Regular Consumption of Green Tea as an Element of Diet Therapy in Drug-Induced Liver Injury (DILI)
Abstract
:1. Introduction
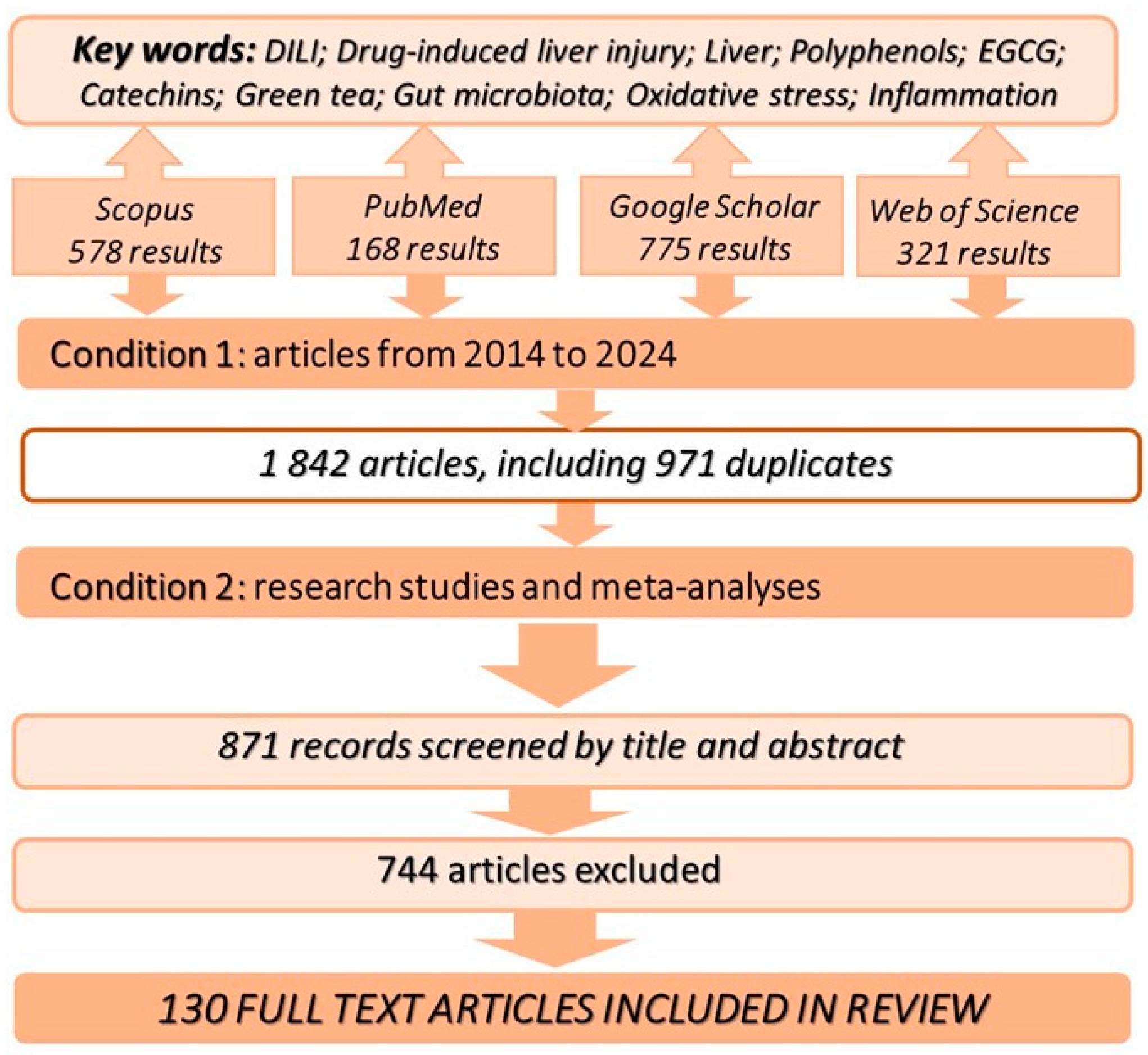
2. Methodology of Information Retrieval in Databases
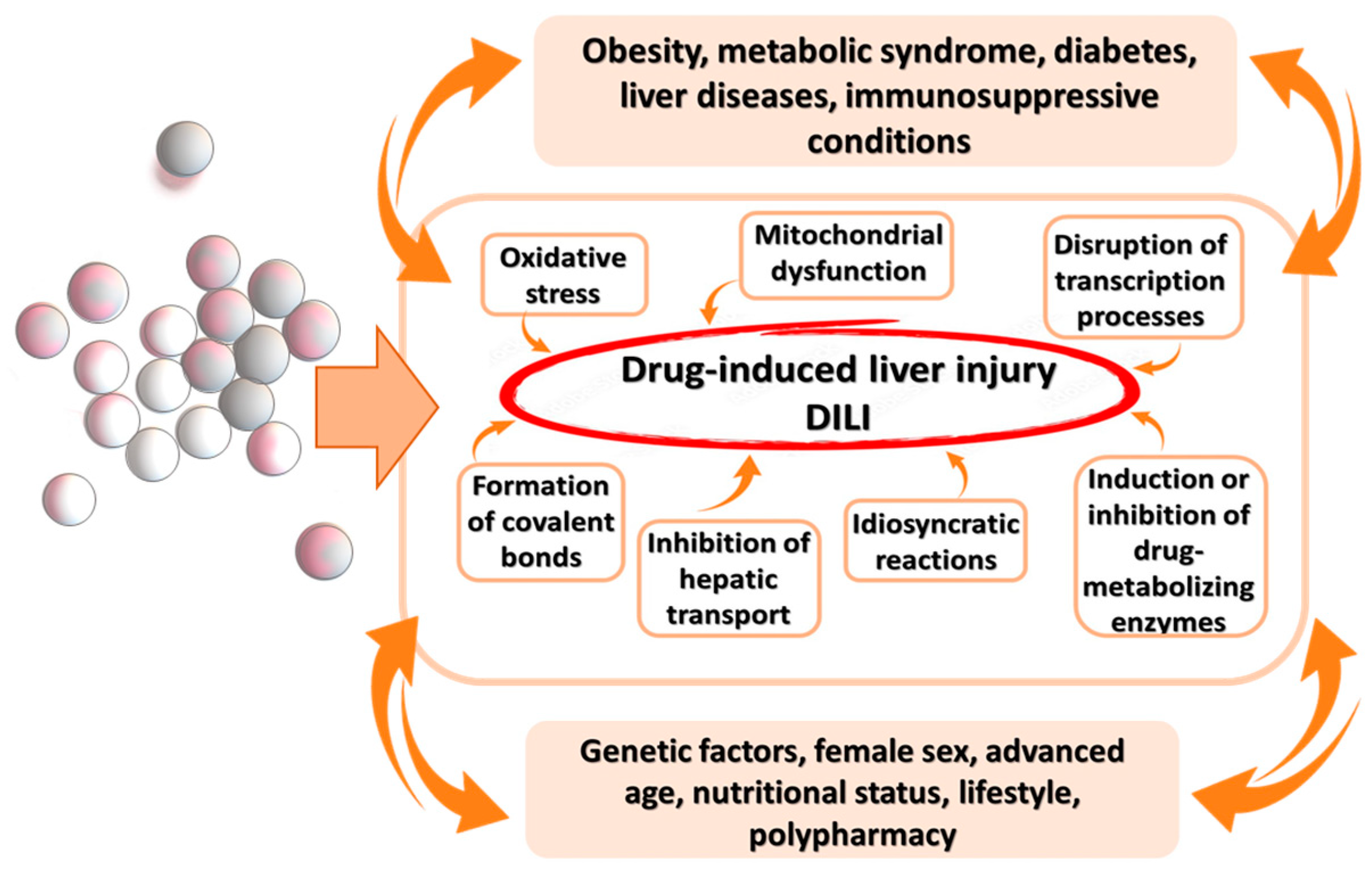
3. Pathophysiological Mechanisms of DILI
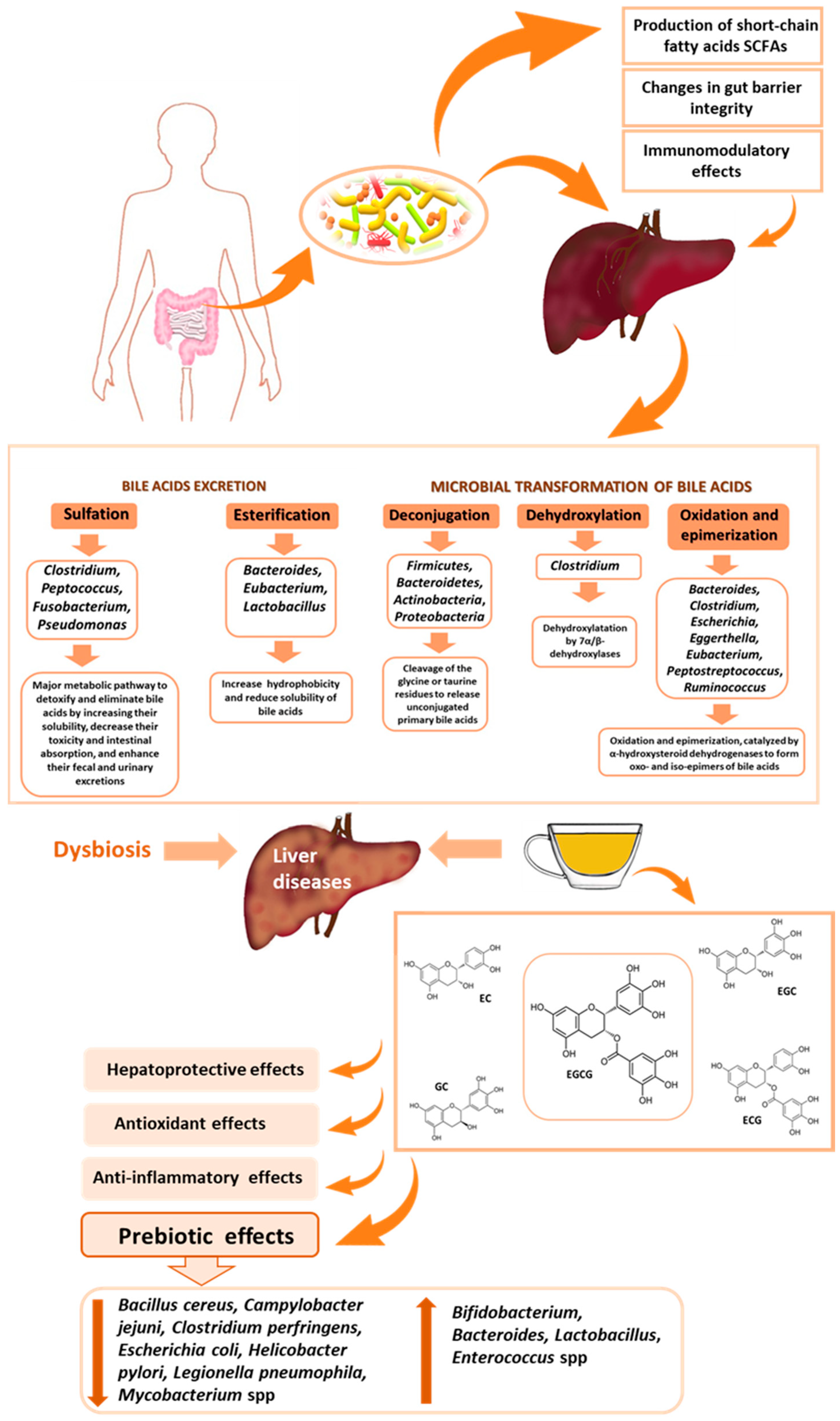
4. Effect of Gut Microbiota on Liver Function
5. The Potential of Green Tea as a Component of Dietotherapy in DILI
5.1. Phenolic Compounds in Green Tea
5.2. Hepatoprotective Effects of Green Tea
5.3. The Antioxidant Action of Green Tea
5.4. Anti-Inflammatory Action of Green Tea
5.5. Anti-Obesogenic and Anti-Diabetic Effects of Green Tea
5.6. Impact of Green Tea on Gut Microbiota
6. Controversies
7. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Zhang, Q.; Zhang, H.Y.; Yu, X.Q.; Cui, Z.J.; Lv, Z.W. Impact of particulate matter 2.5 on the liver function of mice. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4357–4368. [Google Scholar] [CrossRef]
- Wada, T.; Abe, G.; Kudou, T.; Ogawa, E.; Nagai, T.; Tanaka, S.; Hirohata, S. Liver damage in patients with polymyositis and dermatomyositis. Kitasato Med. J. 2016, 46, 40–46. [Google Scholar]
- Meng, Q.; Li, N.; Yuan, L.; Gao, X. Analysis of common causes of liver damage among children 12 years and younger in Weifang. J. Int. Med. Res. 2021, 49, 3000605211006661. [Google Scholar] [CrossRef]
- Navarro, V.J.; Barnhart, H.; Bonkovsky, H.L.; Davern, T.; Fontana, R.J.; Grant, L.; Reddy, K.R.; Seeff, L.B.; Serrano, J.; Sherker, A.H.; et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology 2014, 60, 1399–1408. [Google Scholar] [CrossRef]
- Appiah, J.; Prasad, A.; Shah, V.; Patel, V.; Fareen, N.; Marin, A.C.; Cheriyath, P. Amoxicillin-Clavulanate Induced Liver Injury in a Young Female. Cureus 2023, 15, e33445. [Google Scholar] [CrossRef]
- Cubero, F.J.; Zoubek, M.E.; Hu, W.; Peng, J.; Zhao, G.; Nevzorova, Y.A.; Al Masaoudi, M.; Bechmann, L.P.; Boekschoten, M.V.; Muller, M.; et al. Combined Activities of JNK1 and JNK2 in Hepatocytes Protect Against Toxic Liver Injury. Gastroenterology 2016, 150, 968–981. [Google Scholar] [CrossRef]
- Yu, Y.C.; Mao, Y.M.; Chen, C.W.; Chen, J.J.; Chen, J.; Cong, W.M.; Ding, Y.; Duan, Z.P.; Fu, Q.C.; Guo, X.Y.; et al. Drug-induced Liver Injury (DILI) Study Group; Chinese Society of Hepatology (CSH); Chinese Medical Association (CMA). CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol. Int. 2017, 11, 221–241. [Google Scholar] [CrossRef]
- Kegel, V.; Pfeiffer, E.; Burkhardt, B.; Liu, J.L.; Zeilinger, K.; Nüssler, A.K.; Seehofer, D.; Damm, G. Subtoxic Concentrations of Hepatotoxic Drugs Lead to Kupffer Cell Activation in a Human In Vitro Liver Model: An Approach to Study DILI. Mediat. Inflamm. 2015, 2015, 640631. [Google Scholar] [CrossRef]
- Luo, T.; Yang, S.; Zhao, T.; Zhu, H.; Chen, C.; Shi, X.; Chen, D.; Wang, K.; Jiang, K.; Xu, D.; et al. Hepatocyte DDX3X protects against drug-induced acute liver injury via controlling stress granule formation and oxidative stress. Cell Death Dis. 2023, 14, 400. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wan, X.; Yang, C.S.; Zhang, J. Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure—A rat model study. Regul. Toxicol. Pharmacol. 2015, 73, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Baranowska-Wójcik, E. The Effect of Brewing Time on the Antioxidant Activity of Tea Infusions. Appl. Sci. 2024, 14, 2014. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, J.; Gao, T.; Wu, Y.; Huang, D.; Yan, F.; Weng, Z. Preliminary Study on Hepatoprotective Effect and Mechanism of (-)-Epigallocatechin-3-gallate against Acetaminophen-induced Liver Injury in Rats. Iran. J. Pharm. Res. 2021, 20, 46–56. [Google Scholar] [CrossRef]
- Weng, Z.; Zhou, P.; Salminen, W.F.; Yang, X.; Harrill, A.H.; Cao, Z.; Mattes, W.B.; Mendrick, D.L.; Shi, Q. Green tea epigallocatechin gallate binds to and inhibits respiratory complexes in swelling but not normal rat hepatic mitochondria. Biochem. Biophys. Res. Commun. 2014, 443, 1097–1104. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Maddur, H.; Russo, M.W.; Wong, R.J.; Reddy, K.R.; Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am. J. Gastroenterol. 2021, 116, 878–898. [Google Scholar] [CrossRef] [PubMed]
- Grudziński, I.P.; Rużycka, M.; Cieszanowski, A.; Szeszkowski, W.; Badurek, I.; Małkowska, A.; Bamburowicz-Klimkowska, M. MRI-based preclinical discovery of DILI: A lesson from paracetamol-induced hepatotoxicity. Regul. Toxicol. Pharmacol. 2019, 108, 104478. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.; Abbara, A.; Collin, S.; Henderson, M.; Shehata, M.; Gorgui-Naguib, H.; Lynn, W.; Kon, O.M.; John, L. Predicting drug-induced liver injury from anti-tuberculous medications by early monitoring of liver tests. J. Infect. 2021, 82, 240–244. [Google Scholar] [CrossRef]
- Mendizabal, M.; Silva, M.O. Liver transplantation in acute liver failure: A challenging scenario. World J. Gastroenterol. 2016, 22, 1523–1531. [Google Scholar] [CrossRef]
- Chiew, A.L.; Reith, D.; Pomerleau, A.; Wong, A.; Isoardi, K.Z.; Soderstrom, J.; Buckley, N.A. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med. J. Aust. 2020, 212, 175–183. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J.S.; Kim, H.W.; Kim, Y.H.; Jung, S.S.; Kim, J.W.; Oh, J.Y.; Lee, H.; Kim, S.K.; Kim, S.H.; et al. Metabolic Disorders Are Associated With Drug-Induced Liver Injury During Antituberculosis Treatment: A Multicenter Prospective Observational Cohort Study in Korea. Open Forum Infect. Dis. 2023, 10, ofad422. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, P.; Shao, X.; Zhu, J.; Xiao, J.; Shi, J.; Zhang, L.; Zhu, H.J.; Ma, X.; Manautou, J.E.; et al. Acetaminophen-Induced Liver Injury Alters Expression and Activities of Cytochrome P450 Enzymes in an Age-Dependent Manner in Mouse Liver. Drug Metab. Dispos. 2020, 48, 326–336. [Google Scholar] [CrossRef]
- Sundaram, V.; Björnsson, E.S. Drug-induced cholestasis. Hepatol. Commun. 2017, 1, 726–735. [Google Scholar] [CrossRef]
- Ul Amin Mohsin, N.; Farrukh, M.; Shahzadi, S.; Irfan, M. Drug Metabolism: Phase I and Phase II Metabolic Pathways. In Drug Metabolism and Pharmacokinetics; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- El-Bakry, H.A.; El-Sherif, G.; Rostom, R.M. Therapeutic dose of green tea extract provokes liver damage and exacerbates paracetamol-induced hepatotoxicity in rats through oxidative stress and caspase 3-dependent apoptosis. Biomed. Pharmacother. 2017, 96, 798–811. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Cheng, M.; Yu, L.; Liu, W.; Nie, X.; Wang, M.; Zhou, M.; Chen, W. Lipid peroxidation mediates the association between iron overload and liver injury: Cross-sectional and longitudinal analyses in general Chinese urban adults. Environ. Sci. Pollut. Res. Int. 2023, 30, 60343–60353. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Han, D.; Petrovic, L.M.; Kaplowitz, N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J. Biol. Chem. 2011, 286, 35071–35078. [Google Scholar] [CrossRef]
- Zhang, J.; Min, R.W.M.; Le, K.; Zhou, S.; Aghajan, M.; Than, T.A.; Win, S.; Kaplowitz, N. The role of MAP2 kinases and p38 kinase in acute murine liver injury models. Cell Death Dis. 2017, 8, e2903. [Google Scholar] [CrossRef]
- Kang, S.G.; Choi, Y.Y.; Mo, S.J.; Kim, T.H.; Ha, J.H.; Hong, D.K.; Lee, H.; Park, S.D.; Shim, J.J.; Lee, J.L.; et al. Effect of gut microbiome-derived metabolites and extracellular vesicles on hepatocyte functions in a gut-liver axis chip. Nano Converg. 2023, 10, 5. [Google Scholar] [CrossRef]
- Cao, X.; Zolnikova, O.; Maslennikov, R.; Reshetova, M.; Poluektova, E.; Bogacheva, A.; Zharkova, M.; Ivashkin, V. Low Short-Chain-Fatty-Acid-Producing Activity of the Gut Microbiota Is Associated with Hypercholesterolemia and Liver Fibrosis in Patients with Metabolic-Associated (Non-Alcoholic) Fatty Liver Disease. Gastrointest. Disord. 2023, 5, 464–473. [Google Scholar] [CrossRef]
- Liu, B.; Qian, J.; Wang, Q.; Wang, F.; Ma, Z.; Qiao, Y. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS ONE 2014, 9, e106184. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Jocken, J.W.E.; González Hernández, M.A.; Hoebers, N.T.H.; van der Beek, C.M.; Essers, Y.P.G.; Blaak, E.E.; Canfora, E.E. Short-Chain Fatty Acids Differentially Affect Intracellular Lipolysis in a Human White Adipocyte Model. Front. Endocrinol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.; Li, C.; Guo, X.; Landersdorfer, C.; Shen, H.H.; Peleg, A.Y.; Li, J.; Imoto, S.; Yao, J.; et al. iAMPCN: A deep-learning approach for identifying antimicrobial peptides and their functional activities. Brief. Bioinform. 2023, 24, bbad240. [Google Scholar] [CrossRef]
- Sunkara, L.T.; Jiang, W.; Zhang, G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS ONE 2012, 7, e49558. [Google Scholar] [CrossRef]
- Fei, N.; Miyoshi, S.; Hermanson, J.B.; Miyoshi, J.; Xie, B.; DeLeon, O.; Hawkins, M.; Charlton, W.; D’Souza, M.; Hart, J.; et al. Imbalanced gut microbiota predicts and drives the progression of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in a fast-food diet mouse model. bioRxiv 2023. [Google Scholar] [CrossRef]
- Shu, W.; Shanjian, C.; Jinpiao, L.; Qishui, O. Gut microbiota dysbiosis in patients with hepatitis B virus-related cirrhosis. Ann. Hepatol. 2022, 27, 100676. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, S.D.; Chen, Y.; Li, X.Y.; Zhu, Q.; Nakayama, K.; Zhang, W.Q.; Weng, C.Z.; Zhang, J.; Wang, H.K.; et al. Alterations in gut microbiome and metabolomics in chronic hepatitis B infection-associated liver disease and their impact on peripheral immune response. Gut Microbes 2023, 15, 2155018. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, S.; Fu, Y.; Wu, W.; Chen, T.; Chen, J.; Yang, B.; Ou, Q. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J. Viral Hepat. 2020, 27, 143–155. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Hui, S.; Liu, Y.; Chen, M.; Wang, X.; Lang, H.; Zhou, M.; Yi, L.; Mi, M. Capsaicin Improves Glucose Tolerance and Insulin Sensitivity Through Modulation of the Gut Microbiota-Bile Acid-FXR Axis in Type 2 Diabetic db/db Mice. Mol. Nutr. Food Res. 2019, 63, e1900608. [Google Scholar] [CrossRef]
- Sannasiddappa, T.H.; Lund, P.A.; Clarke, S.R. In Vitro Antibacterial Activity of Unconjugated and Conjugated Bile Salts on Staphylococcus aureus. Front. Microbiol. 2017, 8, 1581. [Google Scholar] [CrossRef]
- Tian, Y.; Gui, W.; Koo, I.; Smith, P.B.; Allman, E.L.; Nichols, R.G.; Rimal, B.; Cai, J.; Liu, Q.; Patterson, A.D. The microbiome modulating activity of bile acids. Gut Microbes 2020, 11, 979–996. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, S.M.; Jung, J. Integrated omics analysis unraveled the microbiome-mediated effects of Yijin-Tang on hepatosteatosis and insulin resistance in obese mouse. Phytomedicine 2020, 79, 153354. [Google Scholar] [CrossRef]
- Han, R.; Qiu, H.; Zhong, J.; Zheng, N.; Li, B.; Hong, Y.; Ma, J.; Wu, G.; Chen, L.; Sheng, L.; et al. Si Miao Formula attenuates non-alcoholic fatty liver disease by modulating hepatic lipid metabolism and gut microbiota. Phytomedicine 2021, 85, 153544. [Google Scholar] [CrossRef]
- Klyarytskaya, I.; Maksymova, H.; Stilid, I.E. Drug-induced Liver Disease in Patients with Diabetes Mellitus. Euroasian J. Hepatogastroenterol. 2015, 5, 83–86. [Google Scholar] [CrossRef]
- Cao, S.Y.; Li, B.Y.; Gan, R.Y.; Mao, Q.Q.; Wang, Y.F.; Shang, A.; Meng, J.M.; Xu, X.Y.; Wei, X.L.; Li, H.B. The In Vivo Antioxidant and Hepatoprotective Actions of Selected Chinese Teas. Foods 2020, 9, 262. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Yuan, L.; Yang, Y.; Zhao, H.; Ho, C.T.; Li, S. Green Tea Catechins Effectively Altered Hepatic Fibrogenesis in Rats by Inhibiting ERK and Smad1/2 Phosphorylation. J. Agric. Food Chem. 2019, 67, 5437–5445. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, X.; Lu, X.; Chen, J.; Zhao, Y. Protective effects of polyphenols-enriched extract from Huangshan Maofeng green tea against CCl4-induced liver injury in mice. Chem. Biol. Interact. 2014, 220, 75–83. [Google Scholar] [CrossRef]
- Pezeshki, A.; Safi, S.; Feizi, A.; Askari, G.; Karami, F. The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int. J. Prev. Med. 2016, 7, 28. [Google Scholar] [CrossRef]
- Hussain, M.; Habib-Ur-Rehman; Akhtar, L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak. J. Med. Sci. 2017, 33, 931–936. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; Chiu, H.F.; Cheng, J.C.; Chang, Y.H.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Tsai, K.S.; Wang, C.K. Comparative studies on the hypolipidemic, antioxidant and hepatoprotective activities of catechin-enriched green and oolong tea in a double-blind clinical trial. Food Funct. 2018, 9, 1205–1213. [Google Scholar] [CrossRef]
- Fukuzawa, Y.; Kapoor, M.P.; Yamasaki, K.; Okubo, T.; Hotta, Y.; Juneja, L.R. Effects of green tea catechins on nonalcoholic steatohepatitis (NASH) patients. J. Funct. Foods 2014, 9, 48–59. [Google Scholar] [CrossRef]
- Tabatabaee, S.M.; Alavian, S.M.; Ghalichi, L.; Miryounesi, S.M.; Mousavizadeh, K.; Jazayeri, S.; Vafa, M.R. Green tea in non-alcoholic fatty liver disease: A double blind randomized clinical trial. Hepat. Mon. 2017, 17, e14993. [Google Scholar] [CrossRef]
- Kheirandish, V.; Mard-Soltani, M.; Mojab, F.; Shakerian, N.; Nanaie, F. The Effect of Milk Thistle, Green Tea, and Cinnamon Beverages on Liver Enzymes of Operating Room Anesthesia Personnel. Trends Med. Sci. 2023, 3, e136000. [Google Scholar] [CrossRef]
- George, J.; Tsuchishima, M.; Tsutsumi, M. Epigallocatechin-3-gallate inhibits osteopontin expression and prevents experimentally induced hepatic fibrosis. Biomed. Pharmacother. 2022, 151, 113111. [Google Scholar] [CrossRef]
- Tang, H.; Hao, S.; Chen, X.; Li, Y.; Yin, Z.; Zou, Y.; Song, X.; Li, L.; Ye, G.; Zhao, L.; et al. Epigallocatechin-3-gallate protects immunity and liver drug-metabolism function in mice loaded with restraint stress. Biomed. Pharmacother. 2020, 129, 110418. [Google Scholar] [CrossRef]
- Xiao, J.; Ho, C.T.; Liong, E.C.; Nanji, A.A.; Leung, T.M.; Lau, T.Y.H.; Fung, M.L.; Tipoe, G.L. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur. J. Nutr. 2014, 53, 187–199. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, X.; Chen, Y.; Deng, Y.; Qian, K. Epigallocatechin gallate attenuated non-alcoholic steatohepatitis induced by methionine- and choline-deficient diet. Eur. J. Pharmacol. 2015, 761, 405–412. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, L.; Chen, R.; Wen, S.; Li, Q.; Lai, X.; Zhang, Z.; Cao, F.; Sun, S. Chinese Tea Alleviates CCl4-Induced Liver Injury through the NF-κB or Nrf2 Signaling Pathway in C57BL-6J Mice. Nutrients 2022, 14, 972. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Wang, T.; Cai, M.; Qian, F.; Sun, Y.; Wang, Y. Green tea polyphenols prevent lipopolysaccharide-induced inflammatory liver injury in mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2019, 10, 3898–3908. [Google Scholar] [CrossRef]
- Shareef, S.H.; Ibrahim, I.A.A.; Alzahrani, A.R.; Al-Medhtiy, M.H.; Abdulla, M.A. Hepatoprotective effects of methanolic extract of green tea against Thioacetamide-Induced liver injury in Sprague Dawley rats. Saudi J. Biol. Sci. 2022, 29, 564–573. [Google Scholar] [CrossRef]
- Ning, K.; Lu, K.; Chen, Q.; Guo, Z.; Du, X.; Riaz, F.; Feng, L.; Fu, Y.; Yin, C.; Zhang, F.; et al. Epigallocatechin Gallate Protects Mice against Methionine–Choline-Deficient-Diet-Induced Nonalcoholic Steatohepatitis by Improving Gut Microbiota To Attenuate Hepatic Injury and Regulate Metabolism. ACS Omega 2020, 5, 20800–20809. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Wang, Y.; Zhang, Q.; Li, J.; Zhong, P.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-Gallate Alleviates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via Inhibition of Apoptosis and Promotion of Autophagy through the ROS/MAPK Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5599997. [Google Scholar] [CrossRef]
- Li, J.H.; Sapper, T.N.; Mah, E.; Rudraiah, S.; Schill, K.E.; Chitchumroonchokchai, C.; Moller, M.V.; McDonald, J.D.; Rohrer, P.R.; Manautou, J.E.; et al. Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFκB pro inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol. Nutr. Food Res. 2016, 60, 858–870. [Google Scholar] [CrossRef]
- Mehri, N.; Felehgari, H.; Larki Harchegani, A.; Behrooj, H.; Kheiripour, N.; Ghasemi, H.; Mirhoseini, M.; Ranjbar, A. Hepatoprotective effect of the root extract of green tea against malathion-induced oxidative stress in rats. J. Herbmed Pharmacol. 2016, 5, 116–119. [Google Scholar]
- Sajjad, F.; Minhas, L.A. Effects of Green Tea (Camellia Sinensis) on Liver Function Tests of Mice on High Fat Diet. Pak. J. Med. Sci. 2014, 8, 550–553. [Google Scholar]
- Wang, D.; Wang, T.; Li, Z.; Guo, Y.; Granato, D. Green Tea Polyphenols Upregulate the Nrf2 Signaling Pathway and Suppress Oxidative Stress and Inflammation Markers in D-Galactose-Induced Liver Aging in Mice. Front. Nutr. 2022, 9, 836112. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Gan, R.Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.P. Microbial metabolism of theaflavin-3,3’-digallate and its gut microbiota composition modulatory effects. J. Agric. Food Chem. 2021, 69, 232–245. [Google Scholar] [CrossRef]
- Vinci, G.; D’Ascenzo, F.; Maddaloni, L.; Prencipe, S.A.; Tiradritti, M. The Influence of Green and Black Tea Infusion Parameters on Total Polyphenol Content and antioxidant Activity by ABTS and DPPH Assays. Beverages 2022, 8, 18. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Teixeira, A.M.; Sousa, C.A. Review on the Biological Activity of Camellia Species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Marzec, A.; Domian, E.; Galus, S.; Ciurzyńska, A.; Brzezińska, R.; Kowalska, H. Influence of Tea Brewing Parameters on the Antioxidant Potential of Infusions and Extracts Depending on the Degree of Processing of the Leaves of Camellia sinensis. Molecules 2021, 26, 4773. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Bąkowski, M.; Krusiński, R.; Jachimowicz-Rogowska, K.; Demkowska-Kutrzepa, M.; Kiczorowska, B.; Krupa, W. Tannic Acid and Tea Prevents the Accumulation of Lead and Cadmium in the Lungs, Heart and Brain of Adolescent Male Wistar Rats-Possible Therapeutic Option. Animals 2022, 12, 2838. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.A. Hepatoprotective Effect of Green Tea Extract against Cyclophosphamide Induced Liver Injury in Albino Rats. Forensic Med. Anat. Res. 2018, 6, 11–19. [Google Scholar] [CrossRef]
- Diao, J.X.; Ou, J.Y.; Dai, H.; Li, H.Y.; Huang, W.; Hua, H.Y.; Xie, T.; Wang, M.; Yang, Y.G. Antioxidant and Antiapoptotic Polyphenols from Green Tea Extract Ameliorate CCl4-Induced Acute Liver Injury in Mice. Chin. J. Integr. Med. 2020, 26, 736–744. [Google Scholar] [CrossRef]
- Al-Gnami, S.A. Effect of polyphenols which extracted from green tea in reduce toxic effects of cadmium sulfate in rat’s liver. IOSR J. Pharm. Biol. Sci. 2014, 9, 53–58. [Google Scholar]
- Hamadouche, N.A.; Guellil, H.; Slimani, M.; Aoues, A. Positive effects of green tea (Camelia sinensis) on hepatic dysfunction induced by lead acetate in male rats. Int. J. Drug Dev. Res. 2014, 6, 87–96. [Google Scholar]
- Yamasaki, S.; Kimura, G.; Koizumi, K.; Dai, N.; Ketema, R.M.; Tomihara, T.; Ueno, Y.; Ohno, Y.; Sato, S.; Kurasaki, M.; et al. Maternal green tea extract intake during lactation attenuates hepatic lipid accumulation in adult male rats exposed to a continuous high-fat diet from the foetal period. Food Nutr. Res. 2020, 64, fnr.v64.5231. [Google Scholar] [CrossRef]
- Chen, B.T.; Li, W.X.; He, R.R.; Li, Y.F.; Tsoi, B.; Zhai, Y.J.; Kurihara, H. Anti-inflammatory effects of a polyphenols-rich extract from tea (Camellia sinensis) flowers in acute and chronic mice models. Oxid. Med. Cell Longev. 2012, 2012, 537923. [Google Scholar] [CrossRef]
- Karolczak, D.; Seget, M.; Bajerska, J.; Błaszczyk, A.; Drzymała-Czyż, S.; Walkowiak, J.; Marszałek, A. Green tea extract prevents the development of nonalcoholic liver steatosis in rats fed a high-fat diet. Pol. J. Pathol. 2019, 70, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Bae, U.J.; Park, J.; Park, I.W.; Chae, B.M.; Oh, M.R.; Jung, S.J.; Ryu, G.S.; Chae, S.W.; Park, B.H. Epigallocatechin-3-Gallate-Rich Green Tea Extract Ameliorates Fatty Liver and Weight Gain in Mice Fed a High Fat Diet by Activating the Sirtuin 1 and AMP Activating Protein Kinase Pathway. Am. J. Chin. Med. 2018, 46, 617–632. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, R.; Lang, J.; Fu, Y.; Yang, L.; Zhao, D. Epigallocatechin-3-gallate ameliorates hepatic damages by relieve FGF21 resistance and promotion of FGF21-AMPK pathway in mice fed a high fat diet. Diabetol. Metab. Syndr. 2022, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.B.; Chu, X.L.; Jin, Y.X.; Jiang, J.J.; Zhao, X.; Yu, M. Epigallocatechin gallate alleviates high-fat diet-induced hepatic lipotoxicity by targeting mitochondrial ROS-mediated ferroptosis. Front. Pharmacol. 2023, 14, 1148814. [Google Scholar] [CrossRef]
- Mostafa-Hedeab, G.; Ewaiss Hassan, M.; Halawa, T.; Ahmed Wani, F. Epigallocatechin gallate ameliorates tetrahydrochloride-induced liver toxicity in rats via inhibition of TGFβ / p-ERK/p-Smad1/2 signaling, antioxidant, anti-inflammatory activity. Saudi Pharm. J. 2022, 30, 1293–1300. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Weng, P. Antioxidant and hepatoprotective effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3″Me) from Chinese oolong tea. J. Agric. Food Chem. 2014, 62, 10046–10054. [Google Scholar] [CrossRef]
- An, Z.; Qi, Y.; Huang, D.; Gu, X.; Tian, Y.; Li, P.; Li, H.; Zhang, Y. EGCG inhibits Cd(2+)-induced apoptosis through scavenging ROS rather than chelating Cd(2+) in HL-7702 cells. Toxicol. Mech. Methods 2014, 24, 259–267. [Google Scholar] [CrossRef]
- Yang, C.; Wu, A.; Tan, L.; Tang, D.; Chen, W.; Lai, X.; Gu, K.; Chen, J.; Chen, D.; Tang, Q. Epigallocatechin-3-Gallate Alleviates Liver Oxidative Damage Caused by Iron Overload in Mice through Inhibiting Ferroptosis. Nutrients 2023, 15, 1993. [Google Scholar] [CrossRef]
- Ivanišová, E.; Hornák, M.; Cech, M.; Harangozo, L.; Kacániová, M.; Grygorieva, O.; Kowalczewski, P.Ł. Polyphenol Content, Mineral Compounds Composition, Antimicrobial and Antioxidant Activities of Selected Medicinal Herbs from Slovak Republic. Appl. Sci. 2023, 13, 1918. [Google Scholar] [CrossRef]
- Shannon, E.; Jaiswal, A.K.; Abu-Ghannam, N. Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas: A kinetic study. Food Res. 2018, 2, 1–11. [Google Scholar] [CrossRef]
- Korir, M.W.; Wachira, F.N.; Wanyoko, J.K.; Ngure, R.M.; Khalid, R. The fortification of tea with sweeteners and milk and its effect on in vitro antioxidant potential of tea product and glutathione levels in an animal model. Food Chem. 2014, 145, 145–153. [Google Scholar] [CrossRef]
- Mahboub, F.A. The Effect of Green Tea (Camellia sinensis) Extract Against Hepato-Toxicity Induced By Tamoxifen in Rats. J. Food Process. Technol. 2016, 7, 625. [Google Scholar] [CrossRef]
- Lv, L.; Shu, H.; Mo, X.; Tian, Y.; Guo, H.; Sun, H.-Y. Activation of the Nrf2 Antioxidant Pathway by Longjing Green Tea Polyphenols in Mice Livers. Nat. Prod. Commun. 2022, 17, 1934578X221139409. [Google Scholar] [CrossRef]
- Ye, F.; Li, X.; Li, L.; Lyu, L.; Yuan, J.; Chen, J. The role of Nrf2 in protection against Pb-induced oxidative stress and apoptosis in SH-SY5Y cells. Food Chem. Toxicol. 2015, 86, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Chandra, S.; Dey, P.; Bhattacharya, S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Rha, C.S.; Jeong, H.W.; Park, S.; Lee, S.; Jung, Y.S.; Kim, D.O. Antioxidative, Anti-Inflammatory, and Anticancer Effects of Purified Flavonol Glycosides and Aglycones in Green Tea. Antioxidants 2019, 8, 278. [Google Scholar] [CrossRef]
- Wein, S.; Schrader, E.; Rimbach, G.; Wolffram, S. Oral green tea catechins transiently lower plasma glucose concentrations in female db/db mice. J. Med. Food. 2013, 16, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Alipour, M.; Motevalli, M.S.; Chebbi, A.; Laher, I.; Zouhal, H. Does green tea extract enhance the anti-inflammatory effects of exercise on fat loss? Br. J. Clin. Pharmacol. 2020, 86, 753–762. [Google Scholar] [CrossRef]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Grubbs, B.; Motevalli, M.S.; Baker, J.S.; Laher, I.; Zouhal, H. Effects of green tea extract supplementation and endurance training on irisin, pro-inflammatory cytokines, and adiponectin concentrations in overweight middle-aged men. Eur. J. Appl. Physiol. 2020, 120, 915–923. [Google Scholar] [CrossRef]
- Mota, M.A.; Landim, J.S.; Targino, T.S.; Silva, S.F.; Silva, S.L.; Pereira, M.R. Evaluation of the anti-inflammatory and analgesic effects of green tea (Camellia sinensis) in mice. Acta Cir. Bras. 2015, 30, 242–246. [Google Scholar] [CrossRef] [PubMed]
- El-Kersh, D.M.; Kotob, S.E.; Ammar, N.M.; Mohawed, O.A.M.; Ahmed, H.H.; Farag, M.A. Unravelling the anti-inflammatory and antioxidant effects of standardized green and black caffeinated coffee, tea, and their mixtures in an obese male rat model: Insights from biochemical, metabolomic, and histopathological analyses. Food Chem. Toxicol. 2023, 179, 113971. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jeong, W.-S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci. Hum. Wellness 2022, 11, 502–511. [Google Scholar] [CrossRef]
- Hagiu, A.; Attin, T.; Schmidlin, P.R.; Ramenzoni, L.L. Dose-dependent green tea effect on decrease of inflammation in human oral gingival epithelial keratinocytes: In vitro study. Clin. Oral Investig. 2020, 24, 2375–2383. [Google Scholar] [CrossRef]
- Carito, V.; Ciafrè, S.; Tarani, L.; Ceccanti, M.; Natella, F.; Iannitelli, A.; Tirassa, P.; Chaldakov, G.N.; Ceccanti, M.; Boccardo, C.; et al. TNF-α and IL-10 modulation induced by polyphenols extracted by olive pomace in a mouse model of paw inflammation. Ann. Ist. Super Sanita 2015, 51, 382–386. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The Anti-Inflammatory Properties of Phytochemicals and Their Effects on Epigenetic Mechanisms Involved in TLR4/NF-κB-Mediated Inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.-C.; Li, S.; Zhan, J.; Ho, C.-T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, M.; Liang, Z. (−)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef]
- Li, J.; Yip, Y.W.Y.; Ren, J.; Hui, W.K.; He, J.N.; Yu, Q.X.; Chu, K.O.; Ng, T.K.; Chan, S.O.; Pang, C.P.; et al. Green tea catechins alleviate autoimmune symptoms and visual impairment in a murine model for human chronic intraocular inflammation by inhibiting Th17-associated pro-inflammatory gene expression. Sci. Rep. 2019, 9, 2301. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutrit. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jakab, J.; Miškić, B.; Mikšić, S.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Balcheva-Sivenova, Z.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Caffeic and chlorogenic acids synergistically activate browning program in human adipocytes: Implications of AMPK- and PPAR-mediated pathways. Int. J. Mol. Sci. 2020, 21, 9740. [Google Scholar] [CrossRef]
- Fu, Q.Y.; Li, Q.S.; Lin, X.M.; Qiao, R.Y.; Yang, R.; Li, X.M.; Dong, Z.B.; Xiang, L.P.; Zheng, X.Q.; Lu, J.L.; et al. Antidiabetic Effects of Tea. Molecules 2017, 22, 849. [Google Scholar] [CrossRef]
- Striegel, L.; Kang, B.; Pilkenton, S.J.; Rychlik, M.; Apostolidis, E. Effect of Black Tea and Black Tea Pomace Polyphenols on α-Glucosidase and α-Amylase Inhibition, Relevant to Type 2 Diabetes Prevention. Front. Nutr. 2015, 2, 3. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, C.C.; Chen, C.C.; Yang, K.J.; Huang, C.Y. Inhibition of Staphylococcus aureus PriA Helicase by Flavonol Kaempferol. Protein J. 2015, 34, 169–172. [Google Scholar] [CrossRef]
- Janssens, P.L.; Penders, J.; Hursel, R.; Budding, A.E.; Savelkoul, P.H.; Westerterp-Plantenga, M.S. Long-term green tea supplementation does not change the human gut microbiota. PLoS ONE 2016, 11, e0153134. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Kaihatsu, K.; Nishino, K.; Ogawa, M.; Kato, N.; Yamaguchi, A. Antibacterial and antifungal activities of new acylated derivatives of epigallocatechin gallate. Front. Microbiol. 2012, 3, 53. [Google Scholar] [CrossRef]
- Bustos, I.; García-Cayuela, T.; Hernández-Ledesma, B.; Peláez, C.; Requena, T.; Martínez-Cuesta, M.C. Effect of flavan-3-ols on the adhesion of potential probiotic Lactobacilli to intestinal cells. J. Agric. Food. Chem. 2012, 60, 9082–9088. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef] [PubMed]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific Opinion on the safety of green tea catechins. EFSA J. 2018, 16, 5239. [Google Scholar] [CrossRef]
- James, K.D.; Kennett, M.J.; Lambert, J.D. Potential role of the mitochondria as a target for the hepatotoxic effects of (-)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2018, 111, 302–309. [Google Scholar] [CrossRef]
- Dong, R.; Wang, D.; Wang, X.; Zhang, K.; Chen, P.; Yang, C.S.; Zhang, J. Epigallocatechin-3-gallate enhances key enzymatic activities of hepatic thioredoxin and glutathione systems in selenium-optimal mice but activates hepatic Nrf2 responses in selenium-deficient mice. Redox Biol. 2016, 10, 221–232. [Google Scholar] [CrossRef]
- James, K.D.; Forester, S.C.; Lambert, J.D. Dietary pretreatment with green tea polyphenol, (-)-epigallocatechin-3-gallate reduces the bioavailability and hepatotoxicity of subsequent oral bolus doses of (-)-epigallocatechin-3-gallate. Food Chem. Toxicol. 2015, 76, 103–108. [Google Scholar] [CrossRef]
- Egea, M.B.; Pierce, G.; Becraft, A.R.; Sturm, M.; Yu, W.; Shay, N.F. Intake of Watermelon and Watermelon Byproducts in Male Mice Fed a Western-Style Obesogenic Diet Alters Hepatic Gene Expression Patterns, as Determined by RNA Sequencing. Curr. Dev. Nutr. 2020, 4, nzaa122. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, Z.; Wang, X.; Wang, L.; Zhang, N.; Liu, H.; Cong, X.; Li, S.; Zhu, Z. Protective Effects of Different Selenium Green Tea Polysaccharides on the Development of Type 2 Diabetes in Mice. Foods 2023, 12, 4190. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Z.; Peng, F.; Xiao, F.; Ren, D.; Xue, H.; Chen, T.; Mushtaq, G.; Kamal, M.A. Combination of selenium-enriched green tea polysaccharides and Huo-ji polysaccharides synergistically enhances antioxidant and immune activity in mice. J. Sci. Food Agric. 2015, 95, 3211–3217. [Google Scholar] [CrossRef]
| Characteristic | Duration of the Study | Disease | Dosage of Green Tea | Antioxidant Parameters | Liver Parameters | Type of Study | Reference |
|---|---|---|---|---|---|---|---|
| Control n = 40 Experimental n = 40 | 12 weeks | Non-alcoholic fatty liver disease | 500 mg/day of GTE | ↓ ALT, ↓ AST | Double-blind, placebo-controlled, randomized clinical trial | [51] | |
| Control n = 40 Experimerntal n = 80 | 12 weeks | Non-alcoholic fatty liver disease | 1000 mg/day of GTE | ↓ ALT, ↓ AST, ↓ hs-CRP | Double-blind, placebo-controlled, randomized clinical trial | [52] | |
| Control n = 20 Experimental n = 20 | 12 weeks | Moderate hypercholesterolemia | 2 × 300 mL catechin-enriched green tea/day | ↑ TEAC, ↑ GSH, ↑ SOD, ↑ CAT, ↑ GPx, ↑ GR | reverting mild fatty liver to the normal hepatic condition | Randomized, controlled trial | [53] |
| Control n = 12 Experimental n = 26 | 6 months | Non-alcoholic steatohepatitis | 600 mg/day catechins | ↓ ALT, ↓ AST | Double-blind, placebo-controlled, randomized clinical trial | [54] | |
| Control n = 24 Experimental n = 21 | 3 months | Non-alcoholic fatty liver disease | 550 mg/day green tea tablets | ↓ AST | Placebo-controlled, randomized clinical trial | [55] | |
| Control n = 16 Experimental n = 16 | 4 weeks | Operating room staff chronically exposed to inhalation anesthetics | ↓ AST, ↓ ALT, ↓ ALP, ↓ bilirubin | Placebo-controlled, randomized clinical trial | [56] |
| Animal Species | Duration of Experiment | Treatments | Dosage of Green Tea | Antioxidant Parameters | Liver Parameters | Anti-inflammatory Indices | Tissues | References | |
|---|---|---|---|---|---|---|---|---|---|
| Control n = 6 Experimental n = 18 | Male Wistar rats | 28 days | Intraperitoneal injections of N-nitrosodimethylamine in a dose of 1 mg/100 g body weight on 3 consecutive days of a week | 0.2 mg EGCG/100 g body weight | ↓ MDA | ↓ ALT, ↓ AST | Serum | [57] | |
| Control n = 5 Experimental n = 25 | Male and female mice ICR | 7 days | Stress-induced liver injury and immunosuppression | 40 mg EGCG/kg | ↓ ALT, ↓ AST | ↓ IL-1β, ↓ IL-2, ↓ IL-6 | Serum, liver | [58] | |
| Control n = 6 Experimental n = 22 | Female Sprague–Dawley rats | 8 weeks | Non-alcoholic fatty liver disease | 50 mg EGCG/kg | ↓ iNOS, ↓ COX-2, ↓ TNF-α | ↓ ALT,↓AST ratio, ↓ number of fatty score, necrosis | ↓ inflammatory foci | Serum, liver | [59] |
| Control n = 8 Experimental n = 32 | Male C57BL/6 mice | 4 weeks | Methionine- and choline-deficient diet-induced non-alcoholic steatohepatitis | 25, 50, or 100 mg EGCG/kg | ↓ ALT, ↓ AST | Serum | [60] | ||
| Control n = 5 Experimental n = 30 | Female C57BL/6 mice | 4 days | CCl4-induced liver injury | 100 mg GTE/kg | ↓ ALT, ↓ AST, ↓ liver index | Serum, liver | [61] | ||
| Control n = 6 Experimental n = 18 | Male ICR mice | 7 days | Lipopolysaccharide-induced inflammatory liver injury | 100 or 200 mg green tea polyphenols/kg body weight | ↓ MDA, ↓ GSH, ↑ SOD | ↓ ALT, ↓ AST | ↓ IL-1β, ↓ IL-18, ↓ IL-6, ↓ TNF-α | Serum, liver | [62] |
| Control n = 6 Experimental n = 24 | Male Sprague–Dawley rats | 2 months | Thioacetamide-induced liver injury | 250 mg/kg or 500 mg/kg daily methanolic GTE | ↓ MDA, ↑ SOD, ↑ CAT | ↓ ALT, ↓ AST, ↓ ALP, ↓ bilirubin | Serum, liver | [63] | |
| Control n = 12 Experimental n = 36 | Male C57BL/6J mice | 4 weeks | Methionine–choline-deficient diet-induced non-alcoholic steatohepatitis | 50 mg/kg EGCG | ↓ ALT | Serum | [64] | ||
| Control n = 12 Experimental n = 6 | Male C57BL/6J mice | 14 weeks | High-fat diet-induced non-alcoholic fatty liver disease | EGCG—50 mg/kg/day | ↓ ROS, ↑ GPx, ↑ SOD, ↑ CAT | ↓ ALT, ↓ AST | Serum, liver | [65] | |
| Control n = 12 Experimental n = 48 | Adult male Wistar rats | 6 or 12 weeks | 7 mg CdCl2 + 50 mg Pb(CH3COO)2 per kg of feed | Green tea infusion (contains 111 mg tannic acid) per 1000 mL H2O2 | ↑ SOD, ↑ CAT, ↑ GPx | Liver | [11] | ||
| Control n = 20 Experimental n = 23 | Male Nrf2-null mice, male C57BL6 WT mice | 8 weeks | High-fat diet-induced non-alcoholic steatohepatitis | 2% GTE | ↓ MDA | ↓ ALT | ↓ TNF-α | Liver | [66] |
| Control n = 8 Experimental n = 24 | Male Wistar rats | 1 week | Halathion 150 mg/kg by gavage | 30 mg/kg green tea through intraperitoneal injection | ↓ LPO, ↑ TAP, ↑ TTG | ↓ ALT, ↓ AST, ↑ ChE | Plasma, liver | [67] | |
| Control n = 20 Experimental n = 40 | Adult mice Balb-C strain | 12 weeks | High-fat and high-cholesterol diet-induced hepatic steatosis | 1% green tea over in food | ↓ ALT, ↓ AST, ↓ ALP | Serum | [68] | ||
| Control n = 10 Experimental n = 30 | Male Kunming mice | 12 weeks | D-galactose-induced liver ageing | 0.05% green tea polyphenols diet | ↑ SOD, ↑ CAT, ↑ GSH, ↑ GST, ↑ TAC, ↓ MDA, ↓ NO | ↓ ALT, ↓ AST, ↓ ALP | ↓ TNF-α, ↓ TGF-β, ↓ IL-1β, ↓ IL-6 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska-Mieczan, A.; Jachimowicz-Rogowska, K.; Kwiecień, M.; Borsuk-Stanulewicz, M.; Tomczyk-Warunek, A.; Stamirowska-Krzaczek, E.; Purwin, C.; Stryjecka, M.; Tomaszewska, M. Regular Consumption of Green Tea as an Element of Diet Therapy in Drug-Induced Liver Injury (DILI). Nutrients 2024, 16, 2837. https://doi.org/10.3390/nu16172837
Winiarska-Mieczan A, Jachimowicz-Rogowska K, Kwiecień M, Borsuk-Stanulewicz M, Tomczyk-Warunek A, Stamirowska-Krzaczek E, Purwin C, Stryjecka M, Tomaszewska M. Regular Consumption of Green Tea as an Element of Diet Therapy in Drug-Induced Liver Injury (DILI). Nutrients. 2024; 16(17):2837. https://doi.org/10.3390/nu16172837
Chicago/Turabian StyleWiniarska-Mieczan, Anna, Karolina Jachimowicz-Rogowska, Małgorzata Kwiecień, Marta Borsuk-Stanulewicz, Agnieszka Tomczyk-Warunek, Ewa Stamirowska-Krzaczek, Cezary Purwin, Małgorzata Stryjecka, and Marzena Tomaszewska. 2024. "Regular Consumption of Green Tea as an Element of Diet Therapy in Drug-Induced Liver Injury (DILI)" Nutrients 16, no. 17: 2837. https://doi.org/10.3390/nu16172837
APA StyleWiniarska-Mieczan, A., Jachimowicz-Rogowska, K., Kwiecień, M., Borsuk-Stanulewicz, M., Tomczyk-Warunek, A., Stamirowska-Krzaczek, E., Purwin, C., Stryjecka, M., & Tomaszewska, M. (2024). Regular Consumption of Green Tea as an Element of Diet Therapy in Drug-Induced Liver Injury (DILI). Nutrients, 16(17), 2837. https://doi.org/10.3390/nu16172837






