Effectiveness of Zinc Supplementation for Sepsis Treatment: A Single-Center Retrospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Collection and Study Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lubna, S.; Ahmad, R. Clinical and biochemical understanding of Zinc interaction during liver diseases: A paradigm shift. J. Trace Elem. Med. Biol. 2023, 77, 127130. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Murakami, M.; Fukada, T.; Nishida, K.; Yamasaki, S.; Suzuki, T. Roles of zinc and zinc signaling in immunity: Zinc as an intracellular signaling molecule. Adv. Immunol. 2008, 97, 149–176. [Google Scholar] [PubMed]
- Olson, L.M.; Coffey, R.; Porter, K.; Thomas, S.; Bailey, J.K.; Jones, L.M.; Murphy, C.V. The impact of serum zinc normalization on clinical outcomes in severe burn patients. Burns 2000, 46, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, S.; Mhalla, Y.; Trabelsi, I.; Sekma, A.; Youssef, R.; Bel Haj Ali, K.; Ben Soltane, H.; Yacoubi, H.; Msolli, M.A.; Stambouli, N.; et al. Twice-daily oral zinc in the treatment of patients with Coronavirus Disease 2019: A randomized double-blind controlled trial. Clin. Infect. Dis. 2023, 76, 185–191. [Google Scholar] [CrossRef]
- Al Sulaiman, K.; Aljuhani, O.; Al Shaya, A.I.; Kharbosh, A.; Kensara, R.; Al Guwairy, A.; Alharbi, A.; Algarni, R.; Al Harbi, S.; Vishwakarma, R.; et al. Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: A two center propensity-score matched study. Crit. Care 2021, 25, 363. [Google Scholar] [CrossRef]
- Khazdouz, M.; Mazidi, M.; Ehsaei, M.R.; Ferns, G.; Kengne, A.P.; Norouzy, A.R. Impact of zinc supplementation on the clinical outcomes of patients with severe head trauma: A double-blind randomized clinical trial. J. Diet. Suppl. 2018, 15, 1–10. [Google Scholar] [CrossRef]
- Gitte, K.V.; Thomas, S.J.; Karen, L.E.; Morten, H.M.; Thordis, T.; Anders, P. Effects of magnesium, phosphate, or zinc supplementation in intensive care unit patients—A systematic review and meta-analysis. Acta Aaesthesiol. Scand. 2023, 67, 264–276. [Google Scholar]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Prescott, H.C.; Harrison, D.A.; Rowan, K.M.; Shankar-Hari, M.; Wunsch, H. Temporal trends in mortality of critically ill patients with sepsis in the UK, 1988–2019. Am. J. Respir. Crit. Care Med. 2024, 209, 507–516. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Tanaka, M.; Naito, Y.; Katayama, K.; Moriyama, M. Japan’s practical guidelines for zinc deficiency with a particular focus on taste disorders, inflammatory bowel disease, and liver cirrhosis. Int. J. Mol. Sci. 2020, 21, 2941. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.; Ohlsson, T.; Hallstadius, L.; Hansson, L.; Abdulla, M.; Strand, S.E.; Jeppsson, B. Organ sequestration of 65Zn during experimental sepsis. Clin. Nutr. 1989, 8, 263–267. [Google Scholar] [CrossRef]
- Besecker, B.Y.; Exline, M.C.; Hollyfield, J.; Phillips, G.; Disilvestro, R.A.; Wewers, M.D.; Knoell, D.L. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am. J. Clin. Nutr. 2011, 93, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Mathew, J.; Kohler, J.E.; Blass, A.L.; Soybel, A.D. Hemorrhagic shock and surgical stress alter distribution of labile zinc within high- and low-molecular-weight plasma fractions. Shock 2012, 38, 314–319. [Google Scholar] [CrossRef]
- Duncan, A.; Yacoubian, C.; Watson, N.; Morrison, I. The risk of copper deficiency in patients prescribed zinc supplements. J. Clin. Pathol. 2015, 68, 723–725. [Google Scholar] [CrossRef]
- Forman, W.B.; Sheehan, D.; Cappelli, S.; Coffman, B. Zinc abuse--an unsuspected cause of sideroblastic anemia. West. J. Med. 1990, 152, 190–192. [Google Scholar]
- Porter, K.G.; McMaster, D.; Elmes, M.E.; Love, A.H. Anaemia and low serum-copper during zinc therapy. Lancet 1977, 2, 774. [Google Scholar] [CrossRef]
- Patterson, W.P.; Winkelmann, M.; Perry, M.C. Zinc-induced copper deficiency: Megamineral sideroblastic anemia. Ann. Intern. Med. 1985, 103, 385–386. [Google Scholar] [CrossRef]
- Igic, P.G.; Lee, E.; Harper, W.; Roach, K.W. Toxic effects associated with consumption of zinc. Mayo Clin. Proc. 2002, 77, 713–716. [Google Scholar] [CrossRef] [PubMed]
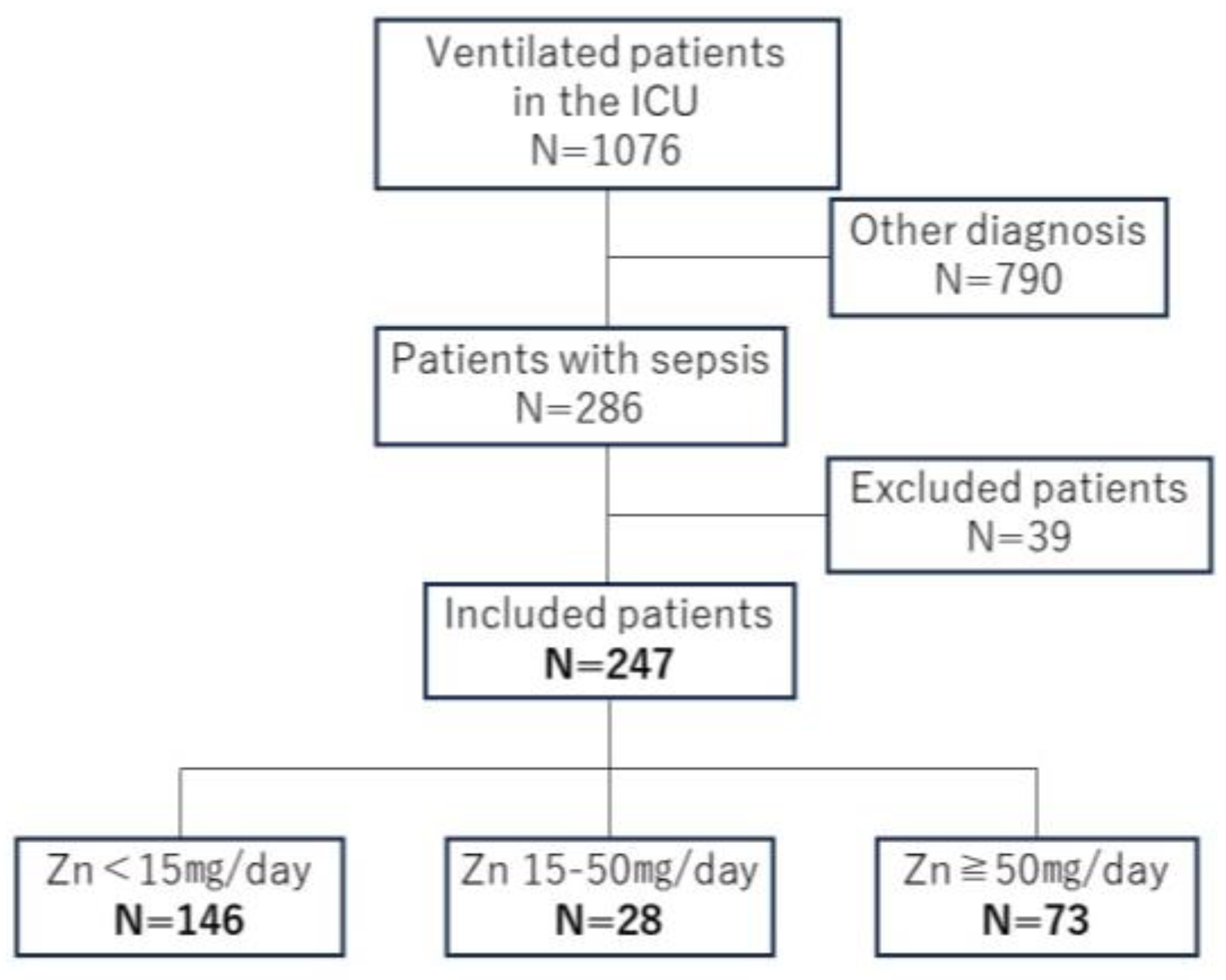

| <15 mg/Day | 15–50 mg/Day | ≥50 mg/Day | p Value | |
|---|---|---|---|---|
| N = 146 | N = 28 | N = 73 | ||
| Age (years) | 68.9 ± 14.1 | 67.8 ± 16.8 | 70.6 ± 13.8 | 0.73 * |
| Sex (male) (%) | 98 (67.1) | 19 (67.9) | 27 (37.0) | 0.81 ** |
| Weight (kg) | 65.9 ± 15.8 | 58.6 ± 13.1 | 64.3 ± 15.7 | 0.12 * |
| Body mass index | 24.7 ± 5.1 | 22.3 ± 3.4 | 24.9 ± 6.3 | 0.04 * |
| CONUT score | 9.8 ± 1.8 | 10.4 ± 1.7 | 10.3 ± 1.7 | 0.03 * |
| Infection focus (%) | ||||
| Lung | 55 (37.7) | 7 (25.0) | 17 (23.3) | 0.07 ** |
| Digestive organs | 55 (37.7) | 14 (50.0) | 39 (53.4) | 0.06 ** |
| Urinary tract | 14 (9.6) | 1 (3.6) | 2 (2.7) | 0.15 ** |
| Skin/Soft tissue | 16 (11.0) | 2 (7.1) | 9 (12.3) | 0.83 ** |
| Others | 6 (4.1) | 4 (14.3) | 6 (8.2) | 0.09 ** |
| Severity of ICU admission | ||||
| SOFA score | 10.5 ± 3.8 | 8.7 ± 3.8 | 10.0 ± 4.1 | 0.07 * |
| APACHE-II score | 31.7 ± 7.0 | 30.1 ± 7.1 | 30.1 ± 7.6 | 0.24 * |
| Total ventilation days | 13.5 ± 16.4 | 17.1 ± 34.6 | 15.5 ± 17.6 | 0.23 * |
| Ventilation days >7 days | 103 (70.5) | 13 (46.4) | 53 (72.6) | 0.04 ** |
| Renal replacement therapy | 61 (41.8) | 9 (32.1) | 31 (42.5) | 0.86 ** |
| L/D on initial arrival (: normal range of our hospital) | ||||
| CRP (mg/dL; <0.14) | 14.2 ± 11.2 | 14.5 ± 8.6 | 12.7 ± 9.2 | 0.61 * |
| Albumin (g/dL: 4.1–5.1) | 2.03 ± 0.5 | 1.9 ± 0.5 | 1.8 ± 0.5 | <0.01 * |
| WBC (×103/µL: 3.3–8.6) | 13.8 ± 11.1 | 12.7 ± 6.2 | 14.3 ± 7.1 | 0.23 * |
| Lymphocyte (/µL) | 893.8 ± 937.8 | 654.1 ± 393.7 | 715.8 ± 445.8 | 0.20 * |
| Platelet (×104/µL: 15.8–34.8) | 16.5 ± 10.7 | 16.5 ± 8.7 | 16.5 ± 19.7 | 0.11 * |
| Total bilirubin (mg/dL: 0.4–1.5) | 1.65 ± 2.5 | 2.1 ± 6.1 | 1.8 ± 2.7 | 0.55 * |
| Creatinine (mg/dL: 0.65–1.07) | 1.58 ± 1.56 | 1.5 ± 1.1 | 1.5 ± 1.7 | 0.98 * |
| Total protein (g/dL: 6.6–8.1) | 4.86 ± 0.78 | 4.5 ± 0.6 | 4.6 ± 0.7 | 0.17 * |
| Total cholesterol (mg/dL: 142–248) | 118.2 ± 38.8 | 104.8 ± 34.3 | 117.0 ± 44.3 | 0.21 * |
| <15 mg/Day | 15–50 mg/Day | ≥50 mg/Day | p Value | |
|---|---|---|---|---|
| Primary outcome | ||||
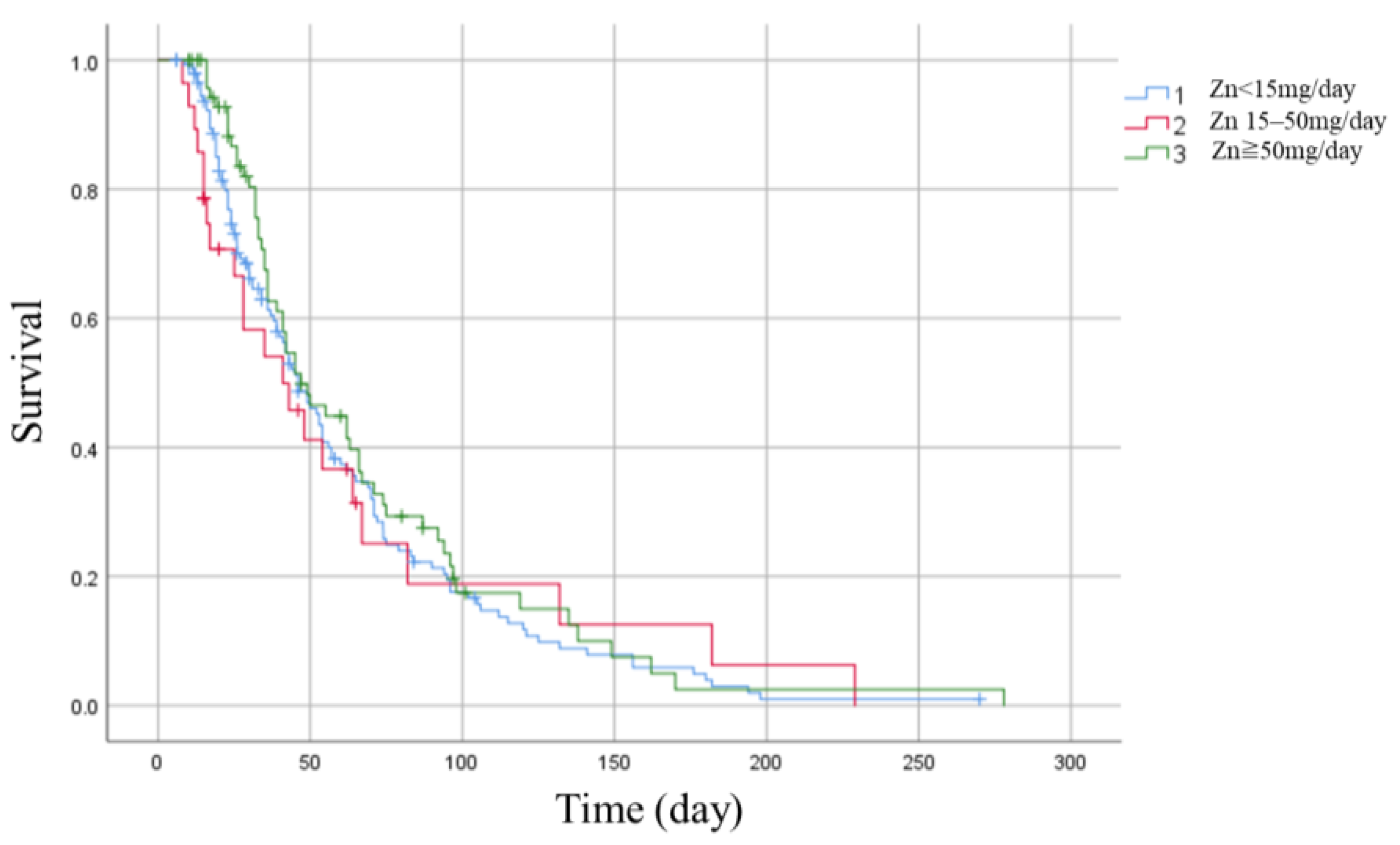
| In-hospital mortality (%) | 28 (19%) | 6 (21%) | 16 (22%) | 0.36 ** |
| Secondary outcome | ||||
| Length of ICU stay (day) | 17.5 ± 13.4 | 18.2 ± 17.0 | 19.5 ± 12.2 | 0.06 * |
| Serum zinc concentration (µg/dL) (normal range of our hospital: 80–130) | ||||
| At the 1st week of admission | 38.5 ± 16.6 | 58.8 ± 19.7 | 74.2 ± 22.5 | <0.01 * |
| At the 2nd week of admission | 58.8 ± 19.7 | 55.3 ± 27.0 | 53.6 ± 19.3 | 0.08 * |
| At the 3rd week of admission | 74.2 ± 22.5 | 65.1 ± 20.3 | 71.0 ± 21.5 | 0.19 * |
| Survival | Death | p Value | |
|---|---|---|---|
| N = 198 | N = 49 | ||
| Age (year old) | 68.6 ± 14.6 | 71.8 ± 12.9 | 0.16 * |
| Sex (male) (%) | 124 (62.6) | 19 (67.9) | 0.05 ** |
| Weight (kg) | 63.5 ± 15.3 | 68.9 ± 16.2 | 0.04 * |
| Body mass index | 24.1 ± 5.37 | 26.1 ± 5.1 | 0.01 * |
| CONUT score | 9.9 ± 1.8 | 10.4 ± 1.6 | 0.04 * |
| Infection Focus (%) | |||
| Lung | 60 (30.3) | 19 (38.8) | 0.26 ** |
| Digestive organs | 91 (46.0) | 17 (34.7) | 0.16 ** |
| Urinary tract | 14 (7.0) | 3 (6.1) | 0.41 ** |
| Skin/Soft tissue | 22 (11.1) | 5 (10.2) | 0.86 ** |
| Others | 11 (5.6) | 5 (10.2) | 0.19 ** |
| SOFA score | 9.8 ± 3.7 | 11.7 ± 4.3 | 0.05 * |
| APACHE II score | 30.6 ± 6.7 | 33 ± 8. | 0.12 * |
| Total ventilation days (days) | 13.3 ± 20.0 | 19.4 ± 16.9 | 0 * |
| Renal replacement therapy (%) | 72 (36.3) | 29 (59.1) | <0.01 ** |
| Dose of zinc supplementation | 46.8 ± 50.1 | 54.4 ± 55.9 | 0.39 * |
| Serum zinc concentration (µg/dL) (normal range of our hospital: 80–130) | |||
| At the 1st week of admission | 34.9 ± 15.1 | 36.9 ± 18.2 | 0.71 * |
| At the 2nd week of admission | 58.2 ± 20.6 | 61.0 ± 19.3 | 0.19 * |
| At the 3rd week of admission | 74.4 ± 21.8 | 61.7 ± 19.7 | <0.01 * |
| Other L/D on initial arrival (normal range of our hospital) | |||
| CRP (mg/dL; <0.14) | 14.5 ± 10.5 | 10.9 ± 8.3 | 0.02 * |
| Albumin (g/dL: 4.1–5.1) | 2.0 ± 0.5 | 1.8 ± 0.5 | <0.01 * |
| WBC (×103/µL: 3.3–8.6) | 13.4 ± 9.9 | 15.7 ± 8.6 | 0.03 * |
| Lymphocyte (/µL) | 833.9 ± 783.4 | 751.7 ± 657 | 0.17 * |
| Platelet (×104/µL: 15.8–34.8) | 16.5 ± 10.6 | 16.7 ± 22.1 | 0.02 * |
| Total bilirubin (mg/dL: 0.4–1.5) | 1.3 ± 2.1 | 3.3 ± 5.3 | <0.01 * |
| Creatinine (mg/dL: 0.65–1.07) | 1.5 ± 1.5 | 1.9 ± 1.5 | 0.02 * |
| Total protein (g/dL: 6.6–8.1) | 4.8 ± 0.8 | 4.7 ± 0.7 | 0.59 * |
| Total cholesterol (mg/dL: 142–248) | 120.3 ± 40.0 | 106 ± 37.2 | 0.02 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Maruhashi, T.; Asari, Y. Effectiveness of Zinc Supplementation for Sepsis Treatment: A Single-Center Retrospective Observational Study. Nutrients 2024, 16, 2841. https://doi.org/10.3390/nu16172841
Kim M, Maruhashi T, Asari Y. Effectiveness of Zinc Supplementation for Sepsis Treatment: A Single-Center Retrospective Observational Study. Nutrients. 2024; 16(17):2841. https://doi.org/10.3390/nu16172841
Chicago/Turabian StyleKim, Muneyoshi, Takaaki Maruhashi, and Yasushi Asari. 2024. "Effectiveness of Zinc Supplementation for Sepsis Treatment: A Single-Center Retrospective Observational Study" Nutrients 16, no. 17: 2841. https://doi.org/10.3390/nu16172841





