Impact of Coffee Intake on Measures of Wellbeing in Mice
Abstract
:1. Introduction
2. Methods
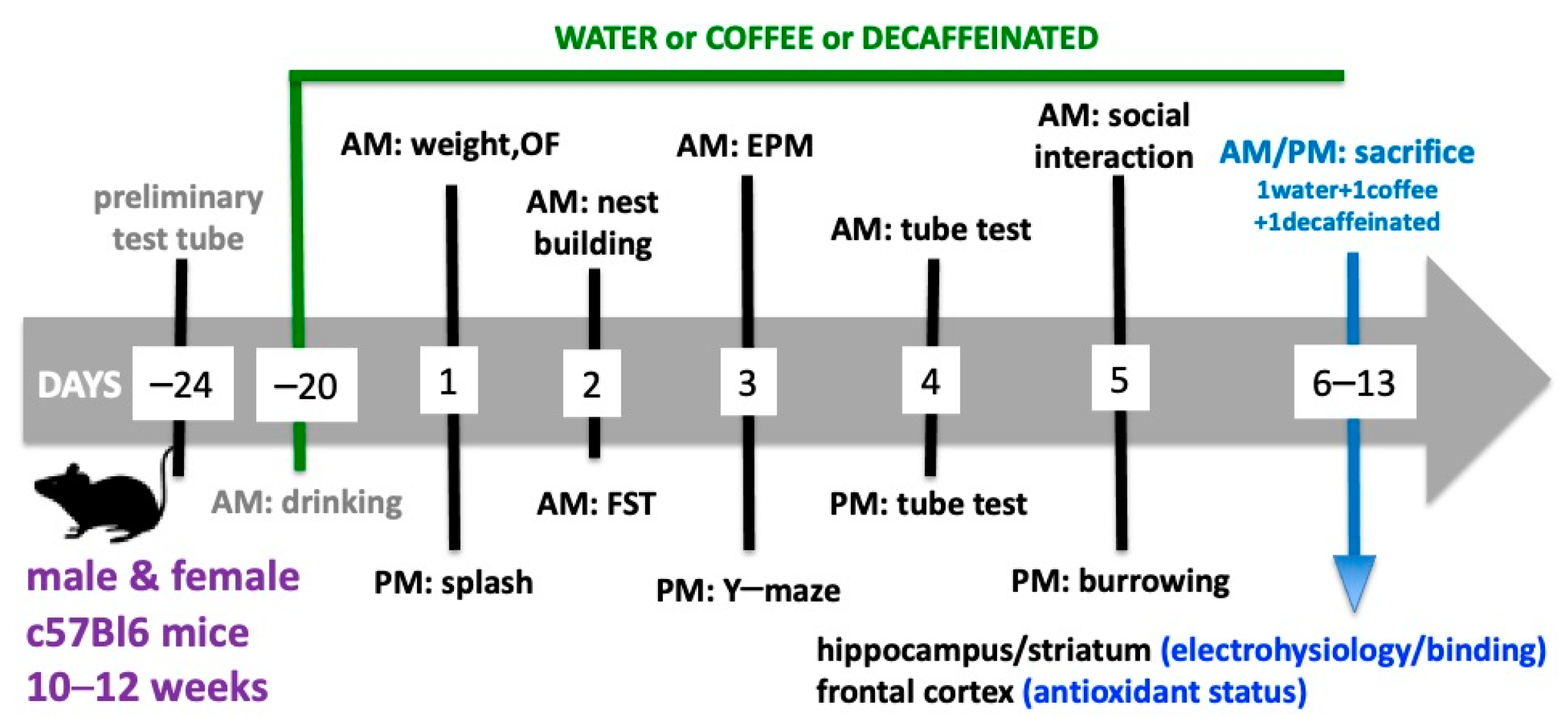
2.1. Animals
2.2. Drugs and Treatments
2.3. Behavioral Testing
2.4. Sacrifice of the Animals
2.5. Antioxidant Potential
2.6. Binding to A1 Receptors
2.7. Slice Electrophysiology
2.8. Statistical Analysis
3. Results
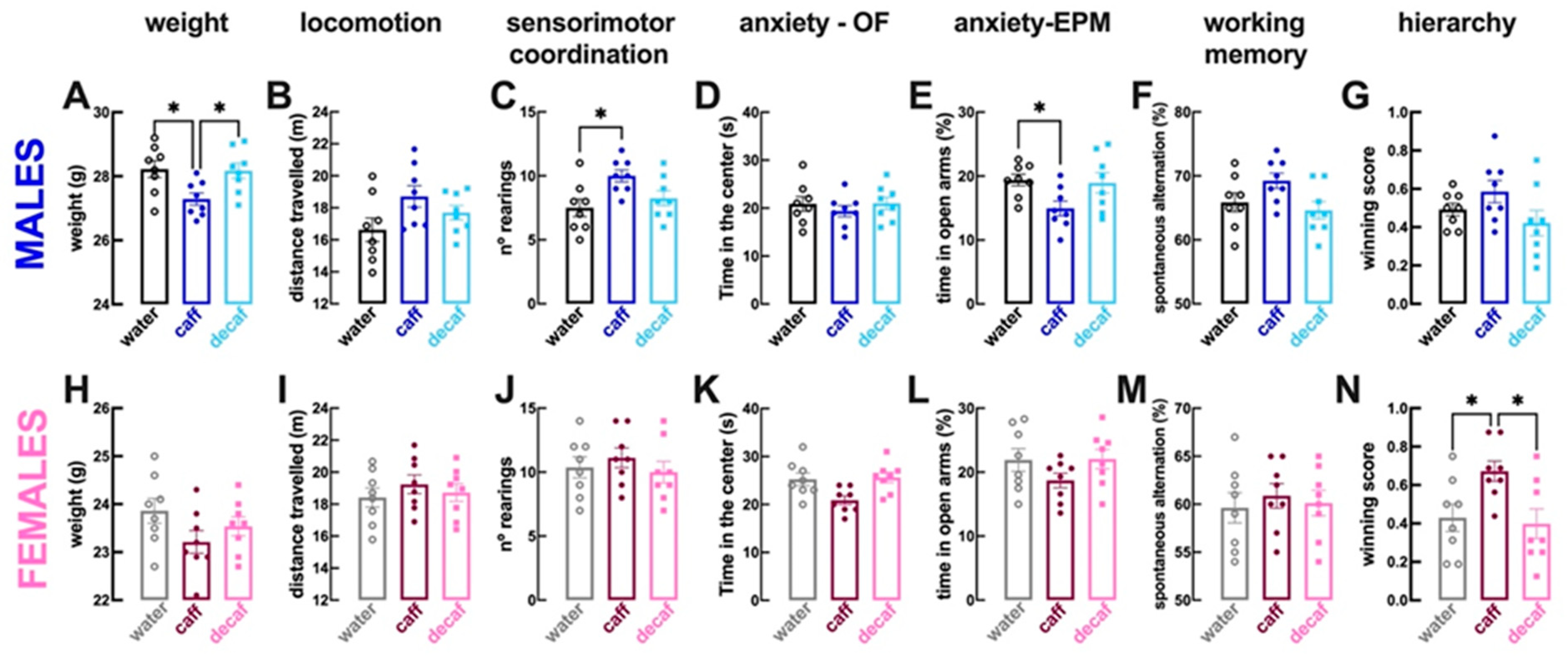
3.1. Behavioral Alterations
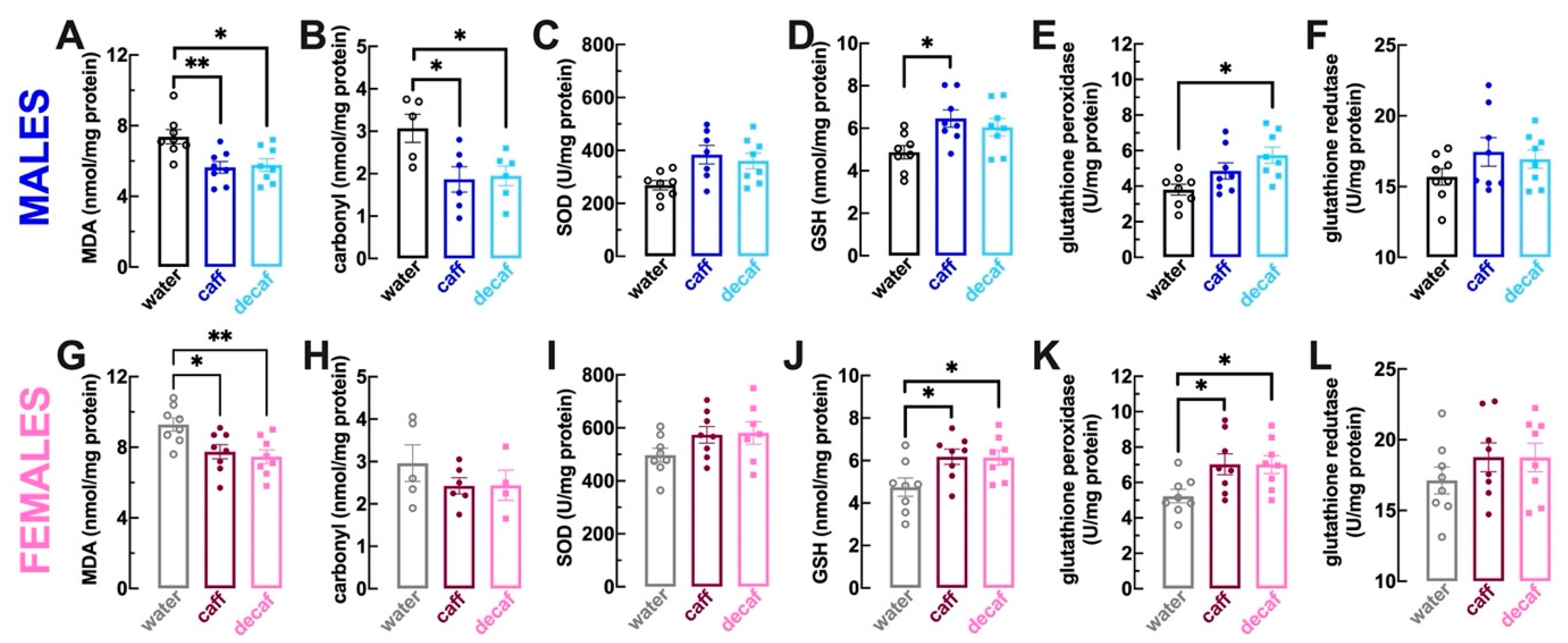
3.2. Alterations of Brain Antioxidant Status
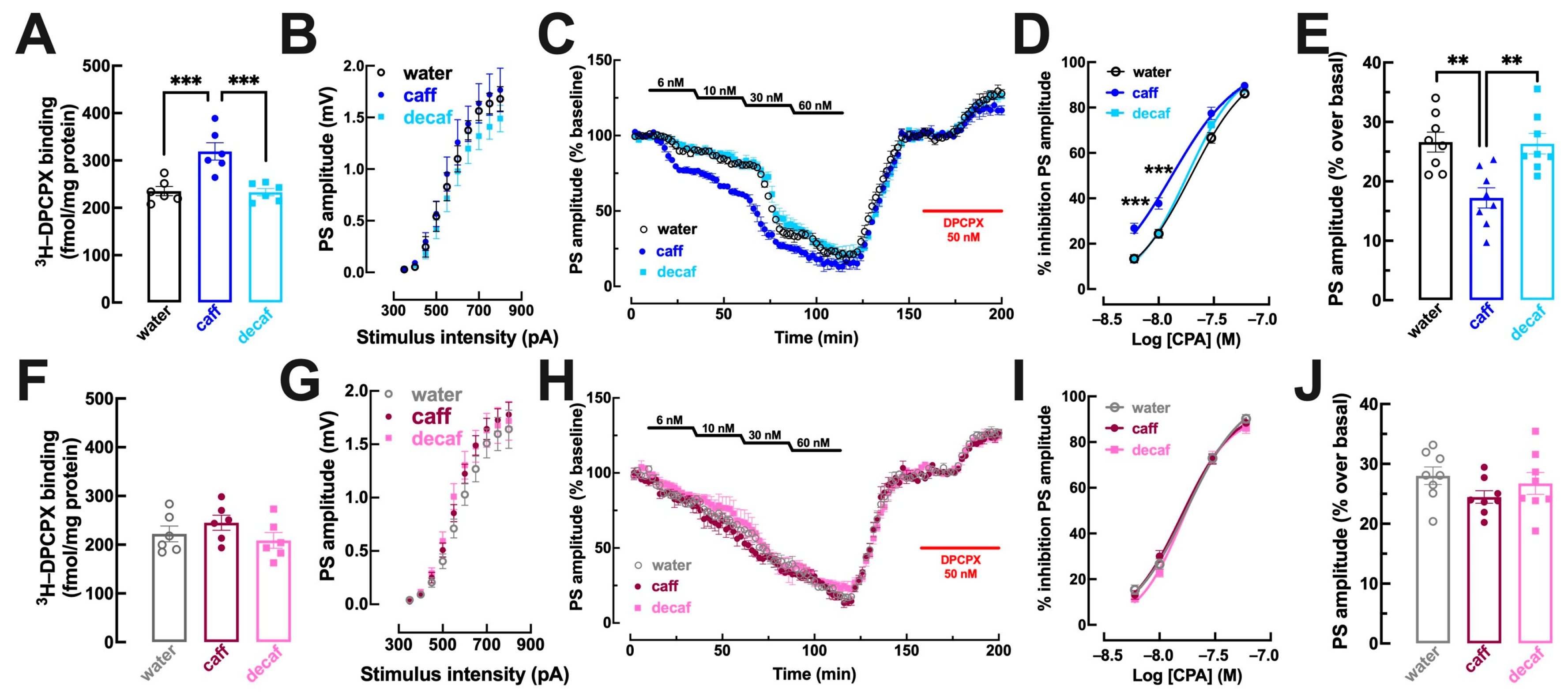
3.3. Alterations of Adenosine Neuromodulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Smith, A. Effects of caffeine on human behavior. Food Chem. Toxicol. 2002, 40, 1243–1255. [Google Scholar] [CrossRef]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, L.; Zhang, C.; Hu, Z.; Tang, J.; Xue, J.; Lu, W. Caffeine intake and anxiety: A meta-analysis. Front. Psychol. 2024, 15, 1270246. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv. Food Nutr. Res. 2020, 91, 65–96. [Google Scholar] [CrossRef]
- de Mendonça, A.; Cunha, R.A. Therapeutic opportunities for caffeine in Alzheimer’s disease and other neurodegenerative disorders. J. Alzheimers Dis. 2010, 20 (Suppl. S1), S1–S2. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, caffeine, and health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Lopes, J.P.; Pliássova, A.; Cunha, R.A. The physiological effects of caffeine on synaptic transmission and plasticity in the mouse hippocampus selectively depend on adenosine A1 and A2A receptors. Biochem. Pharmacol. 2019, 166, 313–321. [Google Scholar] [CrossRef]
- IJzerman, A.P.; Jacobson, K.A.; Müller, C.E.; Cronstein, B.N.; Cunha, R.A. International Union of Basic and Clinical Pharmacology. CXII: Adenosine receptors: A further update. Pharmacol. Rev. 2022, 74, 340–372. [Google Scholar] [CrossRef]
- Cunha, R.A.; Agostinho, P.M. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J. Alzheimers Dis. 2010, 20 (Suppl. S1), S95–S116. [Google Scholar] [CrossRef] [PubMed]
- Paiva, I.; Cellai, L.; Meriaux, C.; Poncelet, L.; Nebie, O.; Saliou, J.M.; Lacoste, A.S.; Papegaey, A.; Drobecq, H.; Le Gras, S.; et al. Caffeine intake exerts dual genome-wide effects on hippocampal metabolism and learning-dependent transcription. J. Clin. Investig. 2022, 132, e149371. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.R.; Oliveira, A.; Gaspar, I.; Rodrigues, M.S.; Santos, J.; Szabó, E.; Silva, H.B.; Tomé, Â.R.; Canas, P.M.; Agostinho, P.; et al. Effects of chronic caffeine consumption on synaptic function, metabolism and adenosine modulation in different brain areas. Biomolecules 2023, 13, 106. [Google Scholar] [CrossRef]
- Magalhães, R.; Picó-Pérez, M.; Esteves, M.; Vieira, R.; Castanho, T.C.; Amorim, L.; Sousa, M.; Coelho, A.; Fernandes, H.M.; Cabral, J.; et al. Habitual coffee drinkers display a distinct pattern of brain functional connectivity. Mol. Psychiatry 2021, 26, 6589–6598. [Google Scholar] [CrossRef] [PubMed]
- Picó-Pérez, M.; Magalhães, R.; Esteves, M.; Vieira, R.; Castanho, T.C.; Amorim, L.; Sousa, M.; Coelho, A.; Moreira, P.S.; Cunha, R.A.; et al. Coffee consumption decreases the connectivity of the posterior Default Mode Network (DMN) at rest. Front. Behav. Neurosci. 2023, 17, 1176382. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.Y.D.; Dobrachinski, F.; Silva, H.B.; Lopes, J.P.; Gonçalves, F.Q.; Soares, F.A.A.; Porciúncula, L.O.; Andrade, G.M.; Cunha, R.A.; Tomé, A.R. Neuromodulation and neuroprotective effects of chlorogenic acids in excitatory synapses of mouse hippocampal slices. Sci. Rep. 2021, 11, 10488. [Google Scholar] [CrossRef]
- Murai, T.; Matsuda, S. The chemopreventive effects of chlorogenic acids, phenolic compounds in coffee, against inflammation, cancer, and neurological diseases. Molecules 2023, 28, 2381. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Fujioka, K.; Shibamoto, T. Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem. 2008, 106, 217–221. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; van Dam, R.M.; Li, T.Y.; Rodriguez-Artalejo, F.; Hu, F.B. The relationship of coffee consumption with mortality. Ann. Intern. Med. 2008, 148, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y.; Giovannucci, E. Coffee consumption and all-cause and cause-specific mortality: A meta-analysis by potential modifiers. Eur. J. Epidemiol. 2019, 34, 731–752. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.J.; Lee, S.G.; Bormate, K.J.; Jung, Y.S. Effect of caffeine consumption on the risk for neurological and psychiatric disorders: Sex differences in human. Nutrients 2020, 12, 3080. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.; Clark, R.E. The medial temporal lobe. Annu. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef]
- Klaus, A.; Alves da Silva, J.; Costa, R.M. What, if, and when to move: Basal ganglia circuits and self-paced action initiation. Annu. Rev. Neurosci. 2019, 42, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Choi, D.S.; Cunha, R.A. Striatopallidal adenosine A2A receptor modulation of goal-directed behavior: Homeostatic control with cognitive flexibility. Neuropharmacology 2023, 226, 109421. [Google Scholar] [CrossRef]
- Price, J.L.; Drevets, W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology 2010, 35, 192–216. [Google Scholar] [CrossRef]
- Watanabe, S.; Takahashi, T.; Ogawa, H.; Uehara, H.; Tsunematsu, T.; Baba, H.; Morimoto, Y.; Tsuneyama, K. Daily coffee intake inhibits pancreatic beta cell damage and nonalcoholic steatohepatitis in a mouse model of spontaneous metabolic syndrome, Tsumura-Suzuki obese diabetic mice. Metab. Syndr. Relat. Disord. 2017, 15, 170–177. [Google Scholar] [CrossRef]
- Rebola, N.; Coelho, J.E.; Costenla, A.R.; Lopes, L.V.; Parada, A.; Oliveira, C.R.; Soares-da-Silva, P.; de Mendonça, A.; Cunha, R.A. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur. J. Neurosci. 2003, 18, 820–828. [Google Scholar] [CrossRef]
- Lopes, C.R.; Gonçalves, F.Q.; Olaio, S.; Tomé, A.R.; Cunha, R.A.; Lopes, J.P. Adenosine A2A receptors shut down adenosine A1 receptor-mediated presynaptic inhibition to promote implementation of hippocampal long-term potentiation. Biomolecules 2023, 13, 715. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, A.M.; Cunha, R.A.; de Mendonça, A.; Ribeiro, J.A. Modification of adenosine modulation of synaptic transmission in the hippocampus of aged rats. Br. J. Pharmacol. 2000, 131, 1629–1634. [Google Scholar] [CrossRef]
- Kaster, M.P.; Machado, N.J.; Silva, H.B.; Nunes, A.; Ardais, A.P.; Santana, M.; Baqi, Y.; Müller, C.E.; Rodrigues, A.L.; Porciúncula, L.O.; et al. Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc. Natl. Acad. Sci. USA 2015, 112, 7833–7838. [Google Scholar] [CrossRef]
- Dias, L.; Lopes, C.R.; Gonçalves, F.Q.; Nunes, A.; Pochmann, D.; Machado, N.J.; Tomé, A.R.; Agostinho, P.; Cunha, R.A. Crosstalk between ATP-P2X7 and adenosine A2A receptors controlling neuroinflammation in rats subject to repeated restraint stress. Front. Cell. Neurosci. 2021, 15, 639322. [Google Scholar] [CrossRef]
- Leffa, D.T.; Pandolfo, P.; Gonçalves, N.; Machado, N.J.; de Souza, C.M.; Real, J.I.; Silva, A.C.; Silva, H.B.; Köfalvi, A.; Cunha, R.A.; et al. Adenosine A2A receptors in the rat prelimbic medial prefrontal cortex control delay-based cost-benefit decision making. Front. Mol. Neurosci. 2018, 11, 475. [Google Scholar] [CrossRef]
- Jirkof, P.; Fleischmann, T.; Cesarovic, N.; Rettich, A.; Vogel, J.; Arras, M. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab. Anim. 2013, 47, 153–161. [Google Scholar] [CrossRef]
- Canas, P.M.; Porciúncula, L.O.; Cunha, G.M.; Silva, C.G.; Machado, N.J.; Oliveira, J.M.; Oliveira, C.R.; Cunha, R.A. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J. Neurosci. 2009, 29, 14741–14751. [Google Scholar] [CrossRef]
- Augusto, E.; Matos, M.; Sévigny, J.; El-Tayeb, A.; Bynoe, M.S.; Müller, C.E.; Cunha, R.A.; Chen, J.F. Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 2013, 33, 11390–11399. [Google Scholar] [CrossRef]
- Fan, Z.; Zhu, H.; Zhou, T.; Wang, S.; Wu, Y.; Hu, H. Using the tube test to measure social hierarchy in mice. Nat. Protoc. 2019, 14, 819–831. [Google Scholar] [CrossRef]
- Moy, S.S.; Nadler, J.J.; Perez, A.; Barbaro, R.P.; Johns, J.M.; Magnuson, T.R.; Piven, J.; Crawley, J.N. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004, 3, 287–302. [Google Scholar] [CrossRef]
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods 2014, 234, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M. Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006, 1, 122–124. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Flohé, L.; Otting, F. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Cunha, R.A.; Constantino, M.D.; Fonseca, E.; Ribeiro, J.A. Age-dependent decrease in adenosine A1 receptor binding sites in the rat brain. Effect of cis unsaturated free fatty acids. Eur. J. Biochem. 2001, 268, 2939–2947. [Google Scholar] [CrossRef]
- Reis, S.L.; Silva, H.B.; Almeida, M.; Cunha, R.A.; Simões, A.P.; Canas, P.M. Adenosine A1 and A2A receptors differently control synaptic plasticity in the mouse dorsal and ventral hippocampus. J. Neurochem. 2019, 151, 227–237. [Google Scholar] [CrossRef]
- Li, W.; Silva, H.B.; Real, J.; Wang, Y.M.; Rial, D.; Li, P.; Payen, M.P.; Zhou, Y.; Muller, C.E.; Tomé, A.R.; et al. Inactivation of adenosine A2A receptors reverses working memory deficits at early stages of Huntington’s disease models. Neurobiol. Dis. 2015, 79, 70–80. [Google Scholar] [CrossRef]
- Gonçalves, F.Q.; Matheus, F.C.; Silva, H.B.; Real, J.I.; Rial, D.; Rodrigues, R.J.; Oses, J.P.; Silva, A.C.; Gonçalves, N.; Prediger, R.D.; et al. Increased ATP release and higher impact of adenosine A2A receptors on corticostriatal plasticity in a rat model of presymptomatic Parkinson’s disease. Mol. Neurobiol. 2023, 60, 1659–1674. [Google Scholar] [CrossRef]
- Anderson, W.W.; Collingridge, G.L. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J. Neurosci. Methods 2007, 162, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichgans, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo’, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee consumption and oxidative stress: A review of human intervention studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Bresciani, G.; da Cruz, I.B.; González-Gallego, J. Manganese superoxide dismutase and oxidative stress modulation. Adv. Clin. Chem. 2015, 68, 87–130. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Xiong, C.; Lucero, J.; Behrens, M.M.; Dugan, L.L.; Quick, K.L. Gender differences in free radical homeostasis during aging: Shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell 2006, 5, 565–574. [Google Scholar] [CrossRef]
- Wang, L.; Ahn, Y.J.; Asmis, R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox Biol. 2020, 31, 101410. [Google Scholar] [CrossRef]
- Serchov, T.; Clement, H.W.; Schwarz, M.K.; Iasevoli, F.; Tosh, D.K.; Idzko, M.; Jacobson, K.A.; de Bartolomeis, A.; Normann, C.; Biber, K.; et al. Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of homer1a. Neuron 2015, 87, 549–562. [Google Scholar] [CrossRef]
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 2007, 83, 277–292. [CrossRef] [PubMed]
- Poole, R.L.; Tordoff, M.G. The taste of caffeine. J. Caffeine Res. 2017, 7, 39–52. [Google Scholar] [CrossRef]
- Yawata, Y.; Shikano, Y.; Ogasawara, J.; Makino, K.; Kashima, T.; Ihara, K.; Yoshimoto, A.; Morikawa, S.; Yagishita, S.; Tanaka, K.F.; et al. Mesolimbic dopamine release precedes actively sought aversive stimuli in mice. Nat. Commun. 2023, 14, 2433. [Google Scholar] [CrossRef] [PubMed]
- Mann, L.G.; Claassen, D.O. Mesial temporal dopamine: From biology to behaviour. Eur. J. Neurosci. 2024, 59, 1141–1152. [Google Scholar] [CrossRef]
- Yin, Y.Q.; Zhang, C.; Wang, J.X.; Hou, J.; Yang, X.; Qin, J. Chronic caffeine treatment enhances the resilience to social defeat stress in mice. Food Funct. 2015, 6, 479–491. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Kamal, M.; Tikamdas, R.; Nouh, R.A.; Tian, J.; Sayed, M. Effects of chronic caffeine administration on behavioral and molecular adaptations to sensory contact model induced stress in adolescent male mice. Behav. Genet. 2020, 50, 374–383. [Google Scholar] [CrossRef]
- Mao, Z.F.; Ouyang, S.H.; Zhang, Q.Y.; Wu, Y.P.; Wang, G.E.; Tu, L.F.; Luo, Z.; Li, W.X.; Kurihara, H.; Li, Y.F.; et al. New insights into the effects of caffeine on adult hippocampal neurogenesis in stressed mice: Inhibition of CORT-induced microglia activation. FASEB J. 2020, 34, 10998–11014. [Google Scholar] [CrossRef]
- Basu Mallik, S.; Mudgal, J.; Hall, S.; Kinra, M.; Grant, G.D.; Nampoothiri, M.; Anoopkumar-Dukie, S.; Arora, D. Remedial effects of caffeine against depressive-like behaviour in mice by modulation of neuroinflammation and BDNF. Nutr. Neurosci. 2022, 25, 1836–1844. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Li, Z.; da Silva, B.S.; Zheng, W.; Xiang, Z.; He, Y.; Xu, T.; Cordeiro, C.; Deng, L.; et al. Lateral septum adenosine A2A receptors control stress-induced depressive-like behaviors via signaling to the hypothalamus and habenula. Nat. Commun. 2023, 14, 1880. [Google Scholar] [CrossRef]
- Rikitake, M.; Notake, S.; Kurokawa, K.; Hata, J.; Seki, F.; Komaki, Y.; Oshiro, H.; Kawaguchi, N.; Haga, Y.; Yoshimaru, D.; et al. Effects of chronic caffeine intake and withdrawal on neural activity assessed via resting-state functional magnetic resonance imaging in mice. Heliyon 2022, 8, e11714. [Google Scholar] [CrossRef]
- Wang, L.; Shen, X.; Wu, Y.; Zhang, D. Coffee and caffeine consumption and depression: A meta-analysis of observational studies. Aust. N. Z. J. Psychiatry 2016, 50, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Castellano, S.; Pajak, A.; Galvano, F. Coffee, tea, caffeine and risk of depression: A systematic review and dose-response meta-analysis of observational studies. Mol. Nutr. Food Res. 2016, 60, 223–234. [Google Scholar] [CrossRef]
- Min, J.; Cao, Z.; Cui, L.; Li, F.; Lu, Z.; Hou, Y.; Yang, H.; Wang, X.; Xu, C. The association between coffee consumption and risk of incident depression and anxiety: Exploring the benefits of moderate intake. Psychiatry Res. 2023, 326, 115307. [Google Scholar] [CrossRef]
- Torabynasab, K.; Shahinfar, H.; Payandeh, N.; Jazayeri, S. Association between dietary caffeine, coffee, and tea consumption and depressive symptoms in adults: A systematic review and dose-response meta-analysis of observational studies. Front. Nutr. 2023, 10, 1051444. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Mirzaei, F.; Pan, A.; Okereke, O.I.; Willett, W.C.; O’Reilly, É.J.; Koenen, K.; Ascherio, A. Coffee, caffeine, and risk of depression among women. Arch. Intern. Med. 2011, 171, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Suga, H.; Kobayashi, S.; Sasaki, S. Three-generation study of women on diets and health study group. intake of coffee associated with decreased depressive symptoms among elderly Japanese women: A multi-center cross-sectional study. J. Epidemiol. 2020, 30, 338–344. [Google Scholar] [CrossRef]
- Amendola, C.A.; Gabrieli, J.D.; Lieberman, H.R. Caffeine’s effects on performance and mood are independent of age and gender. Nutr. Neurosci. 1998, 1, 269–280. [Google Scholar] [CrossRef]
- Lohi, J.J.; Huttunen, K.H.; Lahtinen, T.M.; Kilpeläinen, A.A.; Muhli, A.A.; Leino, T.K. Effect of caffeine on simulator flight performance in sleep-deprived military pilot students. Mil. Med. 2007, 172, 982–987. [Google Scholar] [CrossRef]
- Ullrich, S.; de Vries, Y.C.; Kühn, S.; Repantis, D.; Dresler, M.; Ohla, K. Feeling smart: Effects of caffeine and glucose on cognition, mood and self-judgment. Physiol. Behav. 2015, 151, 629–637. [Google Scholar] [CrossRef]
- Makki, N.M.; Alharbi, S.T.; Alharbi, A.M.; Alsharif, A.S.; Aljabri, A.M. Caffeine consumption and depression, anxiety, and stress levels among university students in Medina: A cross-sectional study. Cureus 2023, 15, e48018. [Google Scholar] [CrossRef]
- Ratliff-Crain, J.; Kane, J. Predictors for altering caffeine consumption during stress. Addict. Behav. 1995, 20, 509–516. [Google Scholar] [CrossRef]
- Giles, G.E.; Spring, A.M.; Urry, H.L.; Moran, J.M.; Mahoney, C.R.; Kanarek, R.B. Caffeine alters emotion and emotional responses in low habitual caffeine consumers. Can. J. Physiol. Pharmacol. 2018, 96, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.S.; Penn, T.M.; Cable, S.T.; Aroke, E.N.; Goodin, B.R. Higher habitual dietary caffeine consumption is related to lower experimental pain sensitivity in a community-based sample. Psychopharmacology 2018, 235, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, F.; Stampfer, M.; Kubzansky, L.D.; Trudel-Fitzgerald, C. Prospective associations between coffee consumption and psychological well-being. PLoS ONE 2022, 17, e0267500. [Google Scholar] [CrossRef]
- Xu, Q.; Anderson, D.; Courtney, M. A longitudinal study of the relationship between lifestyle and mental health among midlife and older women in Australia: Findings from the Healthy Aging of Women Study. Health Care Women Int. 2010, 31, 1082–1096. [Google Scholar] [CrossRef]
- Broughton, K.A.; Payne, L.; Liechty, T. An exploration of older men’s social lives and well-being in the context of a coffee group. Leis. Sci. 2017, 39, 261–276. [Google Scholar] [CrossRef]
- Manippa, V.; Lupo, R.; Tommasi, L.; Brancucci, A. Italian breakfast in mind: The effect of caffeine, carbohydrate and protein on physiological state, mood and cognitive performance. Physiol. Behav. 2021, 234, 113371. [Google Scholar] [CrossRef] [PubMed]
- Quarta, S.; Siculella, L.; Levante, A.; Carluccio, M.A.; Calabriso, N.; Scoditti, E.; Damiano, F.; Lecciso, F.; Pinto, P.; García-Conesa, M.T.; et al. Association between Mediterranean lifestyle and perception of well-being and distress in a sample population of university Italian students. Int. J. Food Sci. Nutr. 2023, 74, 556–567. [Google Scholar] [CrossRef]
- Booth, N.; Saxton, J.; Rodda, S.N. Estimates of caffeine use disorder, caffeine withdrawal, harm and help-seeking in New Zealand: A cross-sectional survey. Addict. Behav. 2020, 109, 106470. [Google Scholar] [CrossRef]
- Abdoli, F.; Davoudi, M.; Momeni, F.; Djafari, F.; Dolatshahi, B.; Hosseinzadeh, S.; Aliyaki, H.; Khalili, Z. Estimate the prevalence of daily caffeine consumption, caffeine use disorder, caffeine withdrawal and perceived harm in Iran: A cross-sectional study. Sci. Rep. 2024, 14, 7644. [Google Scholar] [CrossRef]
- Bodur, M.; Kaya, S.; Ilhan-Esgin, M.; Çakiroğlu, F.P.; Özçelik, A.Ö. The caffeine dilemma: Unraveling the intricate relationship between caffeine use disorder, caffeine withdrawal symptoms and mental well-being in adults. Public Health Nutr. 2024, 27, e57. [Google Scholar] [CrossRef]
- Hines, D.J.; Schmitt, L.I.; Hines, R.M.; Moss, S.J.; Haydon, P.G. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl. Psychiatry 2013, 3, e212. [Google Scholar] [CrossRef]
- Ju, X.; Wang, S.; Yan, P.; Zhu, C.; Hu, X.; Dong, J.; Tan, Z. Rapid eye movement sleep deprivation combined with fluoxetine protects against depression-induced damage and apoptosis in rat hippocampi via A1 adenosine receptor. Front. Psychiatry 2021, 12, 599399. [Google Scholar] [CrossRef] [PubMed]
- Serchov, T.; Schwarz, I.; Theiss, A.; Sun, L.; Holz, A.; Döbrössy, M.D.; Schwarz, M.K.; Normann, C.; Biber, K.; van Calker, D. Enhanced adenosine A1 receptor and Homer1a expression in hippocampus modulates the resilience to stress-induced depression-like behavior. Neuropharmacology 2020, 162, 107834. [Google Scholar] [CrossRef]
- Cunha, R.A. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: Different roles, different sources and different receptors. Neurochem. Int. 2001, 38, 107–125. [Google Scholar] [CrossRef]
- Chen, J.; Rinaldo, L.; Lim, S.J.; Young, H.; Messing, R.O.; Choi, D.S. The type 1 equilibrative nucleoside transporter regulates anxiety-like behavior in mice. Genes Brain Behav. 2007, 6, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.R.; Rowe, M.; Cullen, P.K.; Furst, S. Enhancing brain adenosine signaling with the nucleoside transport blocker NBTI (S-(4-nitrobenzyl)-6-theoinosine) mimics the effects of inescapable shock on later shuttle-escape performance in rats. Behav. Neurosci. 2008, 122, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hong, S.I.; Lee, J.; Peyton, L.; Baker, M.; Choi, S.; Kim, H.; Chang, S.Y.; Choi, D.S. Activation of astrocytes in the dorsomedial striatum facilitates transition from habitual to goal-directed reward-seeking behavior. Biol. Psychiatry 2020, 88, 797–808. [Google Scholar] [CrossRef]
- Hinton, D.J.; Lee, M.R.; Jang, J.S.; Choi, D.S. Type 1 equilibrative nucleoside transporter regulates astrocyte-specific glial fibrillary acidic protein expression in the striatum. Brain Behav. 2014, 4, 903–914. [Google Scholar] [CrossRef]
- Lee, C.C.; Chang, C.P.; Lin, C.J.; Lai, H.L.; Kao, Y.H.; Cheng, S.J.; Chen, H.M.; Liao, Y.P.; Faivre, E.; Buée, L.; et al. Adenosine augmentation evoked by an ENT1 inhibitor improves memory impairment and neuronal plasticity in the APP/PS1 mouse model of Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 8936–8952. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.; Sardinha, V.M.; Guerra-Gomes, S.; Araque, A.; Sousa, N. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 2015, 38, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Rial, D.; Lemos, C.; Pinheiro, H.; Duarte, J.M.; Gonçalves, F.Q.; Real, J.I.; Prediger, R.D.; Gonçalves, N.; Gomes, C.A.; Canas, P.M.; et al. Depression as a glial-based synaptic dysfunction. Front. Cell. Neurosci. 2016, 9, 521. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Verkhratsky, A.; Tang, Y.; Illes, P. Astrocytes and major depression: The purinergic avenue. Neuropharmacology 2022, 220, 109252. [Google Scholar] [CrossRef]
- Agostinho, P.; Madeira, D.; Dias, L.; Simões, A.P.; Cunha, R.A.; Canas, P.M. Purinergic signaling orchestrating neuron-glia communication. Pharmacol. Res. 2020, 162, 105253. [Google Scholar] [CrossRef]
- Camfield, D.A.; Silber, B.Y.; Scholey, A.B.; Nolidin, K.; Goh, A.; Stough, C. A randomised placebo-controlled trial to differentiate the acute cognitive and mood effects of chlorogenic acid from decaffeinated coffee. PLoS ONE 2013, 8, e82897. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Goodman, C.L.; Capps, C.R.; Shue, Z.L.; Arnot, R. Influence of 2-weeks ingestion of high chlorogenic acid coffee on mood state, performance, and postexercise inflammation and oxidative stress: A randomized, placebo-controlled trial. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 55–65. [Google Scholar] [CrossRef]
- Jackson, P.A.; Kenney, C.; Forster, J.; Smith, E.F.; Elcoate, R.; Spittlehouse, B.; Johnson, J.; Kennedy, D.O. Acute cognitive performance and mood effects of coffeeberry extract: A randomized, double blind, placebo-controlled crossover study in healthy humans. Nutrients 2023, 15, 2418. [Google Scholar] [CrossRef]
- Merighi, S.; Travagli, A.; Nigro, M.; Pasquini, S.; Cappello, M.; Contri, C.; Varani, K.; Vincenzi, F.; Borea, P.A.; Gessi, S. Caffeine for prevention of Alzheimer’s disease: Is the A2A adenosine receptor its target? Biomolecules 2023, 13, 967. [Google Scholar] [CrossRef]
- Holloway, W.R., Jr.; Thor, D.H. Testosterone dependent effects of caffeine on social investigation by adult male rats. Physiol. Behav. 1984, 33, 959–964. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Kawamura, M.; Masino, A.S. Adenosine and ketogenic treatments. J. Caffeine Adenosine Res. 2020, 10, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, Y.; Tomi, M.; Shin, B.C.; Thamotharan, S.; Mazarati, A.; Sankar, R.; Wang, E.A.; Cepeda, C.; Levine, M.S.; et al. Sex-specific life course changes in the neuro-metabolic phenotype of glut3 null heterozygous mice: Ketogenic diet ameliorates electroencephalographic seizures and improves sociability. Endocrinology 2017, 158, 936–949. [Google Scholar] [CrossRef] [PubMed]
- López-Cruz, L.; Carbó-Gas, M.; Pardo, M.; Bayarri, P.; Valverde, O.; Ledent, C.; Salamone, J.D.; Correa, M. Adenosine A2A receptor deletion affects social behaviors and anxiety in mice: Involvement of anterior cingulate cortex and amygdala. Behav. Brain Res. 2017, 321, 8–17. [Google Scholar] [CrossRef]
- Santos, N.; Picolo, V.; Domingues, I.; Perillo, V.; Villacis, R.A.R.; Grisolia, C.K.; Oliveira, M. Effects of environmental concentrations of caffeine on adult zebrafish behaviour: A short-term exposure scenario. Environ. Sci. Pollut. Res. Int. 2023, 30, 63776–63787. [Google Scholar] [CrossRef] [PubMed]
- López-Cruz, L.; Salamone, J.D.; Correa, M. Caffeine and selective adenosine receptor antagonists as new therapeutic tools for the motivational symptoms of depression. Front. Pharmacol. 2018, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ruan, Y.; He, Y.; Cai, Q.; Pan, X.; Zhang, Y.; Liu, C.; Pu, Z.; Yang, J.; Chen, M.; et al. Striatopallidal adenosine A2A receptors in the nucleus accumbens confer motivational control of goal-directed behavior. Neuropharmacology 2020, 168, 108010. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Zhou, X.; Zhou, H.; Luo, S.; Wang, Q.; Yao, Z.; Chen, J.F. Neural representation and modulation of volitional motivation in response to escalating efforts. J. Physiol. 2023, 601, 631–645. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M.; Nunes, E.J.; Randall, P.A.; Pardo, M. The behavioral pharmacology of effort-related choice behavior: Dopamine, adenosine and beyond. J. Exp. Anal. Behav. 2012, 97, 125–146. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Speck, A.E.; Canas, P.M.; Cunha, R.A. Neuronal adenosine A2A receptors signal ergogenic effects of caffeine. Sci. Rep. 2020, 10, 13414. [Google Scholar] [CrossRef]
- Alves, A.C.B.; Santos, N.S.; Santos, A.P.T.; da Panatta, G.; Speck, A.E.; Cunha, R.A.; Aguiar, A.S., Jr. Adenosine A2A and dopamine D2 receptor interaction controls fatigue resistance. Front. Pharmacol. 2024, 15, 1390187. [Google Scholar] [CrossRef]
- Pierling, A.L.; Elmenhorst, E.M.; Lange, D.; Hennecke, E.; Baur, D.M.; Beer, S.; Kroll, T.; Neumaier, B.; Aeschbach, D.; Bauer, A.; et al. Cerebral A1 adenosine receptor availability in female and male participants and its relationship to sleep. Neuroimage 2021, 245, 118695. [Google Scholar] [CrossRef]
- Borgus, J.R.; Puthongkham, P.; Venton, B.J. Complex sex and estrous cycle differences in spontaneous transient adenosine. J. Neurochem. 2020, 153, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Ziegler, A.M. Gender differences in subjective and physiological responses to caffeine and the role of steroid hormones. J. Caffeine Res. 2011, 1, 41–48. [Google Scholar] [CrossRef]
- Temple, J.L.; Ziegler, A.M.; Graczyk, A.; Bendlin, A.; Sion, T.; Vattana, K. Cardiovascular responses to caffeine by gender and pubertal stage. Pediatrics 2014, 134, e112–e119. [Google Scholar] [CrossRef]
- Xu, K.; Xu, Y.; Brown-Jermyn, D.; Chen, J.F.; Ascherio, A.; Dluzen, D.E.; Schwarzschild, M.A. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J. Neurosci. 2006, 26, 535–541. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Guan, X.; Yu, M.; Wang, H. β-Estradiol antagonizes the inhibitory effects of caffeine in BMMSCs via the ERβ-mediated cAMP-dependent PKA pathway. Toxicology 2018, 394, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sisti, J.S.; Hankinson, S.E.; Caporaso, N.E.; Gu, F.; Tamimi, R.M.; Rosner, B.; Xu, X.; Ziegler, R.; Eliassen, A.H. Caffeine, coffee, and tea intake and urinary estrogens and estrogen metabolites in premenopausal women. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Schliep, K.C.; Schisterman, E.F.; Wactawski-Wende, J.; Perkins, N.J.; Radin, R.G.; Zarek, S.M.; Mitchell, E.M.; Sjaarda, L.A.; Mumford, S.L. Serum caffeine and paraxanthine concentrations and menstrual cycle function: Correlations with beverage intakes and associations with race, reproductive hormones, and anovulation in the BioCycle Study. Am. J. Clin. Nutr. 2016, 104, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Kabuto, M.; Shimizu, H. Association of coffee, green tea, and caffeine intakes with serum concentrations of estradiol and sex hormone-binding globulin in premenopausal Japanese women. Nutr. Cancer 1998, 30, 21–24. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Eliassen, A.H.; Missmer, S.A.; Hankinson, S.E.; Tworoger, S.S. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer 2009, 115, 2765–2774. [Google Scholar] [CrossRef]
- Wedick, N.M.; Mantzoros, C.S.; Ding, E.L.; Brennan, A.M.; Rosner, B.; Rimm, E.B.; Hu, F.B.; van Dam, R.M. The effects of caffeinated and decaffeinated coffee on sex hormone-binding globulin and endogenous sex hormone levels: A randomized controlled trial. Nutr. J. 2012, 11, 86. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, N.J.; Ardais, A.P.; Nunes, A.; Szabó, E.C.; Silveirinha, V.; Silva, H.B.; Kaster, M.P.; Cunha, R.A. Impact of Coffee Intake on Measures of Wellbeing in Mice. Nutrients 2024, 16, 2920. https://doi.org/10.3390/nu16172920
Machado NJ, Ardais AP, Nunes A, Szabó EC, Silveirinha V, Silva HB, Kaster MP, Cunha RA. Impact of Coffee Intake on Measures of Wellbeing in Mice. Nutrients. 2024; 16(17):2920. https://doi.org/10.3390/nu16172920
Chicago/Turabian StyleMachado, Nuno J., Ana Paula Ardais, Ana Nunes, Eszter C. Szabó, Vasco Silveirinha, Henrique B. Silva, Manuella P. Kaster, and Rodrigo A. Cunha. 2024. "Impact of Coffee Intake on Measures of Wellbeing in Mice" Nutrients 16, no. 17: 2920. https://doi.org/10.3390/nu16172920




