, 5170–5180. [Google Scholar] [CrossRef]
- Zorrilla, E.P.; Sanchez-Alavez, M.; Sugama, S.; Brennan, M.; Fernandez, R.; Bartfai, T.; Conti, B. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc. Natl. Acad. Sci. USA 2007, 104, 11097–11102. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.W.; Spencer, S.J.; Conti, B.; Jasoni, C.L.; Kent, S.; Radler, M.E.; Reyes, T.M.; Sominsky, L. Diet, behavior and immunity across the lifespan. Neurosci. Biobehav. Rev. 2015, 58, 46–62. [Google Scholar] [CrossRef]
- Dai, X.; Shen, L. Advances and Trends in Omics Technology Development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Goodrich, J.A.; Chen, W.; Qiu, C.; Chen, J.C.; Costello, E.; Alderete, T.L.; Chatzi, L.; Gilliland, F.; Chen, Z. Cardiometabolic profiles and proteomics associated with obesity phenotypes in a longitudinal cohort of young adults. Sci. Rep. 2024, 14, 7384. [Google Scholar] [CrossRef]
- Doumatey, A.P.; Zhou, J.; Zhou, M.; Prieto, D.; Rotimi, C.N.; Adeyemo, A. Proinflammatory and lipid biomarkers mediate metabolically healthy obesity: A proteomics study. Obesity 2016, 24, 1257–1265. [Google Scholar] [CrossRef]
- Korduner, J.; Nilsson, P.M.; Melander, O.; Gerl, M.J.; Engstrom, G.; Bachus, E.; Magnusson, M.; Ottosson, F. Proteomic and Metabolomic Characterization of Metabolically Healthy Obesity: A Descriptive Study from a Swedish Cohort. J. Obes. 2021, 2021, 6616983. [Google Scholar] [CrossRef]
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; Garcia-Canaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866.e1826. [Google Scholar] [CrossRef] [PubMed]
- Boi, S.K.; Orlandella, R.M.; Gibson, J.T.; Turbitt, W.J.; Wald, G.; Thomas, L.; Buchta Rosean, C.; Norris, K.E.; Bing, M.; Bertrand, L.; et al. Obesity diminishes response to PD-1-based immunotherapies in renal cancer. J. Immunother. Cancer 2020, 8, e000725. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, G.; Deng, H.; Xiao, G.; Wang, Y.; Zhang, C.; Chen, Y. Obesity enhances the response to neoadjuvant anti-PD1 therapy in oral tongue squamous cell carcinoma. Cancer Med. 2024, 13, e7346. [Google Scholar] [CrossRef]
- Bader, J.E.; Wolf, M.M.; Lupica-Tondo, G.L.; Madden, M.Z.; Reinfeld, B.I.; Arner, E.N.; Hathaway, E.S.; Steiner, K.K.; Needle, G.A.; Hatem, Z.; et al. Obesity induces PD-1 on macrophages to suppress anti-tumour immunity. Nature 2024, 630, 968–975. [Google Scholar] [CrossRef]
- Malavazos, A.E.; Meregalli, C.; Sorrentino, F.; Vignati, A.; Dubini, C.; Scravaglieri, V.; Basilico, S.; Boniardi, F.; Spagnolo, P.; Malagoli, P.; et al. Semaglutide therapy decreases epicardial fat inflammation and improves psoriasis severity in patients affected by abdominal obesity and type-2 diabetes. Endocrinol. Diabetes Metab. Case Rep. 2023, 2023, 23-0017. [Google Scholar] [CrossRef]
- Pan, X.; Yang, L.; Wang, S.; Liu, Y.; Yue, L.; Chen, S. Semaglutide ameliorates obesity-induced cardiac inflammation and oxidative stress mediated via reduction of neutrophil Cxcl2, S100a8, and S100a9 expression. Mol. Cell Biochem. 2024, 479, 1133–1147. [Google Scholar] [CrossRef]
- Sandsdal, R.M.; Juhl, C.R.; Jensen, S.B.K.; Lundgren, J.R.; Janus, C.; Blond, M.B.; Rosenkilde, M.; Bogh, A.F.; Gliemann, L.; Jensen, J.B.; et al. Combination of exercise and GLP-1 receptor agonist treatment reduces severity of metabolic syndrome, abdominal obesity, and inflammation: A randomized controlled trial. Cardiovasc. Diabetol. 2023, 22, 41. [Google Scholar] [CrossRef]
- Bapat, S.P.; Whitty, C.; Mowery, C.T.; Liang, Y.; Yoo, A.; Jiang, Z.; Peters, M.C.; Zhang, L.J.; Vogel, I.; Zhou, C.; et al. Obesity alters pathology and treatment response in inflammatory disease. Nature 2022, 604, 337–342. [Google Scholar] [CrossRef]
- Mayakrishnan, V.; Lee, D.H.; Kim, K.H.; Kim, C.Y. Role of Corn Peptide Powder in Lipopolysaccharide-Induced Inflammatory Responses in 3T3-L1 Adipocytes. Nutrients 2024, 16, 1924. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakinowska, E.; Krompiewski, M.; Boboryko, D.; Kiełbowski, K.; Pawlik, A. The Role of Inflammatory Mediators in the Pathogenesis of Obesity. Nutrients 2024, 16, 2822. https://doi.org/10.3390/nu16172822
Bakinowska E, Krompiewski M, Boboryko D, Kiełbowski K, Pawlik A. The Role of Inflammatory Mediators in the Pathogenesis of Obesity. Nutrients. 2024; 16(17):2822. https://doi.org/10.3390/nu16172822
Chicago/Turabian StyleBakinowska, Estera, Mariusz Krompiewski, Dominika Boboryko, Kajetan Kiełbowski, and Andrzej Pawlik. 2024. "The Role of Inflammatory Mediators in the Pathogenesis of Obesity" Nutrients 16, no. 17: 2822. https://doi.org/10.3390/nu16172822




