The Impact of Pinus koraiensis Leaf Extract Consumption on Postprandial ApoB100 and Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Participants Subjected to an Oral High-Fat Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Study Design
2.3. Participants
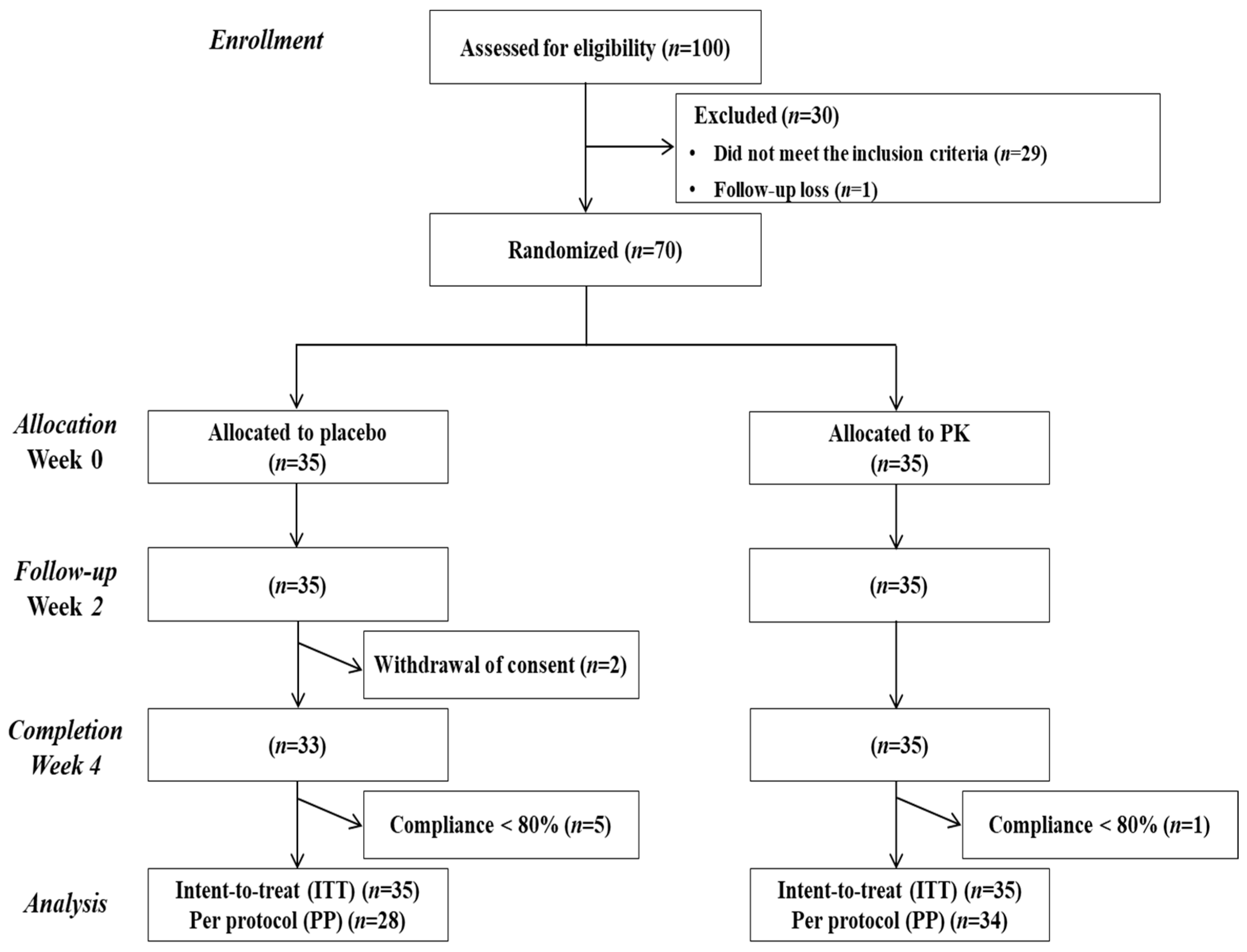
2.4. Protocol
2.5. Assessment of Postprandial Blood Lipid Levels
2.6. Statistical Analysis
3. Results
3.1. Subject Characteristics
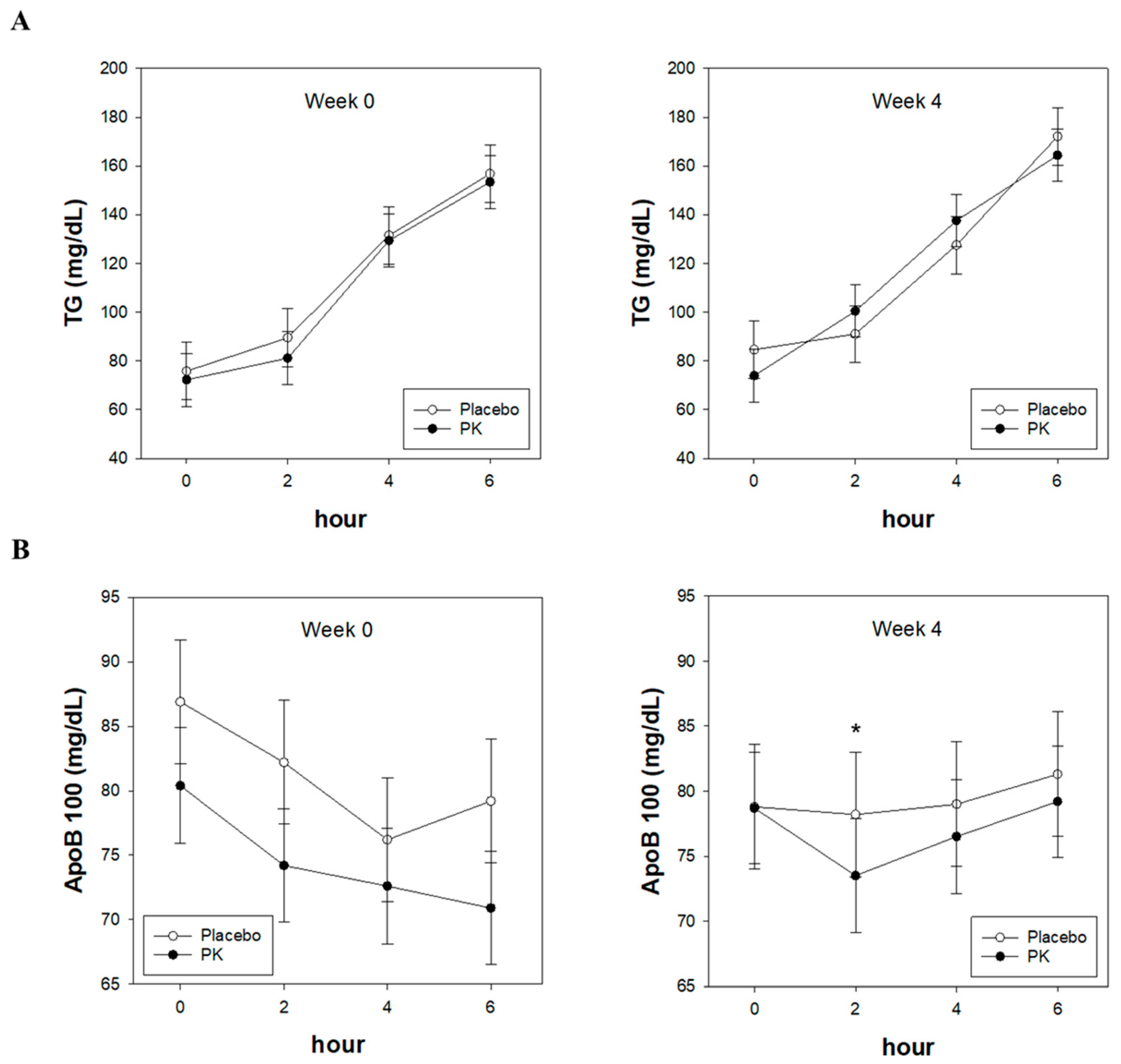
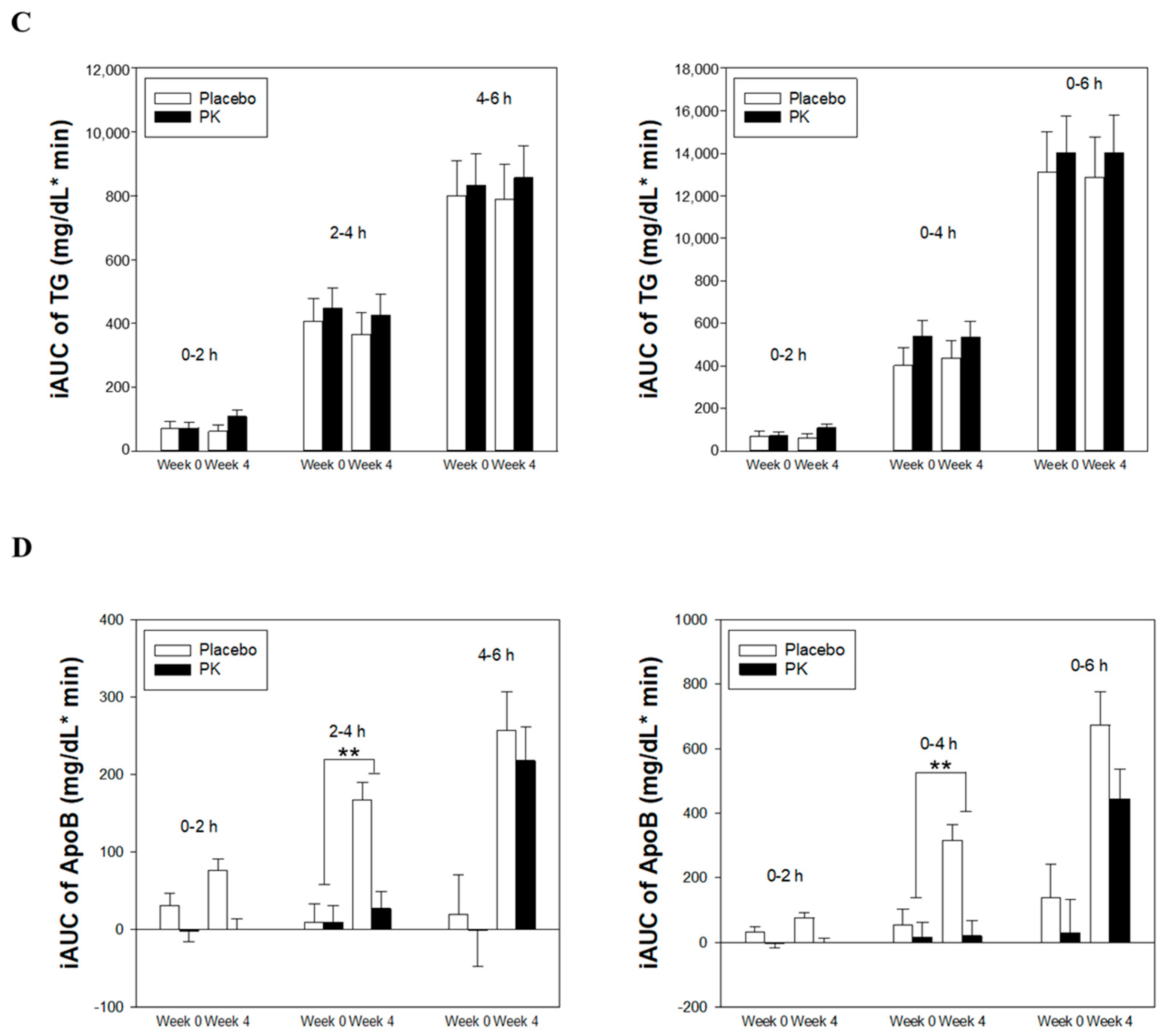
3.2. Effect of PK on Postprandial TG and ApoB100 Levels
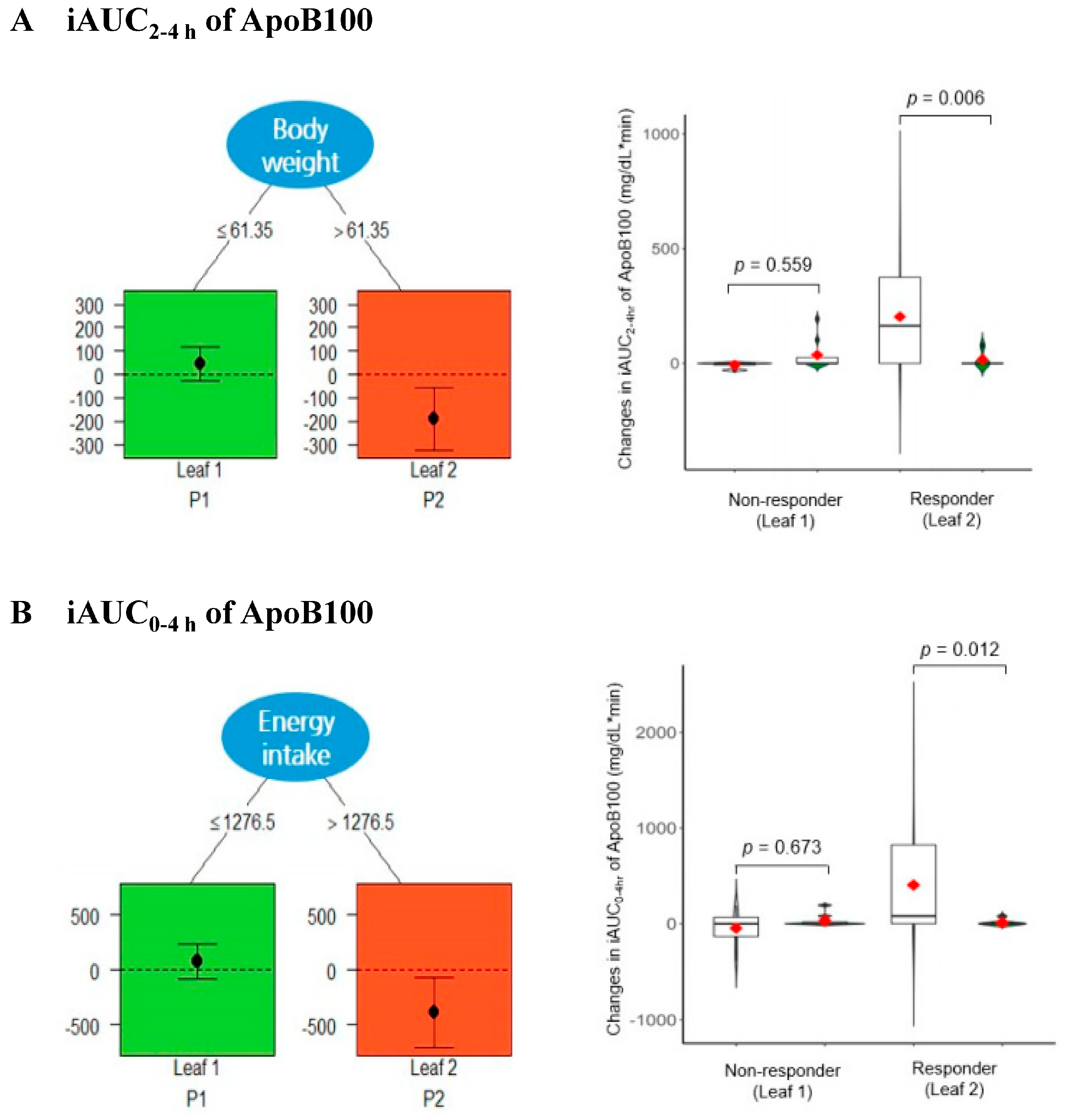
3.3. Qualitative Interaction Tree (QUINT) Analysis of ApoB100 Levels between Responders and Non-Responders
3.4. Correlation Analysis of Postprandial TG and ApoB100 Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Du, A.; Liu, H.; Wang, Z.; Hu, J. Systematic analysis of the global, regional and national burden of cardiovascular diseases from 1990 to 2017. J. Epidemiol. Glob. Health 2022, 12, 92–103. [Google Scholar] [CrossRef]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and cardiovascular disease: Biomarker and potential therapeutic target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action; American College of Cardiology Foundation: Washington DC, USA, 2020; Volume 76, pp. 2980–2981. [Google Scholar]
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Arnao, V.; Pinto, A.; Licata, G. Atherosclerosis as an inflammatory disease. Curr. Pharm. Des. 2012, 18, 4266–4288. [Google Scholar] [CrossRef] [PubMed]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927. [Google Scholar]
- Lee, C.-K.; Liao, C.-W.; Meng, S.-W.; Wu, W.-K.; Chiang, J.-Y.; Wu, M.-S. Lipids and lipoproteins in health and disease: Focus on targeting atherosclerosis. Biomedicines 2021, 9, 985. [Google Scholar] [CrossRef]
- Mehta, A.; Shapiro, M.D. Apolipoproteins in vascular biology and atherosclerotic disease. Nat. Rev. Cardiol. 2022, 19, 168–179. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, Y.; Shin, S.; Han, S.N. Impact of Korean pine nut oil on weight gain and immune responses in high-fat diet-induced obese mice. Nutr. Res. Pract. 2013, 7, 352. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.; Zhu, Y.; Wang, Z. Pinus koraiensis polyphenols: Structural identification, in vitro antioxidant activity, immune function and inhibition of cancer cell proliferation. Food Funct. 2021, 12, 4176–4198. [Google Scholar] [CrossRef]
- Cannac, M.; Pasqualini, V.; Greff, S.; Fernandez, C.; Ferrat, L. Characterization of phenolic compounds in Pinus laricio needles and their responses to prescribed burnings. Molecules 2007, 12, 1614–1622. [Google Scholar] [CrossRef]
- Maimoona, A.; Naeem, I.; Saddiqe, Z.; Ali, N.; Ahmed, G.; Shah, I. Analysis of total flavonoids and phenolics in different fractions of bark and needle extracts of Pinus roxburghii and Pinus wallichiana. J. Med. Plants Res. 2011, 5, 2724–2728. [Google Scholar]
- Stevanovic, T.; Diouf, P.N.; Garcia-Perez, M.E. Bioactive polyphenols from healthy diets and forest biomass. Curr. Nutr. Food Sci. 2009, 5, 264–295. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.; Shin, S.; Cho, J.; Yoo, J.; Kim, I. Effects of dietary pine cone meal on growth performance, serum cholesterol, carcass quality and fatty acid composition and cholesterol content of meat in broiler chickens. J. Anim. Sci. Technol. 2008, 50, 57–68. [Google Scholar]
- Neumann, A. Uber ultramikroskopische Blutuntersuchungen zur Zeit der Fettresorption bei Gesunden und Kranken. Wien. Clin. Wschr. 1907, 20, 851. [Google Scholar]
- Bae, J.-H.; Bassenge, E.; Kim, K.-B.; Kim, Y.-N.; Kim, K.-S.; Lee, H.-J.; Moon, K.-C.; Lee, M.-S.; Park, K.-Y.; Schwemmer, M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 2001, 155, 517–523. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W.; Sevanian, A. Nutritional, dietary and postprandial oxidative stress. J. Nutr. 2005, 135, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernandez-Real, J.M.; Dandona, P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef]
- Esser, D.; Oosterink, E.; Op’t Roodt, J.; Henry, R.M.; Stehouwer, C.D.; Müller, M.; Afman, L.A. Vascular and inflammatory high fat meal responses in young healthy men; a discriminative role of IL-8 observed in a randomized trial. PLoS ONE 2013, 8, e53474. [Google Scholar] [CrossRef]
- Zwaka, T.P.; Hombach, V.; Torzewski, J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: Implications for atherosclerosis. Circulation 2001, 103, 1194–1197. [Google Scholar] [CrossRef]
- Fichtlscherer, S.; Breuer, S.; Heeschen, C.; Dimmeler, S.; Zeiher, A.M. Interleukin-10 serum levels and systemic endothelial vasoreactivity in patients with coronary artery disease. J. Am. Coll. Cardiol. 2004, 44, 44–49. [Google Scholar] [CrossRef]
- Stalenhoef, A.F.; de Graaf, J. Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr. Opin. Lipidol. 2008, 19, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Sinzinger, H.; Berent, R. Platelet function in the postprandial period. Thromb. J. 2012, 10, 19. [Google Scholar] [CrossRef]
- Zilversmit, D.B. Atherogenesis: A postprandial phenomenon. Circulation 1979, 60, 473–485. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.G.; Poulaki, A.; Evangelopoulos, A.; Panagopoulos, F.; Stratigou, T.; Geladari, E.; Karampela, I.; Dalamaga, M. ApoB100 and Atherosclerosis: What’s New in the 21st Century? Metabolites 2024, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, M.C.; Erkelens, D.; Kock, L.; Bruin, T.D. Postprandial apolipoprotein B100 and B48 metabolism in familial combined hyperlipidaemia before and after reduction of fasting plasma triglycerides. Eur. J. Clin. Investig. 1994, 24, 669–678. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I.; Holme, I.; Aastveit, A.H.; Kolar, W.; Steiner, E. High apolipoprotein B, low apolipoprotein AI, and improvement in the prediction of fatal myocardial infarction (AMORIS study): A prospective study. Lancet 2001, 358, 2026–2033. [Google Scholar] [CrossRef]
- No, D.S.; Kim, I.H. Pinolenic acid as a new source of phyto-polyunsaturated fatty acid. Lipid Technol. 2013, 25, 135–138. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Choue, R.; Lim, H. Evaluation of the effects of Pinus koraiensis needle extracts on serum lipid and oxidative stress in adults with borderline dyslipidemia: A randomized, double-blind, and placebo-controlled clinical trial. Evid. Based Complement. Alternat. Med. 2016, 2016, 9594251. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Howard, L.R. Phenolic composition and antioxidant activities of different solvent extracts from pine needles in Pinus species. Prev. Nutr. Food Sci. 2010, 15, 36–43. [Google Scholar] [CrossRef][Green Version]
- Won, S.B.; Jung, G.-y.; Kim, J.; Chung, Y.S.; Hong, E.K.; Kwon, Y.H. Protective effect of Pinus koraiensis needle water extract against oxidative stress in HepG2 cells and obese mice. J. Med. Food 2013, 16, 569–576. [Google Scholar] [CrossRef]
- da Silva Rivas, A.C.; Lopes, P.M.; de Azevedo Barros, M.M.; Costa Machado, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Lee, H.; Woo, M.; Kim, M.; Noh, J.S.; Song, Y.O. Antioxidative and cholesterol-lowering effects of lemon essential oil in hypercholesterolemia-induced rabbits. Prev. Nutr. Food Sci. 2018, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.S.; de Sousa Machado, S.T.; Rodrigues, F.B.; da Silva, Y.A.; Matias, L.C.X.; Lopes, M.J.P.; Gomes, A.D.S.; Ribeiro, T.F.; de Oliveira Garcia, F.A.; Coutinho, H.D.M. Potential anti-inflammatory, hypoglycemic, and hypolipidemic activities of alpha-pinene in diabetic rats. Process Biochem. 2023, 126, 80–86. [Google Scholar] [CrossRef]
- Padilla, J.; Leary, E.; Limberg, J.K. Identifying responders versus non-responders: Incorporation of controls is required for sound statistical inference. Exp. Physiol. 2021, 106, 375–376. [Google Scholar] [CrossRef]
- World Health Organization. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Rifkind, B.; Begg, T. Relationship between relative body weight and serum lipid levels. Br. Med. J. 1966, 2, 208. [Google Scholar] [CrossRef][Green Version]

| Variables | Placebo | PK | p Value 2 | ||||
|---|---|---|---|---|---|---|---|
| Age (yr) | 40.8 | ± | 2.1 | 45.9 | ± | 1.7 | 0.064 |
| Sex (male/female) | 13 | / | 15 | 13 | / | 21 | 0.515 |
| Menstrual status (Y/N/NA) | 12/ | 3 | /13 | 13/ | 8 | /13 | 0.417 |
| Alcohol consumption status (Y/N) | 12 | / | 16 | 19 | / | 15 | 0.307 |
| Alcohol consumption volume (SD/wk) | 4.4 | ± | 1.4 | 5.6 | ± | 1.5 | 0.545 |
| Smoking status (Y/N) | 6 | / | 22 | 3 | / | 31 | 0.277 |
| Smoking amount (cigarette/d) | 1.9 | ± | 0.7 | 0.6 | ± | 0.4 | 0.142 |
| Body weight (kg) | 70.4 | ± | 2.3 | 68.4 | ± | 1.9 | 0.488 |
| BMI (kg/m2) | 25.2 | ± | 0.4 | 25.2 | ± | 0.4 | 0.991 |
| Waist circumference (cm) | 87.1 | ± | 1.9 | 86.2 | ± | 1.4 | 0.722 |
| Blood lipid profile (mg/dL) | |||||||
| TG | 75.6 | ± | 9.0 | 78.9 | ± | 8.5 | 0.794 |
| TC | 208.5 | ± | 7.4 | 211.2 | ± | 6.7 | 0.789 |
| ApoB | 89.0 | ± | 3.4 | 91.2 | ± | 3.4 | 0.662 |
| ApoB100 | 86.3 | ± | 5.2 | 84.3 | ± | 5.5 | 0.798 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-y.; Park, T.g.; Choi, K.; Kim, K.J.; Kim, J.Y. The Impact of Pinus koraiensis Leaf Extract Consumption on Postprandial ApoB100 and Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Participants Subjected to an Oral High-Fat Challenge. Nutrients 2024, 16, 2864. https://doi.org/10.3390/nu16172864
Park S-y, Park Tg, Choi K, Kim KJ, Kim JY. The Impact of Pinus koraiensis Leaf Extract Consumption on Postprandial ApoB100 and Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Participants Subjected to an Oral High-Fat Challenge. Nutrients. 2024; 16(17):2864. https://doi.org/10.3390/nu16172864
Chicago/Turabian StylePark, Soo-yeon, Tae gwon Park, Kwanyong Choi, Kyeong Jin Kim, and Ji Yeon Kim. 2024. "The Impact of Pinus koraiensis Leaf Extract Consumption on Postprandial ApoB100 and Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Participants Subjected to an Oral High-Fat Challenge" Nutrients 16, no. 17: 2864. https://doi.org/10.3390/nu16172864
APA StylePark, S.-y., Park, T. g., Choi, K., Kim, K. J., & Kim, J. Y. (2024). The Impact of Pinus koraiensis Leaf Extract Consumption on Postprandial ApoB100 and Lipid Metabolism: A Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Participants Subjected to an Oral High-Fat Challenge. Nutrients, 16(17), 2864. https://doi.org/10.3390/nu16172864






