Chios Mastic Gum: A Promising Phytotherapeutic for Cardiometabolic Health
Abstract
1. Introduction
History and Traditional Uses of Chios Mastic Gum
2. Evidence for the Utility of Chios Mastic Gum in the Treatment and Management of Cardiometabolic Disease
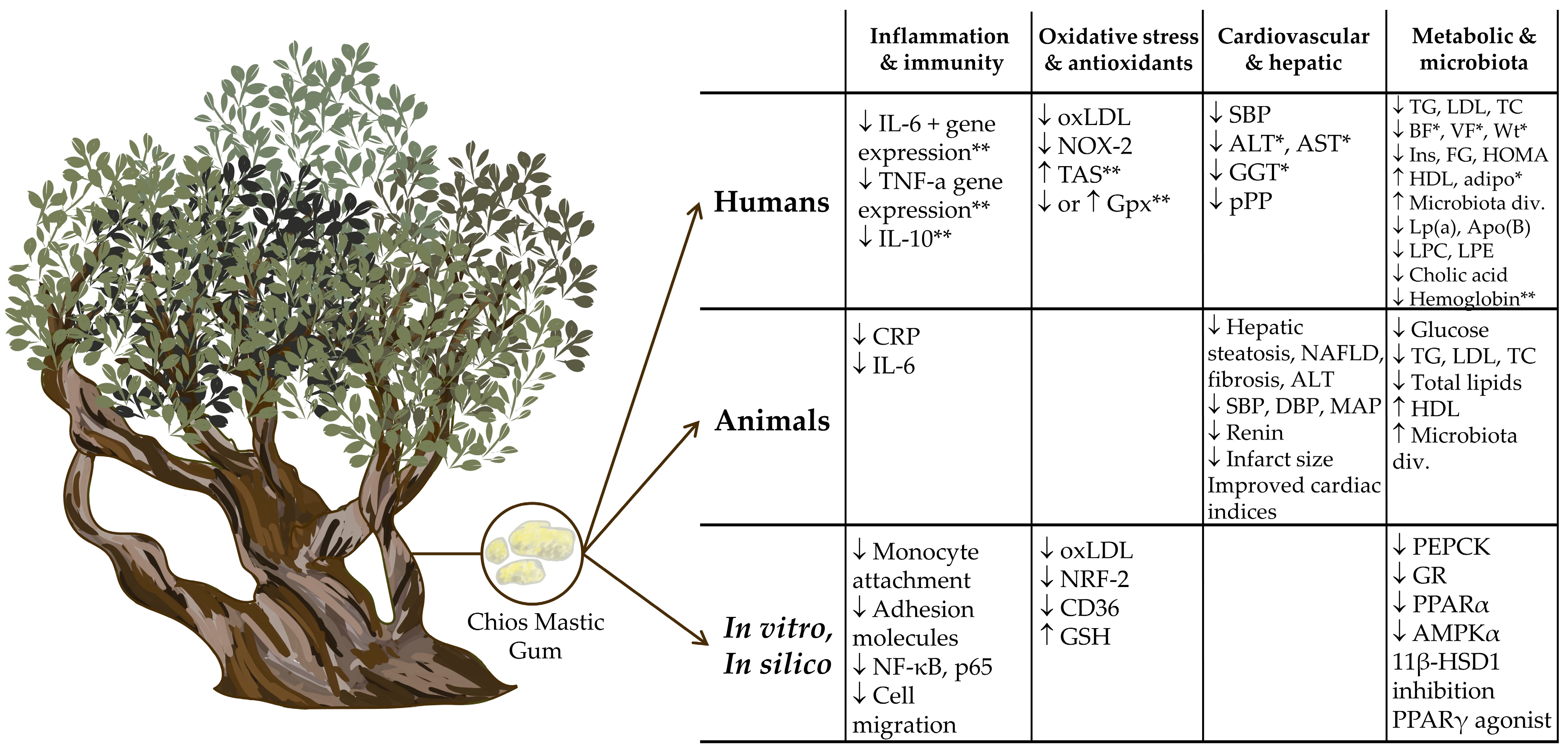
2.1. Human Studies
2.2. Animal Studies
2.3. Proposed Mechanisms of Action from In Vitro and In Silico Studies
3. Critique and Future Directions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Assessment Report on Pistacia lentiscus L., Resina (Mastic). Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-pistacia-lentiscus-l-resin-mastic_en.pdf (accessed on 1 June 2024).
- Huwez, F.U.; Thirlwell, D.; Cockayne, A.; Ala’Aldeen, D.A.A. Mastic Gum Kills Helicobacter pylori. N. Engl. J. Med. 1998, 339, 1946. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, K.J.; van der Horst, J.; Boon, J.J.; Sudeiijer, O.O. Cis-1,4-poly-β-myrcene; the structure of the polymeric fraction of mastic resin (Pistacia lentiscus L.) elucidated. Tetrahedron Lett. 1998, 39, 2645–2648. [Google Scholar] [CrossRef]
- Xynos, N.; Termentzi, A.; Fokialakis, N.; Skaltsounis, L.A.; Aligiannis, N. Supercritical CO2 extraction of mastic gum and chemical characterization of bioactive fractions using LC-HRMS/MS and GC–MS. J. Supercrit. Fluids 2018, 133, 349–356. [Google Scholar] [CrossRef]
- Daferera, D.; Pappas, C.; Tarantilis, P.A.; Polissiou, M. Quantitative analysis of α-pinene and β-myrcene in mastic gum oil using FT-Raman spectroscopy. Food Chem. 2002, 77, 511–515. [Google Scholar] [CrossRef]
- Koutsoudaki, C.; Krsek, M.; Rodger, A. Chemical Composition and Antibacterial Activity of the Essential Oil and the Gum of Pistacia lentiscus Var. chia. J. Agric. Food Chem. 2005, 53, 7681–7685. [Google Scholar] [CrossRef]
- Magiatis, P.; Melliou, E.; Skaltsounis, A.-L.; Chinou, I.B.; Mitaku, S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Medica 1999, 65, 749–752. [Google Scholar] [CrossRef]
- Papanicolaou, D.; Melanitou, M.; Katsaboxakis, K. Changes in chemical composition of the essential oil of Chios “mastic resin” from Pistacia lentiscus var. Chia tree during solidification and storage. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 303–310. [Google Scholar]
- Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical Characterization and Biological Activity of the Mastic Gum Essential Oils of Pistacia lentiscus Var. Chia from Turkey. Molecules 2020, 25, 2136. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R. Characterization of the volatile fraction of mastic oil and mastic gum. Nat. Prod. Res. 2022, 36, 3460–3463. [Google Scholar] [CrossRef]
- Kahaer, G.; Abdulla, R.; Wu, T.; Aisa, H.A. Systematic qualitative analysis of terpenes in mastic (Pistacia lentiscus L.) extract and their fragmentations by UHPLC-Q-Orbitrap-HRMS. Phytochem. Anal. 2024, 35, 1072–1087. [Google Scholar] [CrossRef]
- Ottria, R.; Xynomilakis, O.; Casati, S.; Abbiati, E.; Maconi, G.; Ciuffreda, P. Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future. Int. J. Mol. Sci. 2023, 24, 12038. [Google Scholar] [CrossRef]
- Dimas, K.S.; Pantazis, P.; Ramanujam, R. Review: Chios mastic gum: A plant-produced resin exhibiting numerous diverse pharmaceutical and biomedical properties. In Vivo 2012, 26, 777–785. [Google Scholar]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef] [PubMed]
- Brieudes, V.; Mikropoulou, E.V.; Kallergis, E.; Kaliora, A.C.; Papada, E.; Gkiouvetidis, P.; Angelis, A.; Halabalaki, M. Development, Validation and Application of a UHPLC-MS Method for the Quantification of Chios Mastic Gum Triterpenoids in Human Plasma. Planta Med. 2021, 87, 1101–1109. [Google Scholar] [CrossRef]
- Yu, Y.H.; Feng, Y.P.; Liu, W.; Yuan, T. Diverse Triterpenoids from Mastic Produced by Pistacia lentiscus and Their Anti-Inflammatory Activities. Chem. Biodivers. 2022, 19, e202101012. [Google Scholar] [CrossRef]
- Kaliora, A.; Mylona, A.; Chiou, A.; Petsios, D.; Andrikopoulos, N. Detection and identification of simple phenolics in Pistacia lentiscus resin. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 289–300. [Google Scholar] [CrossRef]
- Serrata, B. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Ahmad, M.A.; Mujeeb, M.; Akhtar, M.; Khushtar, M.; Arif, M.; Haque, M.R. Guggulipid: A Promising Multi-Purpose Herbal Medicinal Agent. Drug Res. 2020, 70, 123–130. [Google Scholar] [CrossRef]
- Brendler, T.; Brinckmann, J.A.; Schippmann, U. Sustainable supply, a foundation for natural product development: The case of Indian frankincense (Boswellia serrata Roxb. ex Colebr.). J. Ethnopharmacol. 2018, 225, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Hatami, E.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Gambogic acid: A shining natural compound to nanomedicine for cancer therapeutics. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188381. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, H.F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Lingbeck, J.M.; O’Bryan, C.A.; Martin, E.M.; Adams, J.P.; Crandall, P.G. Sweetgum: An ancient source of beneficial compounds with modern benefits. Pharmacogn. Rev. 2015, 9, 1–11. [Google Scholar] [CrossRef]
- Sipponen, A.; Jokinen, J.J.; Lohi, J. Resin salve from the Norwegian spruce tree: A ‘novel’ method for the treatment of chronic wounds. J. Wound Care 2007, 16, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Wasef, L.; Teibo, J.O.; Shaheen, H.M.; Zakariya, A.M.; Akinfe, O.A.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbee, A.I.; Alexiou, A.; et al. Commiphora myrrh: A phytochemical and pharmacological update. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Chen, Y. Rosin: A comprehensive review on traditional uses, phytochemistry, and pharmacology. Fitoterapia 2024, 177, 106068. [Google Scholar] [CrossRef] [PubMed]
- Adarsh Krishna, T.P.; Ajeesh Krishna, T.P.; Edachery, B.; Antony Ceasar, S. Guggulsterone—A potent bioactive phytosteroid: Synthesis, structural modification, and its improved bioactivities. RSC Med. Chem. 2024, 15, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Osakabe, N.; Khan, F.; Wenzel, U.; Modafferi, S.; Nicolosi, L.; Fritsch, T.; Jacob, U.M.; Abdelhameed, A.S.; Rashan, L. Frankincense: A neuronutrient to approach Parkinson’s disease treatment. Open Med. 2024, 19, 20240988. [Google Scholar] [CrossRef]
- Papada, E.; Kaliora, A.C. Antioxidant and Anti-Inflammatory Properties of Mastiha: A Review of Preclinical and Clinical Studies. Antioxidants 2019, 8, 208. [Google Scholar] [CrossRef]
- Georgantopoulos, A.; Vougioukas, A.; Kalousi, F.D.; Tsialtas, I.; Psarra, A.G. Comparative Studies on the Anti-Inflammatory and Apoptotic Activities of Four Greek Essential Oils: Involvement in the Regulation of NF-κΒ and Steroid Receptor Signaling. Life 2023, 13, 1534. [Google Scholar] [CrossRef]
- Soulaidopoulos, S.; Tsiogka, A.; Chrysohoou, C.; Lazarou, E.; Aznaouridis, K.; Doundoulakis, I.; Tyrovola, D.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C.; et al. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients 2022, 14, 590. [Google Scholar] [CrossRef]
- Paraschos, S.; Mitakou, S.; Skaltsounis, A.L. Chios gum mastic: A review of its biological activities. Curr. Med. Chem. 2012, 19, 2292–2302. [Google Scholar] [CrossRef]
- Georgiadis, I.; Karatzas, T.; Korou, L.M.; Katsilambros, N.; Perrea, D. Beneficial health effects of Chios Gum Mastic and peroxisome proliferator-activated receptors: Indications of common mechanisms. J. Med. Food 2015, 18, 1–10. [Google Scholar] [CrossRef]
- Milia, E.P.; Sardellitti, L.; Eick, S. Antimicrobial Efficiency of Pistacia lentiscus L. Derivates against Oral Biofilm-Associated Diseases-A Narrative Review. Microorganisms 2023, 11, 1378. [Google Scholar] [CrossRef]
- Alwadi, M.A.M.; Sidhu, A.; Khaled, M.B.; Aboul-Enein, B.H. Mastic (Pistacia lentiscus) gum and oral health: A state-of-the-art review of the literature. J. Nat. Med. 2023, 77, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Mavroudi, A.; Hadjimbei, E.; Giannakou, K.; Chrysostomou, S. The Effect of Mastic Chios Supplementation in Inflammatory Bowel Disease: A Systematic Literature Review. J. Med. Food 2023, 26, 215–223. [Google Scholar] [CrossRef]
- Papazafiropoulou, A.K. Effects of Chios mastic gum on cardiometabolic risk factors. World J. Diabetes 2022, 13, 921–925. [Google Scholar] [CrossRef]
- Giaginis, C.; Theocharis, S. Current evidence on the anticancer potential of Chios mastic gum. Nutr. Cancer 2011, 63, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [CrossRef] [PubMed]
- Chew, N.W.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e413. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef]
- Balling, M.; Nordestgaard, B.G.; Langsted, A.; Varbo, A.; Kamstrup, P.R.; Afzal, S. Small Dense Low-Density Lipoprotein Cholesterol Predicts Atherosclerotic Cardiovascular Disease in the Copenhagen General Population Study. J. Am. Coll. Cardiol. 2020, 75, 2873–2875. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Kannel, W.B. Role of blood pressure in cardiovascular morbidity and mortality. Progress. Cardiovasc. Dis. 1974, 17, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Black, P.H.; Garbutt, L.D. Stress, inflammation and cardiovascular disease. J. Psychosom. Res. 2002, 52, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Chaviaras, N.; Sergentanis, T.N.; Protopapa, E.; Tsaknis, J. Chios mastic gum modulates serum biochemical parameters in a human population. J. Ethnopharmacol. 2007, 111, 43–49. [Google Scholar] [CrossRef]
- Fukazawa, T.; Smyrnioudis, I.; Konishi, M.; Takahashi, M.; Kim, H.K.; Nishimaki, M.; Xiang, M.; Sakamoto, S. Effects of Chios mastic gum and exercise on physical characteristics, blood lipid markers, insulin resistance, and hepatic function in healthy Japanese men. Food Sci. Biotechnol. 2018, 27, 773–780. [Google Scholar] [CrossRef]
- Kartalis, A.; Didagelos, M.; Georgiadis, I.; Benetos, G.; Smyrnioudis, N.; Marmaras, H.; Voutas, P.; Zotika, C.; Garoufalis, S.; Andrikopoulos, G. Effects of Chios mastic gum on cholesterol and glucose levels of healthy volunteers: A prospective, randomized, placebo-controlled, pilot study (CHIOS-MASTIHA). Eur. J. Prev. Cardiol. 2016, 23, 722–729. [Google Scholar] [CrossRef]
- Gioxari, A.; Amerikanou, C.; Valsamidou, E.; Kleftaki, S.A.; Tzavara, C.; Kalaitzopoulou, A.; Stergiou, I.; Smyrnioudis, I.; Kaliora, A.C. Chios mastiha essential oil exhibits antihypertensive, hypolipidemic and anti-obesity effects in metabolically unhealthy adults—A randomized controlled trial. Pharmacol. Res. 2023, 194, 106821. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Qi, Y.; Wang, W.; Wang, M.; Sun, J.; Liu, J.; Li, Y.; Liu, J. Circulating Oxidized Low-Density Lipoprotein Levels Independently Predict 10-Year Progression of Subclinical Carotid Atherosclerosis: A Community-Based Cohort Study. J. Atheroscler. Thromb. 2018, 25, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef]
- Amerikanou, C.; Kanoni, S.; Kaliora, A.C.; Barone, A.; Bjelan, M.; D’Auria, G.; Gioxari, A.; Gosalbes, M.J.; Mouchti, S.; Stathopoulou, M.G.; et al. Effect of Mastiha supplementation on NAFLD: The MAST4HEALTH Randomised, Controlled Trial. Mol. Nutr. Food Res. 2021, 65, e2001178. [Google Scholar] [CrossRef]
- Kanoni, S.; Kumar, S.; Amerikanou, C.; Kurth, M.J.; Stathopoulou, M.G.; Bourgeois, S.; Masson, C.; Kannt, A.; Cesarini, L.; Kontoe, M.S.; et al. Nutrigenetic Interactions Might Modulate the Antioxidant and Anti-Inflammatory Status in Mastiha-Supplemented Patients with NAFLD. Front. Immunol. 2021, 12, 683028. [Google Scholar] [CrossRef]
- Hirsova, P.; Ibrahim, S.H.; Verma, V.K.; Morton, L.A.; Shah, V.H.; LaRusso, N.F.; Gores, G.J.; Malhi, H. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology 2016, 64, 2219–2233. [Google Scholar] [CrossRef]
- Chin, C.F.; Galam, D.; Gao, L.; Tan, B.C.; Wong, B.H.; Chua, G.L.; Loke, R.Y.; Lim, Y.C.; Wenk, M.R.; Lim, M.S.; et al. Blood-derived lysophospholipid sustains hepatic phospholipids and fat storage necessary for hepatoprotection in overnutrition. J. Clin. Investig. 2023, 133, e171267. [Google Scholar] [CrossRef]
- Perakakis, N.; Stefanakis, K.; Mantzoros, C.S. The role of omics in the pathophysiology, diagnosis and treatment of non-alcoholic fatty liver disease. Metabolism 2020, 111, 154320. [Google Scholar] [CrossRef]
- Kontogiannis, C.; Georgiopoulos, G.; Loukas, K.; Papanagnou, E.D.; Pachi, V.K.; Bakogianni, I.; Laina, A.; Kouzoupis, A.; Karatzi, K.; Trougakos, I.P.; et al. Chios mastic improves blood pressure haemodynamics in patients with arterial hypertension: Implications for regulation of proteostatic pathways. Eur. J. Prev. Cardiol. 2019, 26, 328–331. [Google Scholar] [CrossRef]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef]
- Georgiadis, I.; Karatzas, T.; Korou, L.M.; Agrogiannis, G.; Vlachos, I.S.; Pantopoulou, A.; Tzanetakou, I.P.; Katsilambros, N.; Perrea, D.N. Evaluation of Chios mastic gum on lipid and glucose metabolism in diabetic mice. J. Med. Food 2014, 17, 393–399. [Google Scholar] [CrossRef]
- Andreadou, I.; Mitakou, S.; Paraschos, S.; Efentakis, P.; Magiatis, P.; Kaklamanis, L.; Halabalaki, M.; Skaltsounis, L.; Iliodromitis, E.K. “Pistacia lentiscus L.” reduces the infarct size in normal fed anesthetized rabbits and possess antiatheromatic and hypolipidemic activity in cholesterol fed rabbits. Phytomedicine 2016, 23, 1220–1226. [Google Scholar] [CrossRef]
- Kannt, A.; Papada, E.; Kammermeier, C.; D’Auria, G.; Jiménez-Hernández, N.; Stephan, M.; Schwahn, U.; Madsen, A.N.; Østergaard, M.V.; Dedoussis, G.; et al. Mastiha (Pistacia lentiscus) Improves Gut Microbiota Diversity, Hepatic Steatosis, and Disease Activity in a Biopsy-Confirmed Mouse Model of Advanced Non-Alcoholic Steatohepatitis and Fibrosis. Mol. Nutr. Food Res. 2019, 63, e1900927. [Google Scholar] [CrossRef]
- Tzani, A.I.; Doulamis, I.P.; Konstantopoulos, P.S.; Pasiou, E.D.; Daskalopoulou, A.; Iliopoulos, D.C.; Georgiadis, I.V.; Kavantzas, N.; Kourkoulis, S.K.; Perrea, D.N. Chios mastic gum decreases renin levels and ameliorates vascular remodeling in renovascular hypertensive rats. Biomed. Pharmacother. 2018, 105, 899–906. [Google Scholar] [CrossRef]
- Loizou, S.; Paraschos, S.; Mitakou, S.; Chrousos, G.P.; Lekakis, I.; Moutsatsou, P. Chios mastic gum extract and isolated phytosterol tirucallol exhibit anti-inflammatory activity in human aortic endothelial cells. Exp. Biol. Med. 2009, 234, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and α-Pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.K.; Christensen, K.B.; Assimopoulou, A.N.; Fretté, X.; Papageorgiou, V.P.; Kristiansen, K.; Kouskoumvekaki, I. Pharmacophore-driven identification of PPARγ agonists from natural sources. J. Comput. Aided Mol. Des. 2011, 25, 107–116. [Google Scholar] [CrossRef]
- Chigurupati, S.; Dhanaraj, S.A.; Balakumar, P. A step ahead of PPARγ full agonists to PPARγ partial agonists: Therapeutic perspectives in the management of diabetic insulin resistance. Eur. J. Pharmacol. 2015, 755, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, A.; Seibert, J.; Papageorgiou, V.P.; Rollinger, J.M.; Odermatt, A.; Schuster, D.; Assimopoulou, A.N. Pistacia lentiscus Oleoresin: Virtual Screening and Identification of Masticadienonic and Isomasticadienonic Acids as Inhibitors of 11β-Hydroxysteroid Dehydrogenase 1. Planta Med. 2015, 81, 525–532. [Google Scholar] [CrossRef]
- Dedoussis, G.V.; Kaliora, A.C.; Psarras, S.; Chiou, A.; Mylona, A.; Papadopoulos, N.G.; Andrikopoulos, N.K. Antiatherogenic effect of Pistacia lentiscus via GSH restoration and downregulation of CD36 mRNA expression. Atherosclerosis 2004, 174, 293–303. [Google Scholar] [CrossRef]
- Andrikopoulos, N.K.; Kaliora, A.C.; Assimopoulou, A.N.; Papapeorgiou, V.P. Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation. Phytother. Res. 2003, 17, 501–507. [Google Scholar] [CrossRef]
- Kalousi, F.D.; Pollastro, F.; Karra, A.G.; Tsialtas, I.; Georgantopoulos, A.; Salamone, S.; Psarra, A.-M.G. Regulation of Energy Metabolism and Anti-Inflammatory Activities of Mastiha Fractions from Pistacia lentiscus L. var. chia. Foods 2023, 12, 1390. [Google Scholar] [CrossRef]
- Attaye, I.; Pinto-Sietsma, S.-J.; Herrema, H.; Nieuwdorp, M. A crucial role for diet in the relationship between gut microbiota and cardiometabolic disease. Annu. Rev. Med. 2020, 71, 149–161. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Goran, M.I.; Bosy-Westphal, A.; King, J.C.; Schmidt, L.A.; Schwarz, J.M.; Stice, E.; Sylvetsky, A.; Turnbaugh, P.; Bray, G. Pathways and mechanisms linking dietary components to cardiometabolic disease: Thinking beyond calories. Obes. Rev. 2018, 19, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.Y.; Micha, R.; Mozaffarian, D. Dietary fats and cardiometabolic disease: Mechanisms and effects on risk factors and outcomes. Nat. Rev. Cardiol. 2019, 16, 581–601. [Google Scholar] [CrossRef]
- Chang, F.; Jaber, L.A.; Berlie, H.D.; O’Connell, M.B. Evolution of peroxisome proliferator-activated receptor agonists. Ann. Pharmacother. 2007, 41, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.J.; Holder, J.C. PPAR-γ agonists: Therapeutic role in diabetes, inflammation and cancer. Trends Pharmacol. Sci. 2000, 21, 469–474. [Google Scholar] [CrossRef]
- Van Raalte, D.; Ouwens, D.; Diamant, M. Novel insights into glucocorticoid-mediated diabetogenic effects: Towards expansion of therapeutic options? Eur. J. Clin. Investig. 2009, 39, 81–93. [Google Scholar] [CrossRef] [PubMed]
| Fractions | Compound Names |
|---|---|
| Essential oil | Tricyclene, α-pinene, camphene, verbenene, β-pinene, β-myrcene, 2-methylanisole, p-cymeme, limonene, trans-linool oxide, α-campholene aldehyde, trans-pinocarveol, trans-verbenol, pinocamphone, pinocarvone, p-mentha-1,5-dien-8-ol, myrtenal, myrtenol, verbenone, β-carophyllene, α-caryophyllene, carophyllene oxide |
| Polymer | cis-1,4-poly-β-myrcene |
| Triterpene | Oleanonic acid, oleanolic acid, oleanonic aldehyde, oleanolic aldehyde, moronic acid, 28-nor-oleanone, 28-nor-aleanole, 28-hydroxy-β-amyrone, β-amyrine, β-amyrone, germanicol, lupeol, betulonal, lup-20(29)-ene-3-one, 3-oxo-28-norlup-20(29)-ene, 24Z-masticadienonic acid, 24Z-isomasticadienonic acid, 24Z-masticadienolic acid, 24Z-isomasticadienolic acid, mastichadienonal, isomastichadienolal, tirucallol, dammaradienone, mastichinonic acid, butyrospermol, 20(S)-3β-acetoxy-20-hydroxydammar-24-ene, dipterocarpol, 3β-hydroxymalabarica-14(26),17E,21-triene, 3-oxomalabarica-14(26),17E,21-triene, (8R)-3β,8-dihydroxy-polypoda-13E,177E,21-triene, (8R)-3-oxo-8-hydroxypolypoda-13E,17E,21-treiene |
| Reference | Population | Duration and Design | Outcomes |
|---|---|---|---|
| Gioxari [53] | Participants with metabolic disorders (n = 94) | 3 months: CMG EO (adjunct metabolic disease treatment) vs. control | Treatment group: ↓ TG, LDL, oxLDL, SBP, ALT, AST, GGT, weight, BMI, % body fat and visceral fat, and ↑ adiponectin |
| Fukazawa [51] | Healthy male Japanese (n = 21) | 6 months: 3 groups (control, CMG, and CMG + PA) | At 3 months, CMG and CMG + PA: ↓ TG; at 3 and 6 months, CMG + PA ↓ insulin and HOMA-IR; at 6 months CMG ↓ insulin and HOMA-IR |
| Triantafyllou [50] | Healthy volunteers (n = 133) | 18 months high dose vs. 12 months low dose | High dose: ↓ TC, LDL, TC/LDL ratio, lipoprotein (a) apolipoprotein B |
| Kanoni [57] | Obese individuals with NAFLD (n = 98) | 6 months: CMG or placebo | Patients with BMI > 35 kg/m 2 had higher total antioxidant status; CMG–gene interactions were observed with cytokines and antioxidant biomarkers |
| Amerikanou [56] | Patients with NAFLD and obesity (n = 98) | 6 months: CMG or placebo | ↓ inflammatory markers and liver inflammation fibrosis score only in patients with BMI > 35 kg/m2; improved microbiota and lipid metabolite profiles |
| Kartalis [52] | Individuals with TC > 200 mg/dL (n = 156) | 8 weeks: 4 groups (placebo, total mastic, polymer-free mastic, and powder mastic) | In total mastic group only: ↓ TC, fasting blood glucose (the effect was stronger with BMI > 25 kg/m2) |
| Kontogiannis [61] | n = 27 individuals, n = 13 with high blood pressure | Allocated to CMG or placebo. Analysis taken one week apart. | In hypertensive patients, ↓ a/pSBP, pPP; downregulation of the proteostatic and NOx2 pro-oxidant pathway |
| Reference | Animal Model | Duration and Design | Outcomes |
|---|---|---|---|
| Georgiadis [63] | Streptozotocin induced diabetic C57BL/6 mice and controls (n = 27) | 8 weeks: 3 groups (control, low-dose mastic, high-dose mastic) | Low-dose mastic group: ↓ glucose, TC, LDL, TG, and ↑ HDL; high dose exhibited ↓ TG; partially reversed hepatic steatosis in both groups |
| Tzani [66] | 2K1C hypertensive rats and sham (n = 25) | 2 weeks: CMG treatment or control | When comparing 2K1C to 2K1C + CMG rats after CMG administration: ↓ SBP, DBP, mean arterial pressure, renin, CRP, IL-6; improved heart biomechanical indices |
| Vallianou [62] | Hyperlipidemic-induced and naïve rats (n = 18) | 24 h: 2.5%, 4%, 5%, or 7.5% CMG EO and CMG EO constituents | CMG EO administration in naïve rats resulted in ↓ TC, LDL, TG; CMG EO treatment in hyperlipidemic rats resulted in ↓ TC, LDL, TG; camphene in hyperlipidemic rats resulted in ↓ cholesterol, LDL, TG |
| Kannt [65] | NASH-induced C57BL/6J mice and controls (n = 34) | 8 weeks: 3 groups (lean chow, DIO-NASH, DIO-NASH + CMG) | CMG supplementation led to ↓ ALT, liver TC, total lipids, fibrosis, histological NAFLD activity score; ↑ in gut microbiota diversity |
| Andreadou [64] | Rabbits (n = 43) | 6 weeks: 6 groups (SFO controls, ME, and MN across normal-fed and cholesterol-enriched diets) | ME and MN both ↓ infarct size and ↓ MDA in normal-fed rabbits only, hypercholesterolemic rabbits had ↓ TC and LDL when treated with ME and MN |
| Reference | Pathway/Mechanism Influenced | Outcomes |
|---|---|---|
| Loizou [67] | Adhesion molecules, monocytes, NF-κB | ↓ adhesion molecule expression, ↓ monocyte attachment, ↓ phosphorylation of NF-κB p65 |
| Vallianou [62] | HMG-CoA ruled out | ↓ cholesterol, no HMG-CoA reductase activity |
| Xanthis [68] | Cell migration, cytoprotection, antioxidant, NRF-2 | ↑ mRNA of antioxidant genes, ↑ cell viability on oxidative stressor exposure, ↑ wound closure |
| Vuorinen [71] | GR | CMG triterpenoids inhibit 11β-HSD1 |
| Peterson [69] | PPARγ | Oleanolic acid and other CMG subfractions exhibit PPARγ agonism |
| Dedoussis [72] | Antioxidant (GSH), oxLDL (CD36) | Triterpenoids ↑ intracellular glutathione, ↓ CD36 mRNA, ↓ oxLDL |
| Andrikopoulos [73] | oxLDL | ↓ oxLDL |
| Kalousi [74] | GR, AMPKα, PEPCK, PPARα, NF-κΒ, apoptosis | ↓ cell viability and ↑ apoptosis, ↓ transcriptional activation and proteins of multiple metabolic sensors, ↓ inflammation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blomquist, S.A.; Fernandez, M.L. Chios Mastic Gum: A Promising Phytotherapeutic for Cardiometabolic Health. Nutrients 2024, 16, 2941. https://doi.org/10.3390/nu16172941
Blomquist SA, Fernandez ML. Chios Mastic Gum: A Promising Phytotherapeutic for Cardiometabolic Health. Nutrients. 2024; 16(17):2941. https://doi.org/10.3390/nu16172941
Chicago/Turabian StyleBlomquist, Sarah A., and Maria Luz Fernandez. 2024. "Chios Mastic Gum: A Promising Phytotherapeutic for Cardiometabolic Health" Nutrients 16, no. 17: 2941. https://doi.org/10.3390/nu16172941
APA StyleBlomquist, S. A., & Fernandez, M. L. (2024). Chios Mastic Gum: A Promising Phytotherapeutic for Cardiometabolic Health. Nutrients, 16(17), 2941. https://doi.org/10.3390/nu16172941







