6′-Sialyllactose Alleviates Muscle Fatigue through Reduced Blood Lactate Level after Treadmill Exercise in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Treatments
2.2. Serum Analysis
2.3. Treadmill Exercise
2.4. Blood Lactate and Glucose Measurements
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
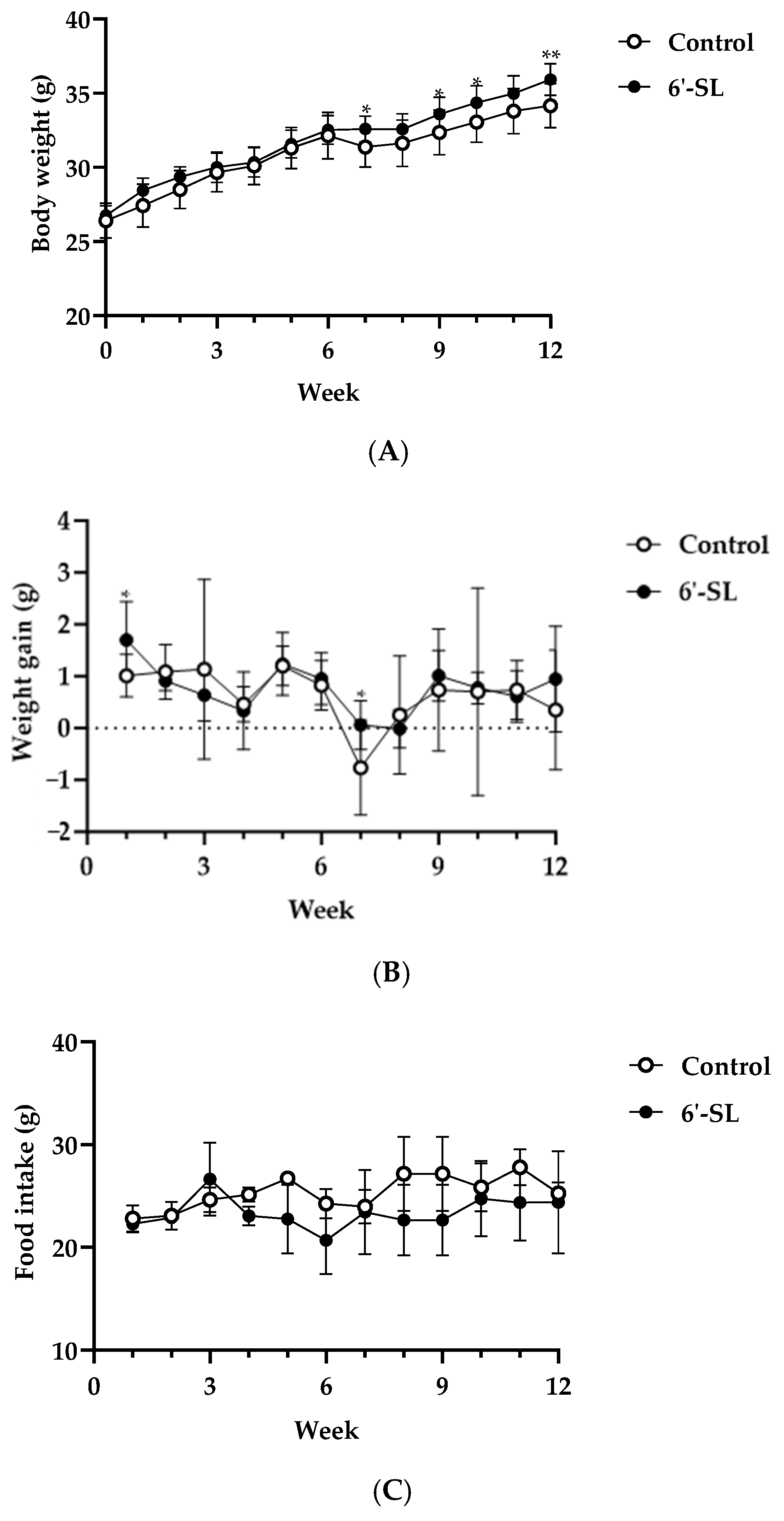
3.1. Effects of 6′-SL on Physiological Characteristics
3.2. 6′-SL Decreases the Level of Fatigue-Related Factors during Exercise
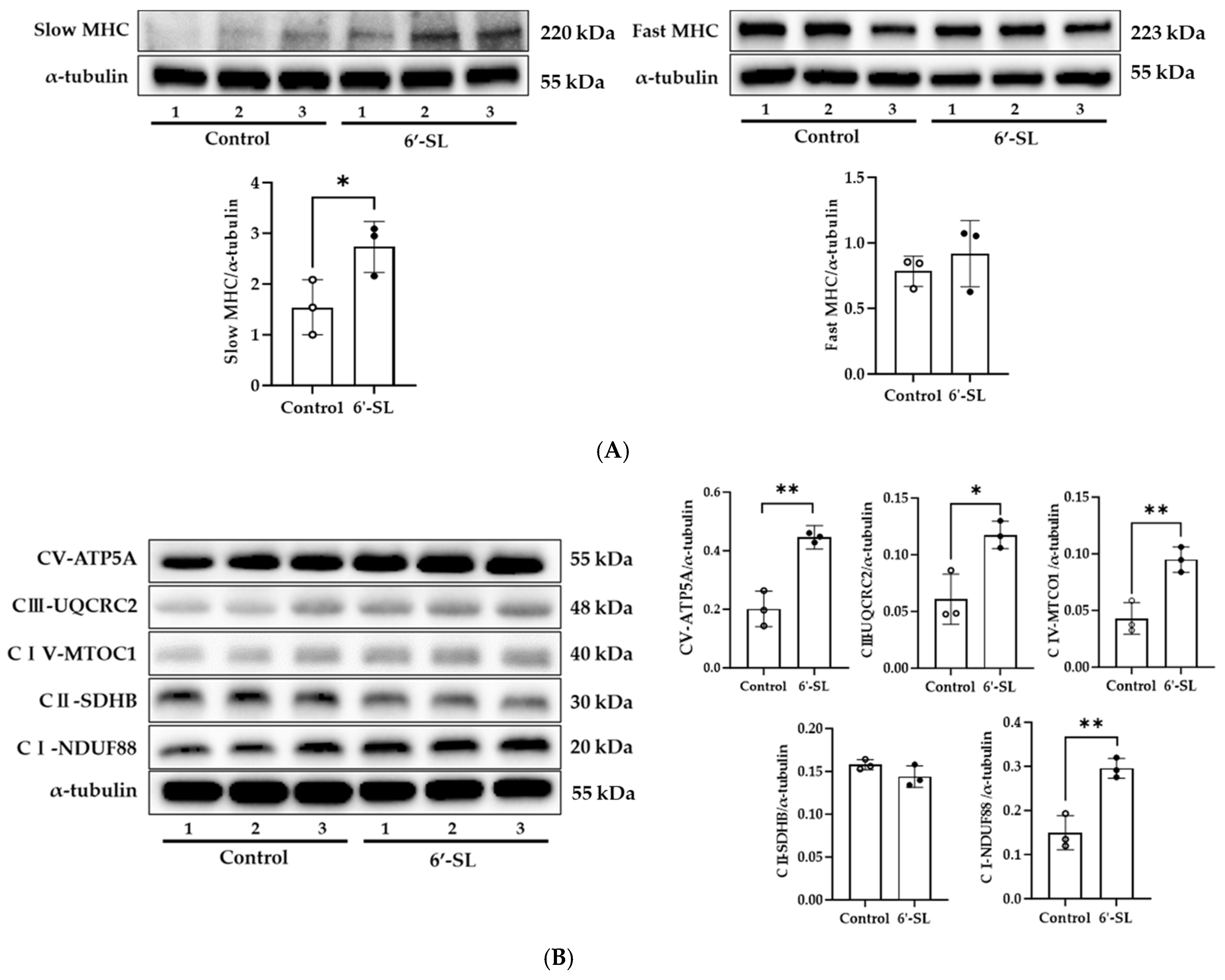
3.3. 6′-SL Increases Energy Metabolism in the GAS Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Carpinteiro, R.P.; Machado, J.; Santos, M.J.; Matos, L.C. Immediate effect of acupuncture on lactate removal after exercise: A pilot study. Rev. Int. Acupunt. 2023, 17, 100260. [Google Scholar] [CrossRef]
- Schirinzi, E.; Ricci, G.; Torri, F.; Mancuso, M.; Siciliano, G. Biomolecules of muscle fatigue in metabolic myopathies. Biomolecules 2024, 14, 50. [Google Scholar] [CrossRef]
- Faghy, M.A.; Lomax, M.; Brown, P.I. Active recovery strategy and lactate clearance in elite swimmers. J. Sports Med. Phys. Fitness 2019, 59, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Dubouchaud, H.; Brown, M.; Sicurello, J.P.; Butz, C.E. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc. Am. Acad. Arts Sci. 1999, 96, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.C.; Bonen, A. Endurance training increases skeletal muscle lactate transport. Acta Physiol. Scand. 1993, 147, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.N.; Hood, D.A. Exercise is mitochondrial medicine for muscle. Sports Med. Health Sci. 2019, 1, 11–18. [Google Scholar] [CrossRef]
- Helvacı, G.; Uçar, A.; Çelebi, M.M.; Çetinkaya, H.; Gündüz, A.Z. Effect of a Mediterranean-style diet on the exercise performance and lactate elimination on adolescent athletes. Nutr. Res. Pract. 2023, 17, 762–779. [Google Scholar] [CrossRef]
- Chai, X.; Pan, M.; Wang, J.; Feng, M.; Wang, Y.; Zhang, Q.; Sun, Y. Cordycepin exhibits anti-fatigue effect via activating TIGAR/SIRT1/PGC-1α signaling pathway. Biochem. Biophys. Res. Commun. 2022, 637, 127–135. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Peeling, P.; Binnie, M.J.; Goods, P.S.R.; Sim, M.; Burke, L.M. Evidence-based supplements for the enhancement of athletic performance. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 178–187. [Google Scholar] [CrossRef]
- Choe, J. Overview of muscle metabolism, muscle fiber characteristics, and meat quality. Korean J. Agriv. Sci. 2018, 45, 50–57. [Google Scholar]
- Takakura, Y.; Suzuki, T.; Hirai, N.; Araki, T.; Ohishi, M.; Sato, H.; Yamaguchi, N.; Takano, H.; Yamaguchi, N. VGLL3 confers slow-twitch muscle differentiation via PGC-1α expression in C2C12 myocytes. Biochem. Biophys. Res. Commun. 2023, 669, 30–37. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.H.; Lee, J.S.; Yang, Y.H.; Nam, J.M.; Kim, B.W.; Ko, Y.G. Mitochondrial oxidative phosphorylation complexes exist in the sarcolemma of skeletal muscle. BMB Rep. 2016, 49, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Zierath, J.R.; Hawley, J.A. Skeletal muscle fiber type: Influence on contractile and metabolic properties. PLoS Biol. 2004, 2, e348. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Guo, B.; Jin, T.; Bao, Z.; Wang, T.; Wen, S.; Huang, F. Garcinol promotes the formation of slow-twitch muscle fibers by inhibiting p300-dependent acetylation of PGC-1α. Int. J. Mol. Sci. 2023, 24, 2702. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Rudloff, S.; Baier, W.; Klein, N.; Strobel, S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu. Rev. Nutr. 2000, 20, 699–722. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Yonekawa, T.; Malicdan, M.C.; Cho, A.; Hayashi, Y.K.; Nonaka, I.; Mine, T.; Yamamoto, T.; Nishino, I.; Noguchi, S. Sialyllactose ameliorates myopathic phenotypes in symptomatic GNE myopathy model mice. Brain 2014, 137, 2670–2679. [Google Scholar] [CrossRef]
- Park, Y.-E.; Park, E.; Choi, J.; Go, H.; Park, D.; Kim, M.-Y.; Sung, N.; Kim, L.; Shin, J.-H. Pharmacokinetics and clinical efficacy of 6′-sialyllactose in patients with GNE myopathy: Randomized pilot trial. Biomed. Pharmacother. 2023, 168, 115689. [Google Scholar] [CrossRef]
- Arellano Spadaro, J.; Hishida, Y.; Matsunaga, Y.; van Es-Remers, M.; Korthout, H.; Kim, H.K.; Poppelaars, E.; Keizer, H.; Iliopoulou, E.; van Duijn, B.; et al. 3′sialyllactose and 6′sialyllactose enhance performance in endurance-type exercise through metabolic adaptation. Food Sci. Nutr. 2023, 11, 6199–6212. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Zheng, P.; Luo, Y.; Yan, H.; Yu, J. Lycopene increases the proportion of slow-twitch muscle fiber by AMPK signaling to improve muscle anti-fatigue ability. J. Nutr. Biochem. 2021, 94, 108750. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2014, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, X.; Zhou, D.; Lai, L.; Xiao, L.; Liu, L.; Fu, T.; Kong, Y.; Zhou, Q.; Vega, R.B.; et al. Coupling of mitochondrial function and skeletal muscle fiber type by a miR-499/Fnip1/AMPK circuit. EMBO Mol. Med. 2016, 8, 1212–1228. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Loenneke, J.P.; Jo, E.; Wilson, G.J.; Zourdos, M.C.; Kim, J.-S. The effects of endurance, strength, and power training on muscle fiber type shifting. J. Strength Cond. Res. 2012, 26, 1724–1729. [Google Scholar] [CrossRef]
- Mishra, P.; Varuzhanyan, G.; Pham, A.H.; Chan, D.C. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metab. 2015, 22, 1033–1044. [Google Scholar] [CrossRef]
- Wu, L.; Ran, L.; Lang, H.; Zhou, M.; Yu, L.; Yi, L.; Zhu, J.; Liu, L.; Mi, M. Myricetin improves endurance capacity by inducing muscle fiber type conversion via miR-499. Nutr Metab. 2019, 16, 27. [Google Scholar] [CrossRef]
- Mo, M.; Zhang, Z.; Wang, X.; Shen, W.; Zhang, L.; Lin, S. Molecular mechanisms underlying the impact of muscle fiber types on meat quality in livestock and poultry. Front. Vet. Sci. 2023, 10, 1284551. [Google Scholar] [CrossRef]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Bendahan, D.; Chatel, B.; Jue, T. Comparative NMR and NIRS analysis of oxygen-dependent metabolism in exercising finger flexor muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R740–R753. [Google Scholar] [CrossRef]
- Nicolson, G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Integr. Med. 2014, 13, 35–43. [Google Scholar]
- Kim, Y.A.; Jin, S.W.; Oh, S.H.; Lee, G.H.; Pham, H.T.; Choi, J.H.; Chung, Y.C.; Lee, W.L.; Kim, S.K.; Jeong, H.G. Platycodon grandiflorum-derived saponin enhances exercise function, skeletal muscle protein synthesis, and mitochondrial function. Food Chem. Toxicol. 2018, 118, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Kotrasová, V.; Keresztesová, B.; Ondrovičová, G.; Bauer, J.A.; Havalová, H.; Pevala, V.; Kutejová, E.; Kunová, N. Mitochondrial Kinases and the Role of Mitochondrial Protein Phosphorylation in Health and Disease. Life 2021, 11, 82. [Google Scholar] [CrossRef]
- Chen, Y.J.; Baskaran, R.; Shibu, M.A.; Lin, W.T. Anti-fatigue and exercise performance improvement effect of Glossogyne tenuifolia extract in mice. Nutrients 2022, 14, 1011. [Google Scholar] [CrossRef]
- Lamou, B.; Taiwe, G.S.; Hamadou, A.; Abene; Houlray, J.; Atour, M.M.; Tan, P.V. Antioxidant and antifatigue properties of the aqueous extract of Moringa oleifera in rats subjected to forced swimming endurance test. Oxid. Med. Cell Longev. 2016, 2016, 3517824. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Wang, Y.; Yu, Z. Anti-fatigue activities of polysaccharides extracted from Hericium erinaceus. Exp. Ther. Med. 2015, 9, 483–487. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Losa-Reyna, J.; Torres-Peralta, R.; Martin-Rincon, M.; Perez-Valera, M.; Curtelin, D.; Ponce-Gonzalez, J.G.; Santana, A.; Calbet, J.A. What limits performance during whole-body incremental exercise to exhaustion in humans? J. Physiol. 2015, 593, 4631–4648. [Google Scholar] [CrossRef]
- Melzer, K. Carbohydrate and fat utilization during rest and physical activity. e-SPEN 2011, 6, e45–e52. [Google Scholar] [CrossRef]
- Kim, J.H.; Yong, S.Y.; Kim, S.H.; Baek, A.; Go, T.H.; Kang, D.R. Randomized, triple-blind, placebo-controlled study to evaluate the safety of 6′-Sialyllactose in healthy adults. Regul. Toxicol. Pharmacol. 2022, 129, 105110. [Google Scholar] [CrossRef] [PubMed]

| Control (n = 8) | 6′-SL (n = 8) | p-Value | |
|---|---|---|---|
| Liver injury marker | |||
| ALT (U/L) | 22.25 ± 12.31 | 14.25 ± 5.50 | 0.1155 |
| AST (U/L) | 113.75 ± 25.76 | 104.75 ± 25.66 | 0.4953 |
| ALP (U/L) | 41.75 ± 2.05 | 41.75 ± 5.60 | >0.9999 |
| T-bilirubin (mg/dL) | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.2663 |
| ALB (d/dL) | 2.78 ± 0.19 | 2.65 ± 0.21 | 0.2298 |
| Total protein (g/dL) | 3.91 ± 0.24 | 3.70 ± 0.21 | 0.0798 |
| Kidney injury marker | |||
| Creatinine (mg/dL) | 0.41 ± 0.08 | 0.37 ± 0.03 | 0.1905 |
| Fatigue marker | |||
| BUN (mg/dL) | 23.25 ± 3.24 | 19.75 ± 2.92 * | 0.0395 |
| Lipid profiles | |||
| LDL (mg/dL) | 12.38 ± 5.24 | 9.75 ± 1.67 | 0.1981 |
| HDL (mg/dL) | 70.13 ± 9.60 | 67.75 ± 6.54 | 0.5722 |
| TG (mg/dL) | 72.25 ± 19.25 | 63.50 ± 15.41 | 0.3325 |
| TC (mg/dL) | 85.13 ± 13.59 | 79.75 ± 8.10 | 0.3529 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.-J.; Kim, L.-L.; Lee, J.-O.; Lee, H.-Y.; Kim, Y.-A.; Go, H. 6′-Sialyllactose Alleviates Muscle Fatigue through Reduced Blood Lactate Level after Treadmill Exercise in Mice. Nutrients 2024, 16, 2957. https://doi.org/10.3390/nu16172957
Park E-J, Kim L-L, Lee J-O, Lee H-Y, Kim Y-A, Go H. 6′-Sialyllactose Alleviates Muscle Fatigue through Reduced Blood Lactate Level after Treadmill Exercise in Mice. Nutrients. 2024; 16(17):2957. https://doi.org/10.3390/nu16172957
Chicago/Turabian StylePark, Eun-Jung, Li-La Kim, Jie-Oh Lee, Hay-Young Lee, Yong-An Kim, and Hiroe Go. 2024. "6′-Sialyllactose Alleviates Muscle Fatigue through Reduced Blood Lactate Level after Treadmill Exercise in Mice" Nutrients 16, no. 17: 2957. https://doi.org/10.3390/nu16172957






