Randomized Clinical Trial to Evaluate the Effect of Probiotic Intake on Androgenic Alopecia
Abstract
1. Introduction
2. Materials and Methods
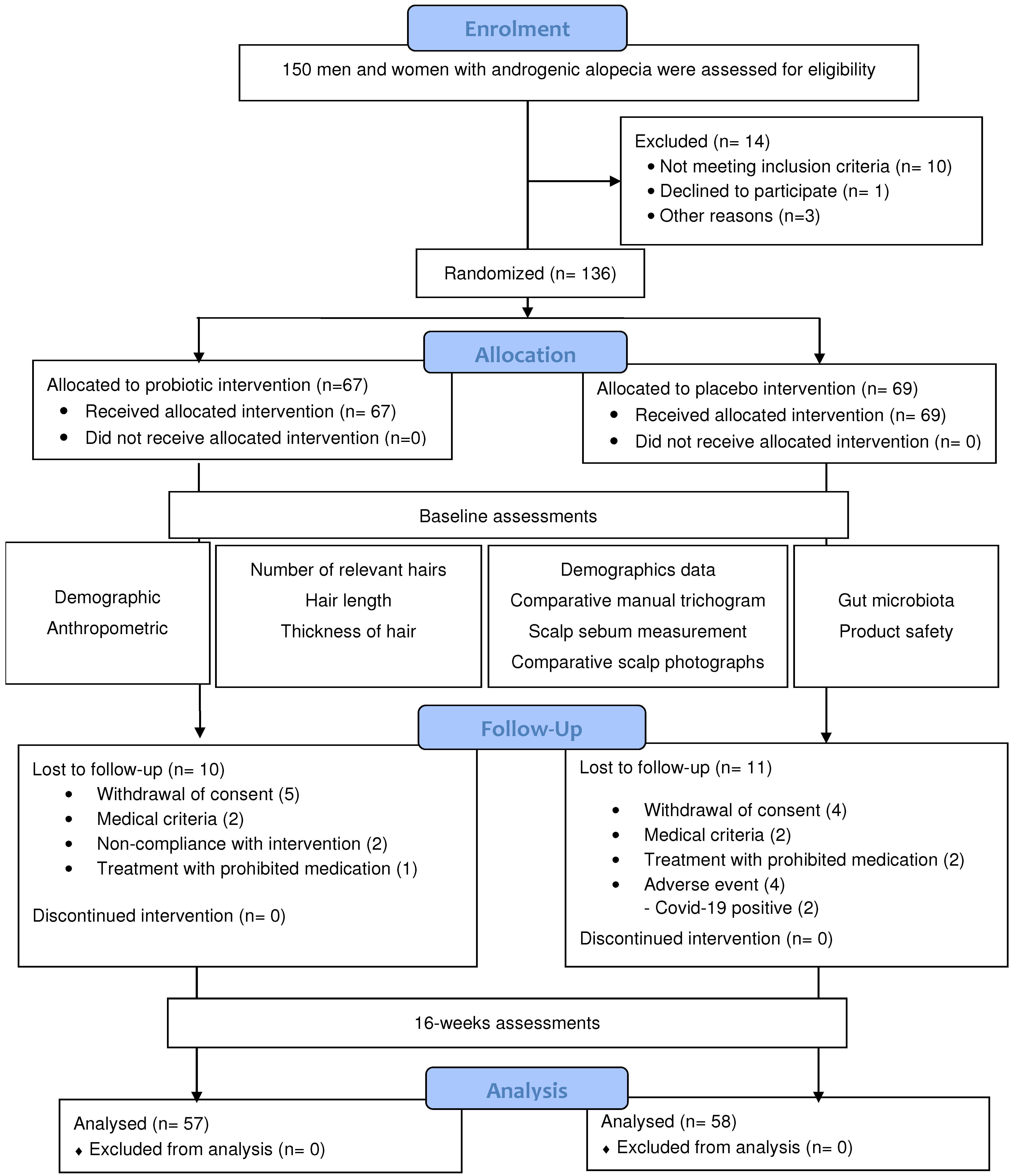
2.1. Study Design
2.2. Study Population
2.3. Randomization, Blinding, Intervention, and Compliance
2.4. Outcomes Measurements
2.4.1. Measurement of the Increase in the Number of Hairs per Square Centimeter
2.4.2. Demographic Data and Anthropometry
2.4.3. Comparative Manual Trichograms
2.4.4. Measurement of Scalp Sebum
2.4.5. Comparative Scalp Photographs
2.4.6. Gut Microbial Analysis
2.4.7. Safety of the Investigational Products
2.5. Data-Quality Assurance
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. Capillary Analysis
3.2. Taxonomic Analysis
3.2.1. Alpha Diversity
3.2.2. Beta Diversity
3.2.3. Microbial Communities
3.3. Adverse Events (AEs)
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Martinez-Jacobo, L.; Villarreal-Villarreal, C.D.; Ortiz-López, R.; Ocampo-Candiani, J.; Rojas-Martínez, A. Genetic and molecular aspects of androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, J.; Grimalt, R.; Hausmann, G.; Lacueva, L.; Moreno, G. Alopecias Guía de Diagnóstico y Tratamiento; 2000 Pulso Ediciones: Sant Cugat del Vallès, Spain, 2007; ISBN 84-86671-58-2. [Google Scholar]
- Vaño, S.; Jaén, P. Manual Práctico de Tricología. #TricoHRC, 1st ed.; Editorial Médica Panamericana: Madrid, Spain, 2019; ISBN 978-84-09-10115-3. [Google Scholar]
- Kibar, M.; Aktan, Ş.; Bilgin, M. Scalp dermatoscopic findings in androgenetic alopecia and their relations with disease severity. Ann. Dermatol. 2014, 26, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef]
- Kosman, M.E. Evaluation of a new antihypertensive agent. Minoxidil. JAMA 1980, 244, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Marubayashi, A.; Nakaya, Y.; Fukui, K.; Li, M.; Arase, S. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: Possible involvement of sulfonylurea receptor 2B as a target of minoxidil. J. Investig. Dermatol. 2001, 117, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Van Zuuren, E.J.; Fedorowicz, Z. Interventions for female pattern hair loss. JAMA Dermatol. 2017, 153, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Mysore, V.; Shashikumar, B.M. Guidelines on the use of finasteride in androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 128–134. [Google Scholar] [CrossRef]
- Jung, D.-R.; Yoo, H.Y.; Kim, M.-J.; Singh, V.; Park, S.H.; Jeong, M.; Park, B.J.; Shin, J.-H. Comparative analysis of scalp and gut microbiome in androgenetic alopecia: A Korean cross-sectional study. Front. Microbiol. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Fu, H.; Xu, T.; Zhao, W.; Jiang, L.; Shan, S. Roles of gut microbiota in androgenetic alopecia: Insights from Mendelian randomization analysis. Front. Microbiol. 2024, 15, 1360445. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- Park, D.W.; Lee, H.S.; Shim, M.S.; Yum, K.J.; Seo, J.T. Do Kimchi and Cheonggukjang Probiotics as a Functional Food Improve Androgenetic Alopecia? A Clinical Pilot Study. World J. Men’s. Health 2020, 38, 95–102. [Google Scholar] [CrossRef]
- Borde, A.; Åstrand, A. Alopecia areata and the gut-the link opens up for novel therapeutic interventions. Expert Opin. Ther. Targets 2018, 22, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuné, J. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br. J. Nutr. 2013, 109, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Borrero, J.; Kelly, E.; O’Connor, P.M.; Kelleher, P.; Scully, C.; Cotter, P.D.; Mahony, J.; van Sinderen, D. Plantaricyclin A, a novel circular bacteriocin produced by Lactobacillus plantarum N1326: Purification, characterization, and heterologous production. Appl. Environ. Microbiol. 2018, 84, e01801-17. [Google Scholar] [CrossRef]
- Mori-Ichioka, A.; Sunada, Y.; Koikeda, T.; Matsuda, H.; Matsuo, S. Effect of applying Lactiplantibacillus plantarum subsp. plantarum N793 to the scalps of men and women with thinning hair: A randomized, double-blind, placebo-controlled, parallel-group study. Biosci. Microbiota Food Health 2024, 43, 192–203. [Google Scholar] [CrossRef]
- Drake, L.; Reyes-Hadsall, S.; Martinez, J.; Heinrich, C.; Huang, K.; Mostaghimi, A. Evaluation of the Safety and Effectiveness of Nutritional Supplements for Treating Hair Loss: A Systematic Review. JAMA Dermatol. 2023, 159, 79–86. [Google Scholar] [CrossRef]
- Olsen, E.A.; Whiting, D.A.; Savin, R.; Rodgers, A.; Johnson-Levonas, A.O.; Round, E.; Rotonda, J.; Kaufman, K.D. Global photographic assessment of men aged 18 to 60 years with male pattern hair loss receiving finasteride 1 mg or placebo. J. Am. Acad. Dermatol. 2012, 67, 379–386. [Google Scholar] [CrossRef]
- Satari, L.; Guillén, A.; Vidal-Verdú, À.; Porcar, M. The wasted chewing gum bacteriome. Sci. Rep. 2020, 10, 1. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Caporaso, J.G.; Douglas, G.M.; Dorrestein, P.C.; Diener, C.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857, Erratum in Nat. Biotechnol. 2019, 37, 1091.. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Andersen, K.S.; Kirkegaard, R.H.; Karst, S.M.; Albertsen, M. ampvis2: An R package to analyse and visualise 16S rRNA amplicon data. bioRxiv 2018. preprint. [Google Scholar] [CrossRef]
- Olsen, E.A.; Dunlap, F.E.; Funicella, T.; Koperski, J.A.; Swinehart, J.M.; Tschen, E.H.; Trancik, R.J. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2002, 47, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. vegan: Community Ecology Package (Version 2.6-4) [Computer Software]. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 19 August 2024).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.P.; Wariboko, M.A.; Hu, X.; Wang, Z.H.; Wu, Q.; Li, Y.M. Factors associated with early-onset androgenetic alopecia: A scoping review. PLoS ONE 2024, 19, e0299212. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, X.; Yao, J.; Cao, W.; Zou, Z.; Wang, L.; Qin, H.; Zhong, D.; Li, Y.; Xue, P.; et al. The role of short-chain fatty acids in inflammatory skin diseases. Front. Microbiol. 2023, 13, 1083432. [Google Scholar] [CrossRef]
- Devjani, S.; Ezemma, O.; Kelley, K.J.; Stratton, E.; Senna, M. Androgenetic Alopecia: Therapy Update. Drugs 2023, 83, 701–715. [Google Scholar] [CrossRef]
- Milani, M.; Colombo, F.; GFM-O-Trial Investigators Group. Efficacy and tolerability of an oral supplement containing amino acids, iron, selenium, and marine hydrolyzed collagen in subjects with hair loss (androgenetic alopecia, AGA or FAGA or telogen effluvium). A prospective, randomized, 3-month, controlled, assessor-blinded study. Skin Res. Technol. 2023, 29, e13381. [Google Scholar] [CrossRef]
- Pekmezci, E.; Dündar, C.; Türkoğlu, M. A proprietary herbal extract against hair loss in androgenetic alopecia and telogen effluvium: A placebo-controlled, single-blind, clinical-instrumental study. Acta Dermatovenerol. Alp. Pannonica Adriat. 2018, 27, 51–57. [Google Scholar] [CrossRef]
- Maftei, N.M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscá, A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Carrión-Gutiérrez, M.; Prieto-Merino, D.; Codoñer-Cortés, F.; Ramón-Vidal, D.; Genovés-Martínez, S.; et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta Derm. Venereol. 2019, 99, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Navarro-López, V.; Ramírez-Boscá, A.; Ramón-Vidal, D.; Ruzafa-Costas, B.; Genovés-Martínez, S.; Chenoll-Cuadros, E.; Carrión-Gutiérrez, M.; de la Parte, J.H.; Prieto-Merino, D.; Codoñer-Cortés, F.M. Effect of Oral Administration of a Mixture of Probiotic Strains on SCORAD Index and Use of Topical Steroids in Young Patients With Moderate Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Feíto-Rodríguez, M.; Ramírez-Boscà, A.; Vidal-Asensi, S.; Fernández-Nieto, D.; Ros-Cervera, G.; Alonso-Usero, V.; Prieto-Merino, D.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Sánchez-Pellicer, P.; et al. Randomized double-blind placebo-controlled clinical trial to evaluate the effect of a mixture of probiotic strains on symptom severity and use of corticosteroids in children and adolescents with atopic dermatitis. Clin. Exp. Dermatol. 2023, 48, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut-Skin Axis: A Review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
- Carrington, A.E.; Maloh, J.; Nong, Y.; Agbai, O.N.; Bodemer, A.A.; Sivamani, R.K. The Gut and Skin Microbiome in Alopecia: Associations and Interventions. J. Clin. Aesthet. Dermatol. 2023, 16, 59–64. [Google Scholar]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A systematic review of the safety of probiotics. Expert. Opin. Drug Saf. 2014, 13, 227–239. [Google Scholar] [CrossRef]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Van Neste, D.; Fuh, V.; Sanchez-Pedreno, P.; Lopez-Bran, E.; Wolff, H.; Whiting, D.; Roberts, J.; Kopera, D.; Stene, J.-J.; Calvieri, S.; et al. Finasteride increases anagen hair in men with androgenetic alopecia. Br. J. Dermatol. 2000, 143, 804–810. [Google Scholar] [CrossRef]
- Vinay, K.; Bhattachajee, R.; Bishnoi, A.; Kaushik, A.; Sachdeva, N.; Pal, A.; Narang, T.; Dogra, S. Clinical and metabolic characteristics of males with early-onset androgenetic alopecia. Indian. J. Dermatol. Venereol. Leprol. 2023, 89, 530–535. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Sinha, S.; Lin, G.; Ferenczi, K. The skin microbiome and the gut-skin axis. Clin. Dermatol. 2021, 39, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Swink, S.M.; Castelo-Soccio, L. A Review of the Use of Biotin for Hair Loss. Skin Appendage Disord. 2017, 3, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Comment on the Use of Biotin for Hair Loss. Skin Appendage Disord. 2018, 4, 345–346. [Google Scholar] [CrossRef] [PubMed]



| Variable | Probiotic | Placebo | p-Value | |
|---|---|---|---|---|
| Hair count | Baseline | 159 ± 56.1 | 167 ± 50.2 | |
| Week 16 | 163 ± 66.8 | 175 ± 61.7 | 0.3638 | |
| p-value | 0.5647 | 0.1423 | ||
| Hair density/cm2 | Baseline | 174 ± 58.3 | 184 ± 55.6 | |
| Week 16 | 178 ± 69.6 | 192 ± 69.3 | 0.5884 | |
| p-value | 0.9509 | 0.4033 | ||
| Number of telogen hairs | Baseline | 34.3 ± 38.1 | 34.5 ± 30.4 | |
| Week 16 | 32.1± 26.9 | 33.1 ± 27.85 | 0.3638 | |
| p-value | 0.0314 | 0.3638 | ||
| Telogen hair density/cm2 | Baseline | 35.5 ± 29.7 | 36.7 ± 30.8 | |
| Week 16 | 35.1 ± 39.2 | 38.1 ± 33.7 | 0.6693 | |
| p-value | 0.2170 | 0.5238 | ||
| Number of anagen hairs | Baseline | 83.8 ± 29.6 | 87.9 ± 30.5 | |
| Week 16 | 82.4 ± 30.6 | 90.8 ± 32 | 0.4177 | |
| p-value | 0.7842 | 0.3806 | ||
| Anagen hair density/cm2 | Baseline | 92.8 ± 32.8 | 97.3 ± 33.8 | |
| Week 16 | 91.3 ± 33.9 | 99.4 ± 33.8 | 0.5146 | |
| p-value | 0.7769 | 0.5262 | ||
| Variable | Probiotic | Placebo | p-Value |
|---|---|---|---|
| Average length (mm) | |||
| Baseline | 1.64 ± 0.313 | 1.62 ± 0.331 | 0.3185 |
| Week 16 | 2.81 ± 8.82 | 1.63 ± 0.275 | |
| p-value * | 0.1593 | 1.000 | |
| Average thickness (mm) | |||
| Baseline | 0.0694 ± 0.0195 | 0.0676 ± 0.0187 | |
| Week 16 | 0.0690 ± 0.0179 | 0.0660 ± 0.01833 | 0.0662 |
| p-value * | 0.6842 | 0.0301 |
| Variable | Probiotic | Placebo | p-Value | Probiotic (n = 33) | Placebo (n = 34) | p-Value |
|---|---|---|---|---|---|---|
| Participants aged 37.5 years or younger | Participants over 37.5 years of age | |||||
| Hair count | ||||||
| Baseline | 153 ± 58.7 | 171 ± 59.0 | 0.0772 | 164 ± 53.9 | 162 ± 40.2 | |
| Week 16 | 146 ± 55.5 | 184 ± 75.6 | 180 ± 73.1 | 167 ± 43.3 | 0.3077 | |
| p-value * | 0.3123 | 0.1275 | 0.0469 | 0.5664 | ||
| Hair density/cm2 | ||||||
| Baseline | 165 ± 56.6 | 189 ± 65.3 | 182 ± 59.7 | 179 ± 44.5 | ||
| Week 16 | 157 ± 47.9 | 201 ± 85.0 | 0.0659 | 199 ± 81.0 | 183 ± 48.6 | 0.2591 |
| p-value * | 0.2430 | 0.1387 | 0.0495 | 0.6941 | ||
| Variables | Probiotic | Placebo | p-Value | Probiotic | Placebo | p-Value |
|---|---|---|---|---|---|---|
| Participants aged 37.5 years or younger | Participants over 37.5 years of age | |||||
| Number of anagen hairs | ||||||
| Baseline | 82.1 ± 31.8 | 90.7 ± 34.5 | 0.0772 | 85.5 ± 27.5 | 85.2 ± 26.3 | |
| Week 16 | 74.8 ± 27.9 | 94.8 ± 37.2 | 89.8 ± 31.7 | 86.6 ± 25.5 | 0.4521 | |
| p-value * | 0.1819 | 0.2281 | 0.2985 | 0.975 | ||
| Number of telogen hairs | ||||||
| Baseline | 28.1 ± 24.8 | 29.1± 22.4 | 36.2 ± 28.6 | 37.1 ± 32.1 | ||
| Week 16 | 22.9 ± 15.4 | 36.9 ± 39.5 | 0.0693 | 45.3 ± 49.4 | 31.9 ± 17.2 | 0.0987 |
| p-value * | 0.2605 | 0.1427 | 0.0521 | 0.6778 | ||
| Density of anagen hairs/cm2 | ||||||
| Baseline | 90.9 ± 35.2 | 100 ± 38.2 | 94.7 ± 30.5 | 94.4 ± 29.1 | ||
| Week 16 | 82.8 ± 30.9 | 103 ± 38.5 | 0.1041 | 99.4 ± 35.1 | 95.9 ± 28.2 | 0.4509 |
| p-value * | 0.1525 | 0.3741 | 0.2994 | 0.9713 | ||
| Telogen hair density/cm2 | ||||||
| Baseline | 31.1 ± 27.5 | 32.2 ± 24.8 | 40.1 ± 31.7 | 41.0 ± 31.7 | ||
| Week 16 | 25.0 ± 16.2 | 40.8 ± 43.7 | 0.0669 | 44.9 ± 51.1 | 35.4 ± 19.0 | 0.3174 |
| p-value * | 0.2456 | 0.1463 | 0.3393 | 0.6416 | ||
| Mean length (mm) | ||||||
| Baseline | 1.73 ± 0.265 | 1.70 ± 0.290 | 1.56 ± 0.340 | 1.55 ± 0.355 | ||
| Week 16 | 1.75 ± 0.220 | 1.63 ± 0.286 | 0.0871 | 3.84 ± 12.4 | 1.63 ± 0.268 | 0.3532 |
| p-value * | 0.4035 | 0.1097 | 0.1733 | 0.966 | ||
| Variables | Probiotic | Placebo | p-Value | Probiotic | Placebo | p-Value |
|---|---|---|---|---|---|---|
| Participants aged 37.5 years or younger | Participants over 37.5 years of age | |||||
| Number of terminal hairs | ||||||
| Baseline | 86.6 ± 32.9 | 101 ± 41.1 | 90.0 ± 36.1 | 83.7 ± 34.2 | ||
| Week 16 | 76.6 ± 30.4 | 102 ± 46.3 | 0.1088 | 98.1 ± 39.3 | 87.6 ± 28.8 | 0.255 |
| p-value * | 0.1011 | 0.511 | 0.0641 | 0.8081 | ||
| Number of vellus hairs | ||||||
| Baseline | 23.6 ± 26.8 | 19.1± 14.2 | 31.7 ± 27.3 | 38.6 ± 41.9 | ||
| Week 16 | 19.2 ± 12.3 | 29.0 ± 30.8 | 0.0338 | 36.6 ± 41.2 | 30.9 ± 24.8 | 0.2683 |
| p-value * | 0.3746 | 0.0314 | 0.5384 | 0.3413 | ||
| Density of terminal hairs/cm2 | ||||||
| Baseline | 95.9 ± 36.5 | 109 ± 48.7 | 99.6 ± 40.0 | 92.7 ± 37.8 | ||
| Week 16 | 84.9 ± 33.7 | 113 ± 51.2 | 0.0644 | 109 ± 43.2 | 97.0 ± 31.9 | 0.2446 |
| p-value * | 0.1064 | 0.2989 | 0.0593 | 0.808 | ||
| Density of vellus hairs/cm2 | ||||||
| Baseline | 26.2 ± 29.7 | 21.2 ± 15.8 | 35.1 ± 30.2 | 42.7 ± 46.3 | ||
| Week 16 | 23.0 ± 19.6 | 32.4 ± 34.3 | 0.0275 | 40.5 ± 45.7 | 34.2 ± 27.5 | 0.2679 |
| p-value * | 0.4861 | 0.0143 | 0.5389 | 0.3403 | ||
| Mean thickness (mm) | ||||||
| Baseline | 0.0726 ± 0.0192 | 0.0739 ± 0.0189 | 0.0661 ± 0.0196 | 0.0614 ± 0.0165 | ||
| Week 16 | 0.0741 ± 0.0182 | 0.0670 ± 0.0154 | 0.0492 | 0.0641 ± 0.0165 | 0.0649 ± 0.211 | 0.3757 |
| p-value * | 0.7278 | 0.0149 | 0.6819 | 0.3989 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Navarro, A.; Vasallo-Morillas, M.I.; Navarro-Belmonte, R.; Vilanova, C.; Torrent, D.; Kilasoniya, A.; Moles-Ugeda, I.; Gallego-Herrera, E.; Ramírez-Boscá, A. Randomized Clinical Trial to Evaluate the Effect of Probiotic Intake on Androgenic Alopecia. Nutrients 2024, 16, 2900. https://doi.org/10.3390/nu16172900
García-Navarro A, Vasallo-Morillas MI, Navarro-Belmonte R, Vilanova C, Torrent D, Kilasoniya A, Moles-Ugeda I, Gallego-Herrera E, Ramírez-Boscá A. Randomized Clinical Trial to Evaluate the Effect of Probiotic Intake on Androgenic Alopecia. Nutrients. 2024; 16(17):2900. https://doi.org/10.3390/nu16172900
Chicago/Turabian StyleGarcía-Navarro, Alejandro, María Isabel Vasallo-Morillas, Roge Navarro-Belmonte, Cristina Vilanova, Daniel Torrent, Alina Kilasoniya, Isabel Moles-Ugeda, Estefanía Gallego-Herrera, and Ana Ramírez-Boscá. 2024. "Randomized Clinical Trial to Evaluate the Effect of Probiotic Intake on Androgenic Alopecia" Nutrients 16, no. 17: 2900. https://doi.org/10.3390/nu16172900
APA StyleGarcía-Navarro, A., Vasallo-Morillas, M. I., Navarro-Belmonte, R., Vilanova, C., Torrent, D., Kilasoniya, A., Moles-Ugeda, I., Gallego-Herrera, E., & Ramírez-Boscá, A. (2024). Randomized Clinical Trial to Evaluate the Effect of Probiotic Intake on Androgenic Alopecia. Nutrients, 16(17), 2900. https://doi.org/10.3390/nu16172900





