Okinawa-Based Nordic Diet Decreases Plasma Glial Fibrillary Acidic Protein Levels in Type 2 Diabetes Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Included in the Study
2.2. Clinical Characteristics
2.3. Analysis of Variables
2.4. Statistical Analyses
3. Results
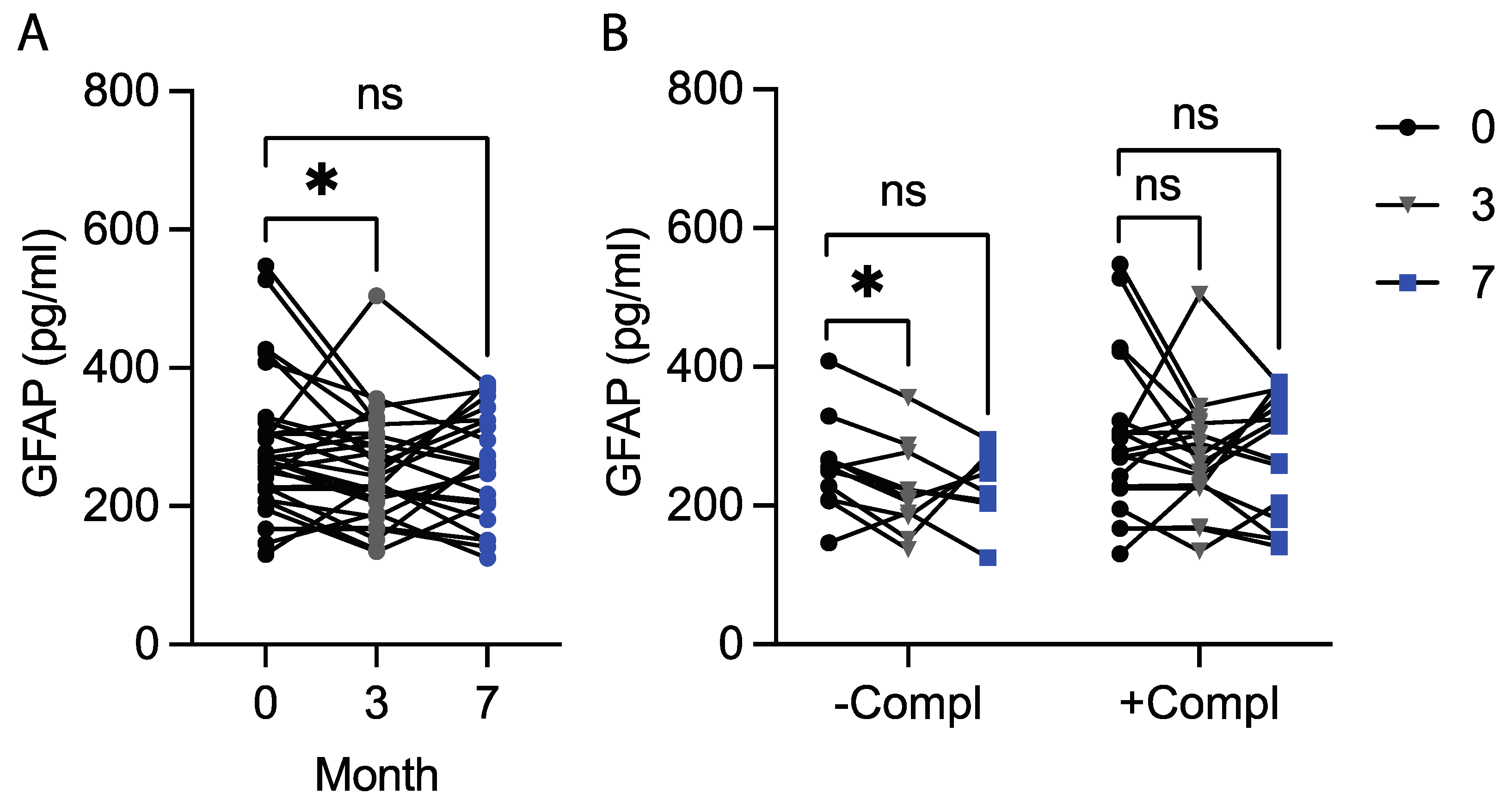
3.1. The Okinawa-Based Nordic Diet Decreases Plasma GFAP Levels
3.2. Plasma GFAP Levels at Baseline Correlate Positively with Plasma NfL Levels but Not with Psychological Well-Being
3.3. Plasma GFAP Levels Correlate Positively with Plasma Levels of Metabolic and Peripheral Inflammation Markers but Not with Gut Leakage Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, R.Z.; Lee, K.Y.; Qi, Z.X.; Wang, Z.; Xu, Z.Y.; Wu, X.H.; Mao, Y. Neuroinflammation Following Traumatic Brain Injury: Take It Seriously or Not. Front. Immunol. 2022, 13, 855701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Valles, S.L.; Singh, S.K.; Campos-Campos, J.; Colmena, C.; Campo-Palacio, I.; Alvarez-Gamez, K.; Caballero, O.; Jorda, A. Functions of Astrocytes under Normal Conditions and after a Brain Disease. Int. J. Mol. Sci. 2023, 24, 8434. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Doorn, K.J.; Goudriaan, A.; Blits-Huizinga, C.; Bol, J.G.; Rozemuller, A.J.; Hoogland, P.V.; Lucassen, P.J.; Drukarch, B.; van de Berg, W.D.; van Dam, A.M. Increased amoeboid microglial density in the olfactory bulb of Parkinson’s and Alzheimer’s patients. Brain Pathol. 2014, 24, 152–165. [Google Scholar] [CrossRef]
- Morrison, H.; Young, K.; Qureshi, M.; Rowe, R.K.; Lifshitz, J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci. Rep. 2017, 7, 13211. [Google Scholar] [CrossRef]
- Adrian, M.; Weber, M.; Tsai, M.C.; Glock, C.; Kahn, O.I.; Phu, L.; Cheung, T.K.; Meilandt, W.J.; Rose, C.M.; Hoogenraad, C.C. Polarized microtubule remodeling transforms the morphology of reactive microglia and drives cytokine release. Nat. Commun. 2023, 14, 6322. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Nichols, N.R.; Day, J.R.; Laping, N.J.; Johnson, S.A.; Finch, C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol. Aging 1993, 14, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.E.; Xie, Z.; Goldsmith, S.; Yoshida, T.; Lanzrein, A.S.; Stone, D.; Rozovsky, I.; Perry, G.; Smith, M.A.; Finch, C.E. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 1999, 89, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Jonesco, D.S.; Hassager, C.; Frydland, M.; Kjaergaard, J.; Karsdal, M.; Henriksen, K. A caspase-6-cleaved fragment of Glial Fibrillary Acidic Protein as a potential serological biomarker of CNS injury after cardiac arrest. PLoS ONE 2019, 14, e0224633. [Google Scholar] [CrossRef]
- Lee, Y.B.; Du, S.; Rhim, H.; Lee, E.B.; Markelonis, G.J.; Oh, T.H. Rapid increase in immunoreactivity to GFAP in astrocytes in vitro induced by acidic pH is mediated by calcium influx and calpain I. Brain Res. 2000, 864, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Plog, B.A.; Dashnaw, M.L.; Hitomi, E.; Peng, W.; Liao, Y.; Lou, N.; Deane, R.; Nedergaard, M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 2015, 35, 518–526. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Papa, L.; Lewis, L.M.; Falk, J.L.; Zhang, Z.; Silvestri, S.; Giordano, P.; Brophy, G.M.; Demery, J.A.; Dixit, N.K.; Ferguson, I.; et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 2012, 59, 471–483. [Google Scholar] [CrossRef]
- Ayala-Guerrero, L.; García-delaTorre, P.; Sánchez-García, S.; Guzmán-Ramos, K. Serum Levels of Glial Fibrillary Acidic Protein Association with Cognitive Impairment and Type 2 Diabetes. Arch. Med. Res. 2022, 53, 501–507. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef]
- Oeckl, P.; Halbgebauer, S.; Anderl-Straub, S.; Steinacker, P.; Huss, A.M.; Neugebauer, H.; von Arnim, C.A.F.; Diehl-Schmid, J.; Grimmer, T.; Kornhuber, J.; et al. Glial Fibrillary Acidic Protein in Serum is Increased in Alzheimer’s Disease and Correlates with Cognitive Impairment. J. Alzheimers Dis. 2019, 67, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Kushiyama, A.; Sun, J.; Kikuchi, T.; Yamazaki, H.; Iwamoto, Y.; Koriyama, H.; Yoshida, S.; Shimamura, M.; Higuchi, M.; et al. Glial fibrillary acidic protein (GFAP) is a novel biomarker for the prediction of autoimmune diabetes. FASEB J. 2017, 31, 4053–4063. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Ryan, J.P.; Fine, D.F.; Rosano, C. Type 2 diabetes and cognitive impairment: Contributions from neuroimaging. J. Geriatr. Psychiatry Neurol. 2014, 27, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Darwish, L.; Beroncal, E.; Sison, M.V.; Swardfager, W. Depression in people with type 2 diabetes: Current perspectives. Diabetes Metab. Syndr. Obes. 2018, 11, 333–343. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Jiang, Y.Y.; Long, C.Y.; Peng, Q.; Yue, R.S. The gut microbiota-astrocyte axis: Implications for type 2 diabetic cognitive dysfunction. CNS Neurosci. Ther. 2023, 29 (Suppl. 1), 59–73. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Karampatsi, D.; Zabala, A.; Wilhelmsson, U.; Dekens, D.; Vercalsteren, E.; Larsson, M.; Nyström, T.; Pekny, M.; Patrone, C.; Darsalia, V. Diet-induced weight loss in obese/diabetic mice normalizes glucose metabolism and promotes functional recovery after stroke. Cardiovasc. Diabetol. 2021, 20, 240. [Google Scholar] [CrossRef]
- Ohlsson, B. An Okinawan-based Nordic diet improves glucose and lipid metabolism in health and type 2 diabetes, in alignment with changes in the endocrine profile, whereas zonulin levels are elevated. Exp. Ther. Med. 2019, 17, 2883–2893. [Google Scholar] [CrossRef]
- Garcia, K.; Ferreira, G.; Reis, F.; Viana, S. Impact of Dietary Sugars on Gut Microbiota and Metabolic Health. Diabetology 2022, 3, 549–560. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Q.; Liao, X.; Elbelt, U.; Weylandt, K.H. The effects of omega-3 fatty acids in type 2 diabetes: A systematic review and meta-analysis. Prostaglandins Leukot. Essent. Fat. Acids 2022, 182, 102456. [Google Scholar] [CrossRef]
- Pocevičiūtė, D.; Roth, B.; Ohlsson, B.; Wennström, M. Okinawa-Based Nordic Diet Decreases Plasma Levels of IAPP and IgA against IAPP Oligomers in Type 2 Diabetes Patients. Int. J. Mol. Sci. 2024, 25, 7665. [Google Scholar] [CrossRef]
- Steinacker, P.; Al Shweiki, M.R.; Oeckl, P.; Graf, H.; Ludolph, A.C.; Schonfeldt-Lecuona, C.; Otto, M. Glial fibrillary acidic protein as blood biomarker for differential diagnosis and severity of major depressive disorder. J. Psychiatr. Res. 2021, 144, 54–58. [Google Scholar] [CrossRef]
- Michel, M.; Fiebich, B.L.; Kuzior, H.; Meixensberger, S.; Berger, B.; Maier, S.; Nickel, K.; Runge, K.; Denzel, D.; Pankratz, B.; et al. Increased GFAP concentrations in the cerebrospinal fluid of patients with unipolar depression. Transl. Psychiatry 2021, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, G.; Höglund, P.; Roth, B.; Larsson, E.; Sjöberg, T.; Wohlfart, B.; Steen, S.; Ohlsson, B. An Okinawan-based Nordic diet improves anthropometry, metabolic control, and health-related quality of life in Scandinavian patients with type 2 diabetes: A pilot trial. Food Nutr. Res. 2016, 60, 32594. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Nilholm, C.; Roth, B.; Linninge, C.; Höglund, P.; Nyman, M.; Ohlsson, B. Anthropometric and metabolic improvements in human type 2 diabetes after introduction of an Okinawan-based Nordic diet are not associated with changes in microbial diversity or SCFA concentrations. Int. J. Food Sci. Nutr. 2018, 69, 729–740. [Google Scholar] [CrossRef]
- Nilholm, C.; Roth, B.; Höglund, P.; Blennow, K.; Englund, E.; Hansson, O.; Zetterberg, H.; Ohlsson, B. Dietary intervention with an Okinawan-based Nordic diet in type 2 diabetes renders decreased interleukin-18 concentrations and increased neurofilament light concentrations in plasma. Nutr. Res. 2018, 60, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B.; Darwiche, G.; Roth, B.; Bengtsson, M.; Höglund, P. High Fiber Fat and Protein Contents Lead to Increased Satiety Reduced Sweet Cravings and Decreased Gastrointestinal Symptoms Independently of Anthropometric Hormonal and Metabolic Factors. J. Diabetes Metab. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Ohlsson, B.; Darwiche, G.; Roth, B.; Höglund, P. Alignments of endocrine, anthropometric, and metabolic parameters in type 2 diabetes after intervention with an Okinawa-based Nordic diet. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Ohlsson, B.; Roth, B.; Larsson, E.; Höglund, P. Calprotectin in serum and zonulin in serum and feces are elevated after introduction of a diet with lower carbohydrate content and higher fiber, fat and protein contents. Biomed. Rep. 2017, 6, 411–422. [Google Scholar] [CrossRef]
- Huebschmann, N.A.; Luoto, T.M.; Karr, J.E.; Berghem, K.; Blennow, K.; Zetterberg, H.; Ashton, N.J.; Simrén, J.; Posti, J.P.; Gill, J.M.; et al. Comparing Glial Fibrillary Acidic Protein (GFAP) in Serum and Plasma Following Mild Traumatic Brain Injury in Older Adults. Front. Neurol. 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Vågberg, M.; Norgren, N.; Dring, A.; Lindqvist, T.; Birgander, R.; Zetterberg, H.; Svenningsson, A. Levels and Age Dependency of Neurofilament Light and Glial Fibrillary Acidic Protein in Healthy Individuals and Their Relation to the Brain Parenchymal Fraction. PLoS ONE 2015, 10, e0135886. [Google Scholar] [CrossRef] [PubMed]
- Heimfarth, L.; Passos, F.R.S.; Monteiro, B.S.; Araujo, A.A.S.; Quintans Junior, L.J.; Quintans, J.S.S. Serum glial fibrillary acidic protein is a body fluid biomarker: A valuable prognostic for neurological disease—A systematic review. Int. Immunopharmacol. 2022, 107, 108624. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Guo, Z.; Yin, Z.; Qiu, Y.; Zhou, B. Gut Microbiota Was Involved in the Process of Liver Injury During Intra-Abdominal Hypertension. Front. Physiol. 2021, 12, 790182. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, L.; Roth, B.; Bang, C.; Stenlund, H.; Ohlsson, B. An Okinawan-Based Nordic Diet Leads to Profound Effects on Gut Microbiota and Plasma Metabolites Linked to Glucose and Lipid Metabolism. Nutrients 2023, 15, 3273. [Google Scholar] [CrossRef]
- Persson, G.R.; Widen, C.; Wohlfart, B.; Sjoberg, K.; Steen, S.; Coleman, M.D.; Holmer, H. Impact of an Okinawa/Nordic based diet on endocrinological and periodontal conditions in individuals with type 2 diabetes. A randomized case-control study. BMC Oral. Health 2023, 23, 544. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Perez-Dominguez, M.; Avila-Munoz, E.; Dominguez-Rivas, E.; Zepeda, A. The detrimental effects of lipopolysaccharide-induced neuroinflammation on adult hippocampal neurogenesis depend on the duration of the pro-inflammatory response. Neural Regen. Res. 2019, 14, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Wennström, M.; Janelidze, S.; Bay-Richter, C.; Minthon, L.; Brundin, L. Pro-inflammatory cytokines reduce the proliferation of NG2 cells and increase shedding of NG2 in vivo and in vitro. PLoS ONE 2014, 9, e109387. [Google Scholar] [CrossRef]
- Kang, J.B.; Park, D.J.; Shah, M.A.; Kim, M.O.; Koh, P.O. Lipopolysaccharide induces neuroglia activation and NF-kappaB activation in cerebral cortex of adult mice. Lab. Anim. Res. 2019, 35, 19. [Google Scholar] [CrossRef]
- von Boyen, G.B.; Schulte, N.; Pfluger, C.; Spaniol, U.; Hartmann, C.; Steinkamp, M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 2011, 11, 3. [Google Scholar] [CrossRef]
- Bradley, J.S., Jr.; Parr, E.J.; Sharkey, K.A. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 1997, 289, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.M.; He, S.; Quintana, E.; Kim, Y.G.; Nunez, G.; Morrison, S.J. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Investig. 2011, 121, 3398–3411. [Google Scholar] [CrossRef]
- Boesmans, W.; Lasrado, R.; Vanden Berghe, P.; Pachnis, V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 2015, 63, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Simo-Servat, O.; Porta, M.; Grauslund, J.; Harding, S.P.; Frydkjaer-Olsen, U.; Garcia-Arumi, J.; Ribeiro, L.; Scanlon, P.; Cunha-Vaz, J.; et al. Serum glial fibrillary acidic protein and neurofilament light chain as biomarkers of retinal neurodysfunction in early diabetic retinopathy: Results of the EUROCONDOR study. Acta Diabetol. 2023, 60, 837–844. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ohlsson, B. The brief Visual Analogue Scale for Irritable Bowel Syndrome questionnaire can be used to evaluate psychological well-being in patients with irritable bowel syndrome. Eur. J. Intern. Med. 2013, 24, e82–e83. [Google Scholar] [CrossRef]
| Plasma GFAP Levels, pg/mL | |||
|---|---|---|---|
| Baseline | After 3 Months of Diet | After Unrestricted Eating | |
| Sex | |||
| Male | 249.6 (200.8, 297.1) | 229.2 (188.5, 266.5) | 206.7 (180.4, 324.2) |
| Female | 277.5 (226.3, 317.7) | 287.5 (197.7, 325.0) | 268.8 (227.8, 365.5) |
| p-value | p = 0.300 | p = 0.133 | p = 0.211 |
| APOE4 status | |||
| Carrier | 255.4 (242.1, 322.5) | 272.9 (210.0, 328.3) | 217.9 (180.4, 295.4) |
| Non-carrier | 269.6 (206.7, 306.3) | 243.8 (184.2, 302.5) | 268.8 (205.1, 350.8) |
| p-value | p = 0.703 | p = 0.471 | p = 0.249 |
| Compl. status | |||
| At least one | 277.5 (225.0, 322.5) | 272.9 (229.2, 321.7) | 315.0 (180.4, 359.6) |
| None | 252.9 (208.3, 267.1) | 210.0 (184.2, 277.1) | 232.5 (204.2, 271.2) |
| p-value | p = 0.287 | p = 0.047 | p = 0.190 |
| Baseline | a Baseline | b Baseline | |
|---|---|---|---|
| Age | 0.482 ** | - | - |
| IAPP | ns | 0.500 ** | 0.478 * |
| C-peptide | 0.375 * | ns | ns |
| HDL | ns | ns | −0.397 * |
| Triglycerides | 0.418 * | ns | ns |
| IL1α | ns | 0.453 * | 0.440 * |
| IL4 | ns | 0.465 * | 0.461 * |
| IL12p70 | ns | 0.500 ** | 0.490 ** |
| IFNγ | ns | 0.546 ** | 0.530 ** |
| TNFα | ns | 0.459 * | 0.446 * |
| NfL | 0.400 * | 0.463 * | 0.456 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pocevičiūtė, D.; Wennström, M.; Ohlsson, B. Okinawa-Based Nordic Diet Decreases Plasma Glial Fibrillary Acidic Protein Levels in Type 2 Diabetes Patients. Nutrients 2024, 16, 2847. https://doi.org/10.3390/nu16172847
Pocevičiūtė D, Wennström M, Ohlsson B. Okinawa-Based Nordic Diet Decreases Plasma Glial Fibrillary Acidic Protein Levels in Type 2 Diabetes Patients. Nutrients. 2024; 16(17):2847. https://doi.org/10.3390/nu16172847
Chicago/Turabian StylePocevičiūtė, Dovilė, Malin Wennström, and Bodil Ohlsson. 2024. "Okinawa-Based Nordic Diet Decreases Plasma Glial Fibrillary Acidic Protein Levels in Type 2 Diabetes Patients" Nutrients 16, no. 17: 2847. https://doi.org/10.3390/nu16172847





