From Waste to Health: Olive Mill Wastewater for Cardiovascular Disease Prevention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Plant Material
2.2. Chemical Analysis
2.3. Determination of the Total Phenolic Content (TPC)
2.4. Antioxidant Assay: DPPH Assay Procedure
2.5. Ex Vivo Cardiovascular Experiments
2.5.1. Animals
2.5.2. Guinea Pig Heart
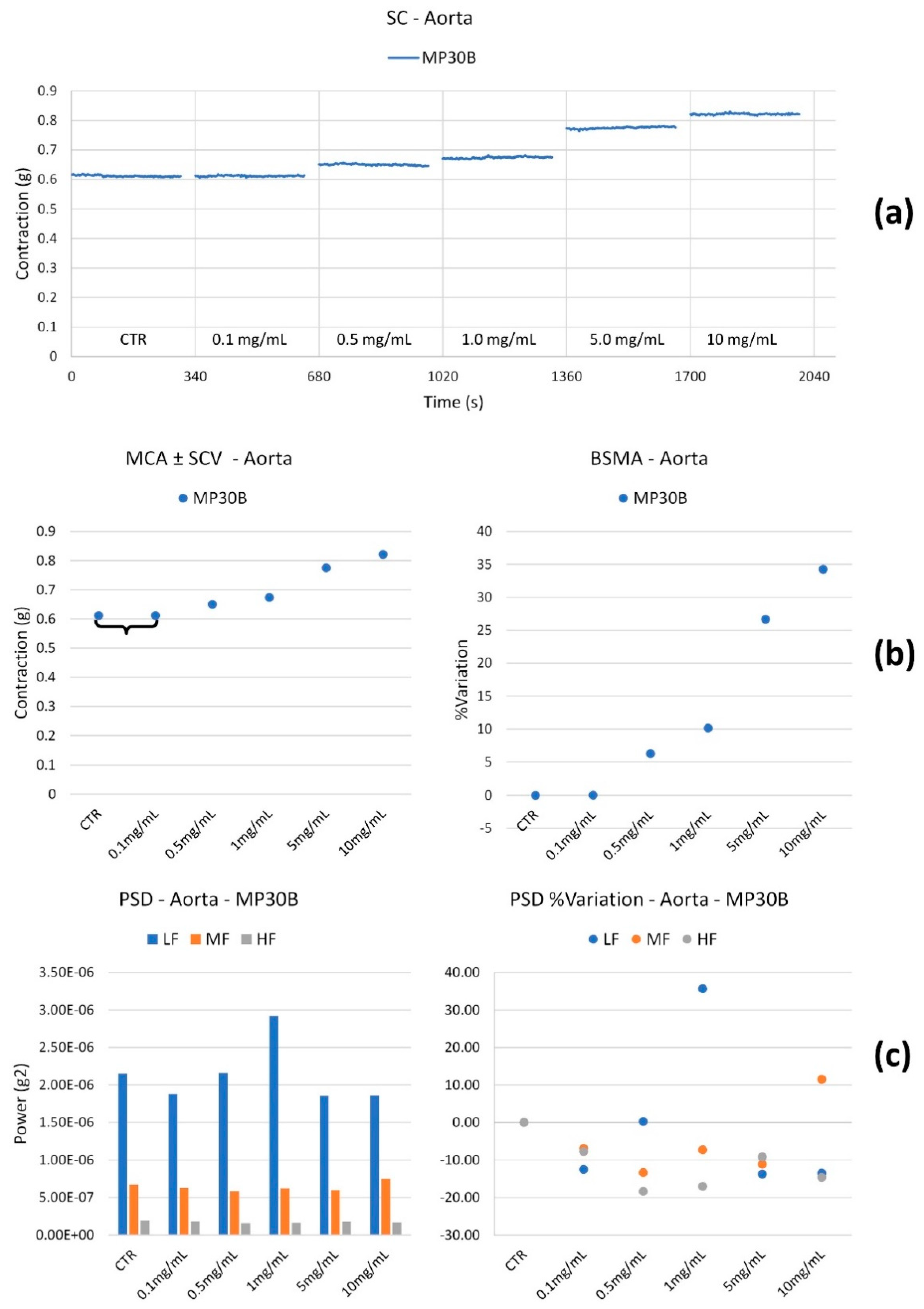
2.5.3. Guinea Pig Aorta
- The Mean Contraction Amplitude (MCA), evaluated as the mean force value (g);
- The standard deviations of the force values over the period as an index of the Spontaneous Contraction Variability (SCV);
- The Basal Spontaneous Motor Activity (BSMA) as the percentage (%) variation in each mean force value (g) for the control period.
2.6. Effect on “In Vitro” Vascular Endothelial Function
2.6.1. Cell Culture
2.6.2. Cell Viability Bioassay
2.6.3. Intracellular Total Oxidant Fluorescent Detection
2.6.4. RNA Extraction
2.6.5. Real-Time PCR
2.7. Determination of iNOS Inhibition in a Cell-Free System
2.7.1. General
2.7.2. Procedure
2.8. Antimicrobial Studies
2.8.1. Microorganisms
2.8.2. Determination of MIC and MBC
2.9. Statistical Analysis
3. Results
3.1. Chemical Assay for the Measurement of the Total Polyphenolic Content (TPC) and Scavenging Activity of MP30B
3.2. Cardiovascular Studies
3.2.1. Cardiac Activity
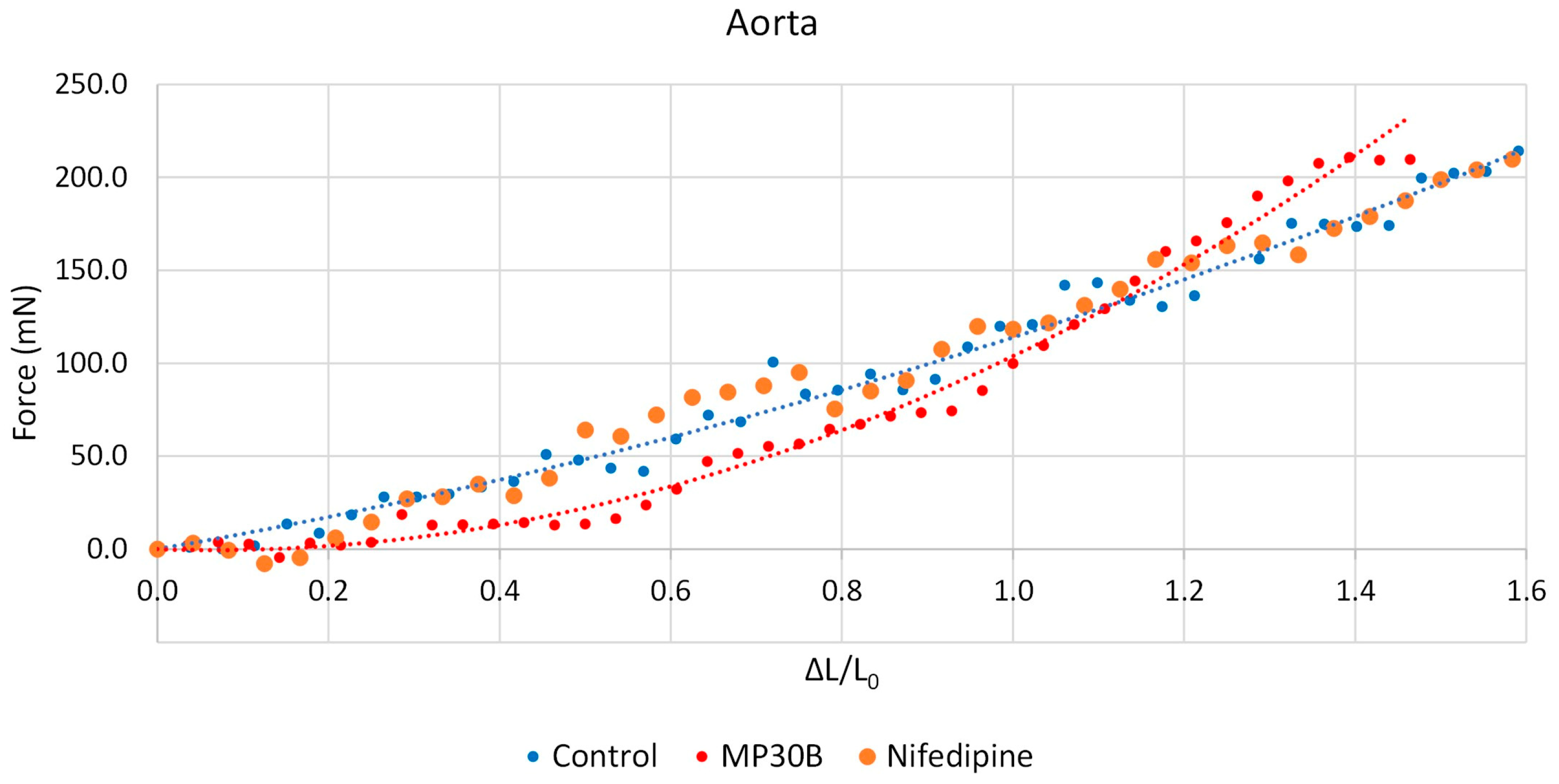
3.2.2. Vascular Activity
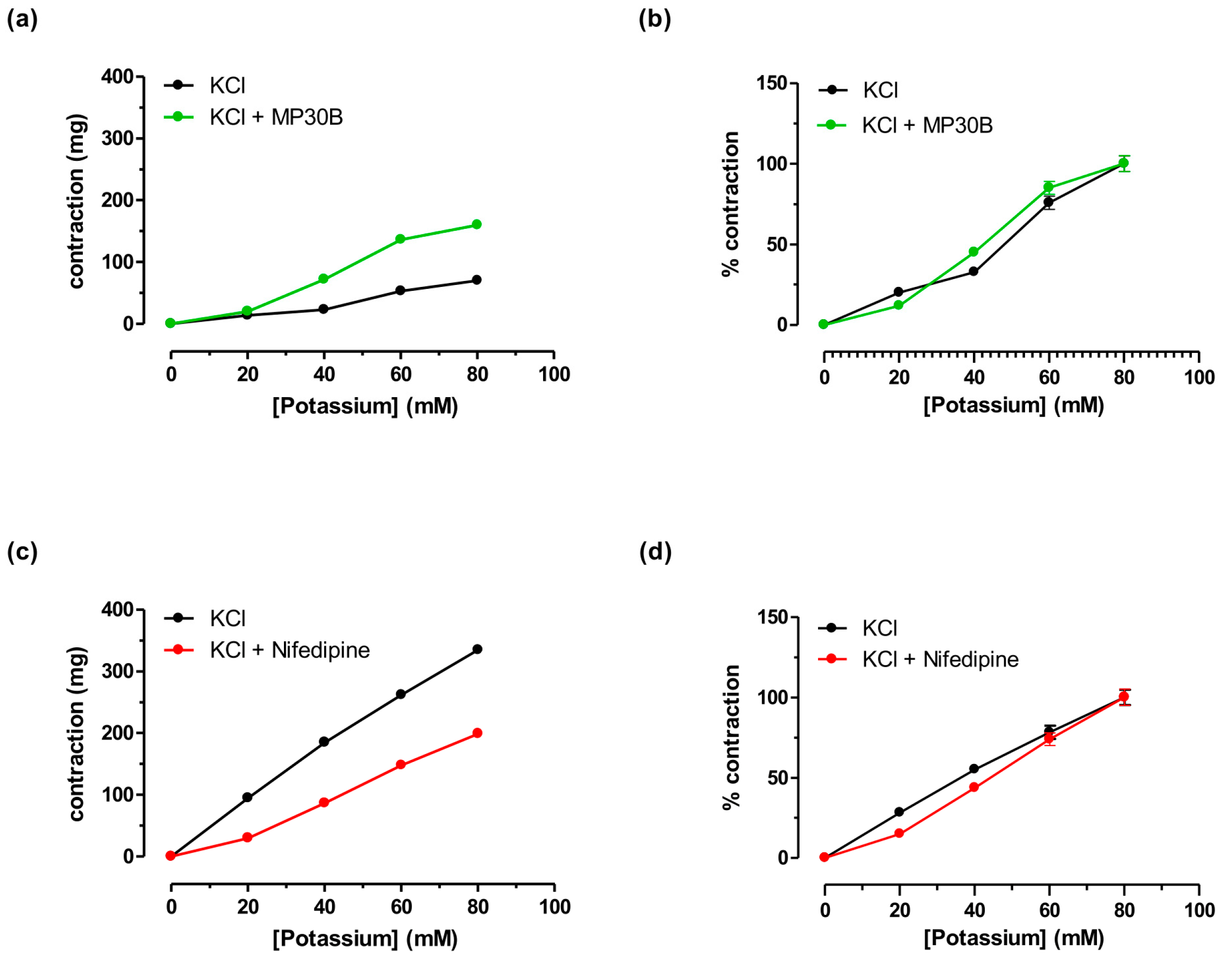
3.2.3. Antispastic Activity on Guinea Pig Aorta Smooth Muscle vs. K+
3.3. Antioxidant and Anti-Inflammatory Activities on Human Umbilical Vein Endothelial Cells
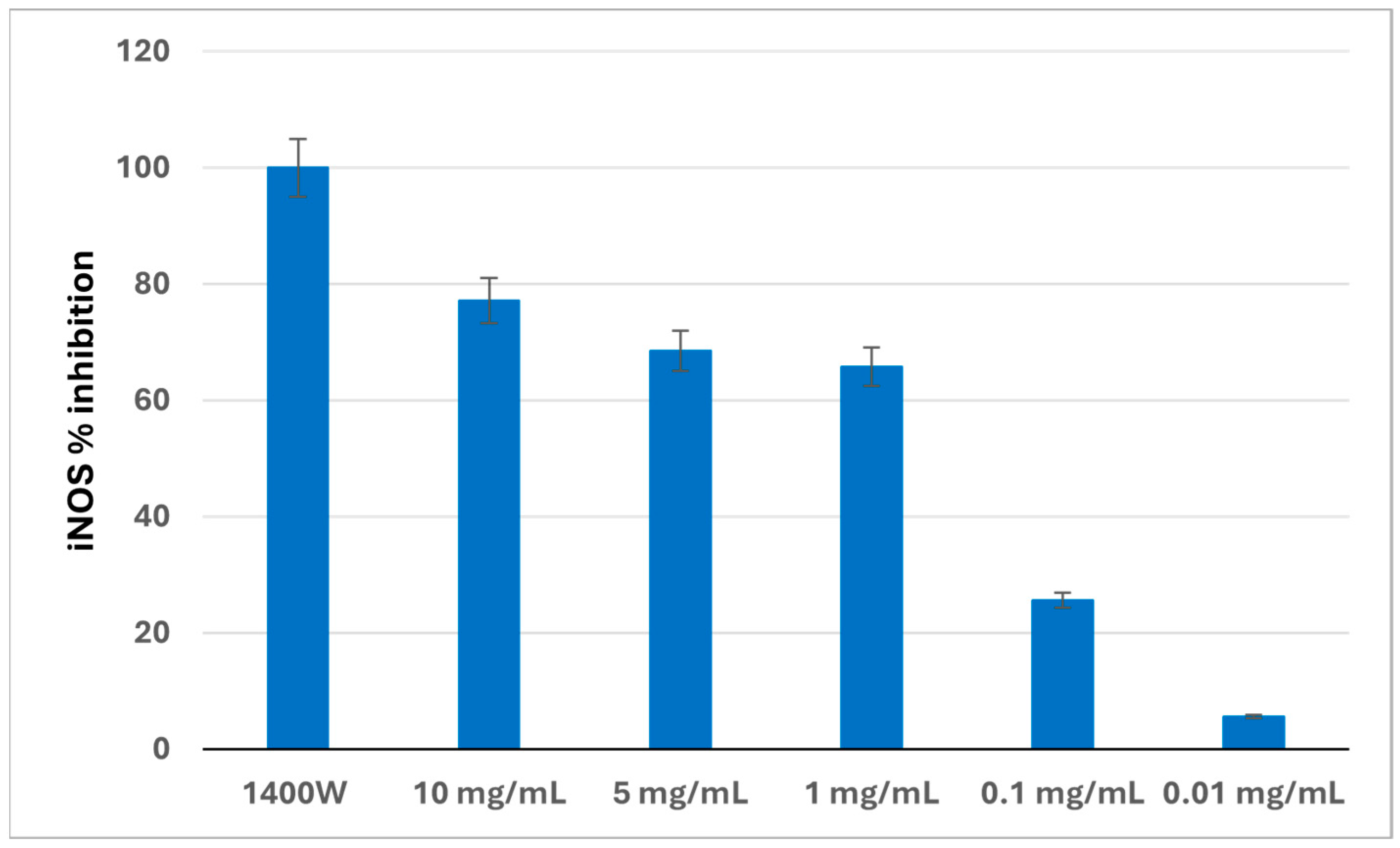
3.4. iNOS Activity in a Cell-Free System
3.5. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Werf, P.; Gilliland, J. A Systematic Review of Food Losses and Food Waste Generation in Developed Countries. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2017, 170, 66–77. [Google Scholar] [CrossRef]
- Tomiyama, J.-M.; Takagi, D.; Kantar, M.B. The Effect of Acute and Chronic Food Shortage on Human Population Equilibrium in a Subsistence Setting. Agric. Food Secur. 2020, 9, 6. [Google Scholar] [CrossRef]
- Garza-Reyes, J.A.; Kumar, V.; Batista, L.; Cherrafi, A.; Rocha-Lona, L. From Linear to Circular Manufacturing Business Models. J. Manuf. Technol. Manag. 2019, 30, 554–560. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative Extraction Technologies for Development of Functional Ingredients Based on Polyphenols from Olive Leaves. Foods 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Malik, K.; Moore, J.; Kamboj, B.; Malik, S.; Malik, V.; Arya, S.; Singh, K.; Mahanta, S.; Bishnoi, D. Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules 2024, 29, 2055. [Google Scholar] [CrossRef]
- Valencia-Hernandez, L.J.; Wong-Paz, J.E.; Ascacio-Valdés, J.A.; Chávez-González, M.L.; Contreras-Esquivel, J.C.; Aguilar, C.N. Procyanidins: From Agro-Industrial Waste to Food as Bioactive Molecules. Foods 2021, 10, 3152. [Google Scholar] [CrossRef]
- Schiebel, C.S.; Bueno, L.R.; Pargas, R.B.; de Mello Braga, L.L.V.; da Silva, K.S.; Fernandes, A.C.V.U.; dos Santos Maia, M.H.; de Oliveira, N.M.T.; Bach, C.; Maria-Ferreira, D. Exploring the Biological Activities and Potential Therapeutic Applications of Agro-Industrial Waste Products through Non-Clinical Studies: A Systematic Review. Sci. Total Environ. 2024, 950, 175317. [Google Scholar] [CrossRef]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef]
- Zeka, K.; Marrazzo, P.; Micucci, M.; Ruparelia, K.C.; Arroo, R.R.J.; Macchiarelli, G.; Annarita Nottola, S.; Continenza, M.A.; Chiarini, A.; Angeloni, C.; et al. Activity of Antioxidants from Crocus sativus L. Petals: Potential Preventive Effects towards Cardiovascular System. Antioxidants 2020, 9, 1102. [Google Scholar] [CrossRef]
- Mattioli, L.B.; Frosini, M.; Amoroso, R.; Maccallini, C.; Chiano, E.; Aldini, R.; Urso, F.; Corazza, I.; Micucci, M.; Budriesi, R. Olea europea L. Leaves and Hibiscus sabdariffa L. Petals Extracts: Herbal Mix from Cardiovascular Network Target to Gut Motility Dysfunction Application. Nutrients 2022, 14, 463. [Google Scholar] [CrossRef] [PubMed]
- El-Guendouz, S.; Aazza, S.; Anahi Dandlen, S.; Majdoub, N.; Lyoussi, B.; Raposo, S.; Dulce Antunes, M.; Gomes, V.; Graça Miguel, M. Antioxidant Activity of Thyme Waste Extract in O/W Emulsions. Antioxidants 2019, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Naeem, M.Y.; Tardugno, R.; Limongelli, F.; Corbo, F. The Main Phenolic Compounds Responsible for the Antioxidant Capacity of Sweet Cherry (Prunus avium L.) Pulp. LWT 2023, 185, 115085. [Google Scholar] [CrossRef]
- Cavalluzzi, M.M.; Lamonaca, A.; Rotondo, N.P.; Miniero, D.V.; Muraglia, M.; Gabriele, P.; Corbo, F.; De Palma, A.; Budriesi, R.; De Angelis, E.; et al. Microwave-Assisted Extraction of Bioactive Compounds from Lentil Wastes: Antioxidant Activity Evaluation and Metabolomic Characterization. Molecules 2022, 27, 7471. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Zallocco, L.; Beghelli, D.; Ronci, M.; Scortichini, S.; Digiacomo, M.; Macchia, M.; Mazzoni, M.R.; Fiorini, D.; Lucacchini, A.; et al. Antioxidant and Neuroprotective Activity of Extra Virgin Olive Oil Extracts Obtained from Quercetano Cultivar Trees Grown in Different Areas of the Tuscany Region (Italy). Antioxidants 2021, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive Mill Wastes: From Wastes to Resources. Environ. Sci. Pollut. Res. 2024, 31, 20853–20880. [Google Scholar] [CrossRef] [PubMed]
- Abunab, H.; Dator, W.L.; Hawamdeh, S. Effect of Olive Leaf Extract on Glucose Levels in Diabetes-Induced Rats: A Systematic Review and Meta-Analysis. J. Diabetes 2017, 9, 947–957. [Google Scholar] [CrossRef]
- Perrinjaquet-Moccetti, T.; Busjahn, A.; Schmidlin, C.; Schmidt, A.; Bradl, B.; Aydogan, C. Food Supplementation with an Olive (Olea europaea L.) Leaf Extract Reduces Blood Pressure in Borderline Hypertensive Monozygotic Twins. Phytother. Res. 2008, 22, 1239–1242. [Google Scholar] [CrossRef]
- Micucci, M.; Malaguti, M.; Toschi, T.G.; Di Lecce, G.; Aldini, R.; Angeletti, A.; Chiarini, A.; Budriesi, R.; Hrelia, S. Cardiac and Vascular Synergic Protective Effect of Olea europea L. Leaves and Hibiscus sabdariffa L. Flower Extracts. Oxid. Med. Cell Longev. 2015, 2015, 318125. [Google Scholar] [CrossRef]
- Micucci, M.; Angeletti, A.; Cont, M.; Corazza, I.; Aldini, R.; Donadio, E.; Chiarini, A.; Budriesi, R. Hibiscus sabdariffa L. Flowers and Olea europea L. Leaves Extract-Based Formulation for Hypertension Care: In Vitro Efficacy and Toxicological Profile. J. Med. Food 2016, 19, 504–512. [Google Scholar] [CrossRef]
- Rodríguez-Llorente, D.; Martín-Gutiérrez, D.; Suárez-Rodríguez, P.; Navarro, P.; Álvarez-Torrellas, S.; García, J.; Larriba, M. Sustainable Recovery of Phenolic Antioxidants from Real Olive Vegetation Water with Natural Hydrophobic Eutectic Solvents and Terpenoids. Environ. Res. 2023, 220, 115207. [Google Scholar] [CrossRef]
- Curci, F.; Corbo, F.; Clodoveo, M.L.; Salvagno, L.; Rosato, A.; Corazza, I.; Budriesi, R.; Micucci, M.; Mattioli, L.B. Polyphenols from Olive-Mill Wastewater and Biological Activity: Focus on Irritable Bowel Syndrome. Nutrients 2022, 14, 1264. [Google Scholar] [CrossRef]
- Arnoldi, A.; Clodoveo, M.L.; Corbo, F.F.R.; Franchini, C.; Lammi, C.; Lentini, G.; Lorenzo, V.; Massari, C.D.; Milani, G.; Moretti, P.; et al. Processo Produttivo Di Complessi Polifenolici Da Acque Di Vegetazione Olearie Con Processo Fermentativo e Relativi Complessi Polifenolici Prodotti; Bioenutra srl: Ginosa, Italy, 2021. [Google Scholar]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for Reporting Experiments Involving Animals: The ARRIVE Guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Corazza, I.; Pinardi, C.; Manco, L.; Bianchini, D.; Cercenelli, L.; Marcelli, E.; Zannoli, R. Biomechanical Approach to the Clinical Treatment of Pulmonary Arterial Hypertension. J. Mech. Med. Biol. 2013, 13, 1340005. [Google Scholar] [CrossRef]
- Duranova, H.; Kuzelova, L.; Borotova, P.; Simora, V.; Fialkova, V. Human Umbilical Vein Endothelial Cells as a Versatile Cellular Model System in Diverse Experimental Paradigms: An Ultrastructural Perspective. Microsc. Microanal. 2024, 30, 419–439. [Google Scholar] [CrossRef]
- Caliceti, C.; Aquila, G.; Pannella, M.; Morelli, M.B.; Fortini, C.; Pinton, P.; Bonora, M.; Hrelia, S.; Pannuti, A.; Miele, L.; et al. 17β-Estradiol Enhances Signalling Mediated by VEGF-A-Delta-like Ligand 4-Notch1 Axis in Human Endothelial Cells. PLoS ONE 2013, 8, e71440. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium Salts and Formazan Products in Cell Biology: Viability Assessment, Fluorescence Imaging, and Labeling Perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J.; Vina, J.; et al. Even Free Radicals Should Follow Some Rules: A Guide to Free Radical Research Terminology and Methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring Reactive Oxygen and Nitrogen Species with Fluorescent Probes: Challenges and Limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef]
- Rizzo, B.; Zambonin, L.; Angeloni, C.; Leoncini, E.; Dalla Sega, F.V.; Prata, C.; Fiorentini, D.; Hrelia, S. Steviol Glycosides Modulate Glucose Transport in Different Cell Types. Oxid. Med. Cell Longev. 2013, 2013, 348169. [Google Scholar] [CrossRef] [PubMed]
- Marracino, L.; Punzo, A.; Severi, P.; Nganwouo Tchoutang, R.; Vargas-De-la-Cruz, C.; Fortini, F.; Vieceli Dalla Sega, F.; Silla, A.; Porru, E.; Simoni, P.; et al. Fermentation of Vaccinium floribundum Berries with Lactiplantibacillus plantarum Reduces Oxidative Stress in Endothelial Cells and Modulates Macrophages Function. Nutrients 2022, 14, 1560. [Google Scholar] [CrossRef]
- Bonvicini, F.; Pagnotta, E.; Punzo, A.; Calabria, D.; Simoni, P.; Mirasoli, M.; Passerini, N.; Bertoni, S.; Ugolini, L.; Lazzeri, L.; et al. Effect of Lactobacillus acidophilus Fermented Broths Enriched with Eruca sativa Seed Extracts on Intestinal Barrier and Inflammation in a Co-Culture System of an Enterohemorrhagic Escherichia coli and Human Intestinal Cells. Nutrients 2020, 12, 3064. [Google Scholar] [CrossRef] [PubMed]
- Maccallini, C.; Gallorini, M.; Sisto, F.; Akdemir, A.; Ammazzalorso, A.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Carradori, S.; Cataldi, A.; et al. New Azolyl-Derivatives as Multitargeting Agents against Breast Cancer and Fungal Infections: Synthesis, Biological Evaluation and Docking Study. J. Enzym. Inhib. Med. Chem. 2021, 36, 1632–1645. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (Ed.) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A10; Approved Standard, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; ISBN 978-1-56238-987-1. [Google Scholar]
- Proto, M.R.; Biondi, E.; Baldo, D.; Levoni, M.; Filippini, G.; Modesto, M.; Di Vito, M.; Bugli, F.; Ratti, C.; Minardi, P.; et al. Essential Oils and Hydrolates: Potential Tools for Defense against Bacterial Plant Pathogens. Microorganisms 2022, 10, 702. [Google Scholar] [CrossRef]
- Tallarida, R.J.; Murray, R.B. Manual of Pharmacologic Calculations; Springer: New York, NY, USA, 1986; ISBN 978-1-4612-9380-4. [Google Scholar]
- Motulsky, H. Prism 5 Statistics Guide 2007. GraphPad Softw. 2007, 31, 39–42. [Google Scholar]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press: New York, NY, USA, 2004; ISBN 978-0-19-517179-2. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox. Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Vijakumaran, U.; Shanmugam, J.; Heng, J.W.; Azman, S.S.; Yazid, M.D.; Haizum Abdullah, N.A.; Sulaiman, N. Effects of Hydroxytyrosol in Endothelial Functioning: A Comprehensive Review. Molecules 2023, 28, 1861. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M. Arterial Stiffening and Vascular/Ventricular Interaction. J. Hum. Hypertens. 1994, 8 (Suppl. S1), S9–S15. [Google Scholar]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive Oil Phenols Are Absorbed in Humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human Absorption of a Supplement Containing Purified Hydroxytyrosol, a Natural Antioxidant from Olive Oil, and Evidence for Its Transient Association with Low-Density Lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Carrión, M.D.; Rubio-Ruiz, B.; Franco-Montalban, F.; Amoia, P.; Zuccarini, M.C.; De Simone, C.; Camacho, M.E.; Amoroso, R.; Maccallini, C. New Amidine-Benzenesulfonamides as iNOS Inhibitors for the Therapy of the Triple Negative Breast Cancer. Eur. J. Med. Chem. 2023, 248, 115112. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Marks, J.S.; Stroup, D.F.; Gerberding, J.L. Actual Causes of Death in the United States, 2000. JAMA 2004, 291, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.K.; Rafiq, T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef]
- Petersen, K.S.; Kris-Etherton, P.M. Diet Quality Assessment and the Relationship between Diet Quality and Cardiovascular Disease Risk. Nutrients 2021, 13, 4305. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Becerra-Tomás, N.; García-Gavilán, J.F.; Bulló, M.; Barrubés, L. Mediterranean Diet and Cardiovascular Disease Prevention: What Do We Know? Prog. Cardiovasc. Dis. 2018, 61, 62–67. [Google Scholar] [CrossRef]
- Lampignano, L.; Donghia, R.; Sila, A.; Bortone, I.; Tatoli, R.; De Nucci, S.; Castellana, F.; Zupo, R.; Tirelli, S.; Giannoccaro, V.; et al. Mediterranean Diet and Fatty Liver Risk in a Population of Overweight Older Italians: A Propensity Score-Matched Case-Cohort Study. Nutrients 2022, 14, 258. [Google Scholar] [CrossRef]
- De Santis, S.; Clodoveo, M.L.; Corbo, F. Correlation between Chemical Characterization and Biological Activity: An Urgent Need for Human Studies Using Extra Virgin Olive Oil. Antioxidants 2022, 11, 258. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Liu, G.; Li, Y.; Sampson, L.; Manson, J.E.; Salas-Salvadó, J.; Martínez-González, M.A.; Stampfer, M.J.; Willett, W.C.; Sun, Q.; et al. Olive Oil Consumption and Cardiovascular Risk in U.S. Adults. J. Am. Coll. Cardiol. 2020, 75, 1729–1739. [Google Scholar] [CrossRef]
- Lammi, C.; Bellumori, M.; Cecchi, L.; Bartolomei, M.; Bollati, C.; Clodoveo, M.L.; Corbo, F.; Arnoldi, A.; Mulinacci, N. Extra Virgin Olive Oil Phenol Extracts Exert Hypocholesterolemic Effects through the Modulation of the LDLR Pathway: In Vitro and Cellular Mechanism of Action Elucidation. Nutrients 2020, 12, 1723. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Fianchini, L.; D’Amico, A.; Novo, M.; Castellani, V.; Stefanini, L.; Violi, F.; Carnevale, R. Extra Virgin Olive Oil and Cardiovascular Diseases: Benefits for Human Health. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef]
- De Santis, S.; Cariello, M.; Piccinin, E.; Sabbà, C.; Moschetta, A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients 2019, 11, 2085. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra Virgin Olive Oil: More than a Healthy Fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- Gonçalves, M.L.M.B.B.; Maximo, G.J. Circular Economy in the Food Chain: Production, Processing and Waste Management. Circ. Econ. Sustain. 2022, 3, 1405–1423. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA Health Claim on Olive Oil Polyphenols: Acid Hydrolysis Validation and Total Hydroxytyrosol and Tyrosol Determination in Italian Virgin Olive Oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, M.; Bollati, C.; Li, J.; Arnoldi, A.; Lammi, C. Assessment of the Cholesterol-Lowering Effect of MOMAST®: Biochemical and Cellular Studies. Nutrients 2022, 14, 493. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Santos-Sánchez, G.; Ponce-España, E.; Bollati, C.; d’Adduzio, L.; Bartolomei, M.; Li, J.; Carrillo-Vico, A.; Lammi, C. MOMAST® Reduces the Plasmatic Lipid Profile and Oxidative Stress and Regulates Cholesterol Metabolism in a Hypercholesterolemic Mouse Model: The Proof of Concept of a Sustainable and Innovative Antioxidant and Hypocholesterolemic Ingredient. Antioxidants 2023, 12, 1335. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Menghini, L.; Ferrante, C.; Di Cesare Mannelli, L.; Ghelardini, C.; Brunetti, L.; Leone, S. Protective Effects Induced by Two Polyphenolic Liquid Complexes from Olive (Olea europaea, Mainly Cultivar Coratina) Pressing Juice in Rat Isolated Tissues Challenged with LPS. Molecules 2019, 24, 3002. [Google Scholar] [CrossRef] [PubMed]
- Boronat, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodríguez-Morató, J.; Varon, C.; Muñoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Cardiovascular Benefits of Tyrosol and Its Endogenous Conversion into Hydroxytyrosol in Humans. A Randomized, Controlled Trial. Free Radic. Biol. Med. 2019, 143, 471–481. [Google Scholar] [CrossRef] [PubMed]
- López de las Hazas, M.-C.; Rubió, L.; Kotronoulas, A.; de la Torre, R.; Solà, R.; Motilva, M.-J. Dose Effect on the Uptake and Accumulation of Hydroxytyrosol and Its Metabolites in Target Tissues in Rats. Mol. Nutr. Food Res. 2015, 59, 1395–1399. [Google Scholar] [CrossRef]
- Perrone, M.A.; Gualtieri, P.; Gratteri, S.; Ali, W.; Sergi, D.; Muscoli, S.; Cammarano, A.; Bernardini, S.; Di Renzo, L.; Romeo, F. Effects of Postprandial Hydroxytyrosol and Derivates on Oxidation of LDL, Cardiometabolic State and Gene Expression: A Nutrigenomic Approach for Cardiovascular Prevention. J. Cardiovasc. Med. 2019, 20, 419–426. [Google Scholar] [CrossRef]
- Dong, Y.-Z.; Li, L.; Espe, M.; Lu, K.-L.; Rahimnejad, S. Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef]
- Scheffler, A.; Rauwald, H.W.; Kampa, B.; Mann, U.; Mohr, F.W.; Dhein, S. Olea europaea Leaf Extract Exerts L-Type Ca(2+) Channel Antagonistic Effects. J. Ethnopharmacol. 2008, 120, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Hermans, M.P.; Lempereur, P.; Salembier, J.-P.; Maes, N.; Albert, A.; Jansen, O.; Pincemail, J. Supplementation Effect of a Combination of Olive (Olea europea L.) Leaf and Fruit Extracts in the Clinical Management of Hypertension and Metabolic Syndrome. Antioxidants 2020, 9, 872. [Google Scholar] [CrossRef]
- Palmerini, C.A.; Carlini, E.; Saccardi, C.; Servili, M.; Montedoro, G.; Arienti, G. Activity of Olive Oil Phenols on Lymphomonocyte Cytosolic Calcium. J. Nutr. Biochem. 2005, 16, 109–113. [Google Scholar] [CrossRef]
- Cohn, J.N.; Finkelstein, S.; McVeigh, G.; Morgan, D.; LeMay, L.; Robinson, J.; Mock, J. Noninvasive Pulse Wave Analysis for the Early Detection of Vascular Disease. Hypertension 1995, 26, 503–508. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic Stiffness Is an Independent Predictor of All-Cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Zannoli, R.; Corazza, I.; Branzi, A.; Rossi, P.L. Aortoventricular Mechanical Matching: Simulation of Normal and Pathological Conditions. J. Mech. Med. Biol. 2008, 8, 109–120. [Google Scholar] [CrossRef]
- London, G.M.; Pannier, B.; Guerin, A.P.; Marchais, S.J.; Safar, M.E.; Cuche, J.L. Cardiac Hypertrophy, Aortic Compliance, Peripheral Resistance, and Wave Reflection in End-Stage Renal Disease. Comparative Effects of ACE Inhibition and Calcium Channel Blockade. Circulation 1994, 90, 2786–2796. [Google Scholar] [CrossRef] [PubMed]
- Dudenbostel, T.; Glasser, S.P. Effects of Antihypertensive Drugs on Arterial Stiffness. Cardiol. Rev. 2012, 20, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Fernández, R.; López-Plaza, B.; Bermejo, L.M.; Palma-Milla, S.; Gómez-Candela, C. Supplementation with Hydroxytyrosol and Punicalagin Improves Early Atherosclerosis Markers Involved in the Asymptomatic Phase of Atherosclerosis in the Adult Population: A Randomized, Placebo-Controlled, Crossover Trial. Nutrients 2019, 11, 640. [Google Scholar] [CrossRef]
- He, F.J.; Nowson, C.A.; Lucas, M.; MacGregor, G.A. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Coronary Heart Disease: Meta-Analysis of Cohort Studies. J. Hum. Hypertens. 2007, 21, 717–728. [Google Scholar] [CrossRef]
- Widmer, R.J.; Lerman, A. Endothelial Dysfunction and Cardiovascular Disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Caliceti, C.; Malaguti, M.; Marracino, L.; Barbalace, M.C.; Rizzo, P.; Hrelia, S. Agri-Food Waste from Apple, Pear, and Sugar Beet as a Source of Protective Bioactive Molecules for Endothelial Dysfunction and Its Major Complications. Antioxidants 2022, 11, 1786. [Google Scholar] [CrossRef]
- Velotti, F.; Bernini, R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients 2023, 15, 1774. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative Stress Drives Heart Failure with Preserved Ejection Fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Chowdhury, N.; Tisha, A.; Sarker, J.; Nath, P.; Ahmed, N.; Abdullah, S.; Farooq, T.; Sujan, W.; Mohib, M.; Sagor, M. Targeting Inducible Nitric Oxide Synthase (iNOS) in the Prevention of Vascular Damage and Cardiac Inflammation in CVD. J. Angiother. 2018, 2, E067–E077. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol Inhibits Pro-Inflammatory Cytokines, iNOS, and COX-2 Expression in Human Monocytic Cells. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Melis, M.P.; Corona, G.; Deiana, M. Modulation of LPS-Induced Nitric Oxide Production in Intestinal Cells by Hydroxytyrosol and Tyrosol Metabolites: Insight into the Mechanism of Action. Food Chem. Toxicol. 2019, 125, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Chamat-Hedemand, S.; Dahl, A.; Hassager, C.; Arpi, M.; Østergaard, L.; Bundgaard, H.; Lauridsen, T.K.; Oestergaard, L.B.; Gislason, G.; Fosbøl, E.; et al. Streptococcal Infective Endocarditis: Clinical Features and Outcomes According to Species. Infection 2023, 51, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Rajani, R.; Klein, J.L. Infective Endocarditis: A Contemporary Update. Clin. Med. 2020, 20, 31–35. [Google Scholar] [CrossRef]
- Chamat-Hedemand, S.; Dahl, A.; Østergaard, L.; Arpi, M.; Fosbøl, E.; Boel, J.; Oestergaard, L.B.; Lauridsen, T.K.; Gislason, G.; Torp-Pedersen, C.; et al. Prevalence of Infective Endocarditis in Streptococcal Bloodstream Infections Is Dependent on Streptococcal Species. Circulation 2020, 142, 720–730. [Google Scholar] [CrossRef]
- Kim, S.L.; Gordon, S.M.; Shrestha, N.K. Distribution of Streptococcal Groups Causing Infective Endocarditis: A Descriptive Study. Diagn. Microbiol. Infect. Dis. 2018, 91, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Fujiwara, T.; Kilian, M. Use of Phylogenetic and Phenotypic Analyses to Identify Nonhemolytic Streptococci Isolated from Bacteremic Patients. J. Clin. Microbiol. 2005, 43, 6073–6085. [Google Scholar] [CrossRef]
- Luque Paz, D.; Lakbar, I.; Tattevin, P. A Review of Current Treatment Strategies for Infective Endocarditis. Expert Rev. Anti. Infect. Ther. 2021, 19, 297–307. [Google Scholar] [CrossRef]
- Naso, F.; Calafiore, A.M.; Gaudino, M.; Zilla, P.; Haverich, A.; Colli, A.; Melder, R.J.; Gandaglia, A. Polyphenols Could Be Effective in Exerting a Disinfectant-Like Action on Bioprosthetic Heart Valves, Counteracting Bacterial Adhesiveness. Cardiol. Cardiovasc. Med. 2022, 6, 487–492. [Google Scholar] [CrossRef]
- Souissi, M.; Ben Lagha, A.; Chaieb, K.; Grenier, D. Effect of a Berry Polyphenolic Fraction on Biofilm Formation, Adherence Properties and Gene Expression of Streptococcus Mutans and Its Biocompatibility with Oral Epithelial Cells. Antibiotics 2021, 10, 46. [Google Scholar] [CrossRef]
- Zayed, S.M.; Aboulwafa, M.M.; Hashem, A.M.; Saleh, S.E. Biofilm Formation by Streptococcus Mutans and Its Inhibition by Green Tea Extracts. AMB Express 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K.; Kobayashi, M.; Suzuki, J.; Sanda, A.; Kodera, Y.; Fukuyama, M. Inhibitory Activity of Hydroxytyrosol against Streptolysin O-Induced Hemolysis. Biocontrol. Sci. 2018, 23, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M.; et al. Hydroxytyrosol Ameliorates Oxidative Stress and Mitochondrial Dysfunction in Doxorubicin-Induced Cardiotoxicity in Rats with Breast Cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef] [PubMed]

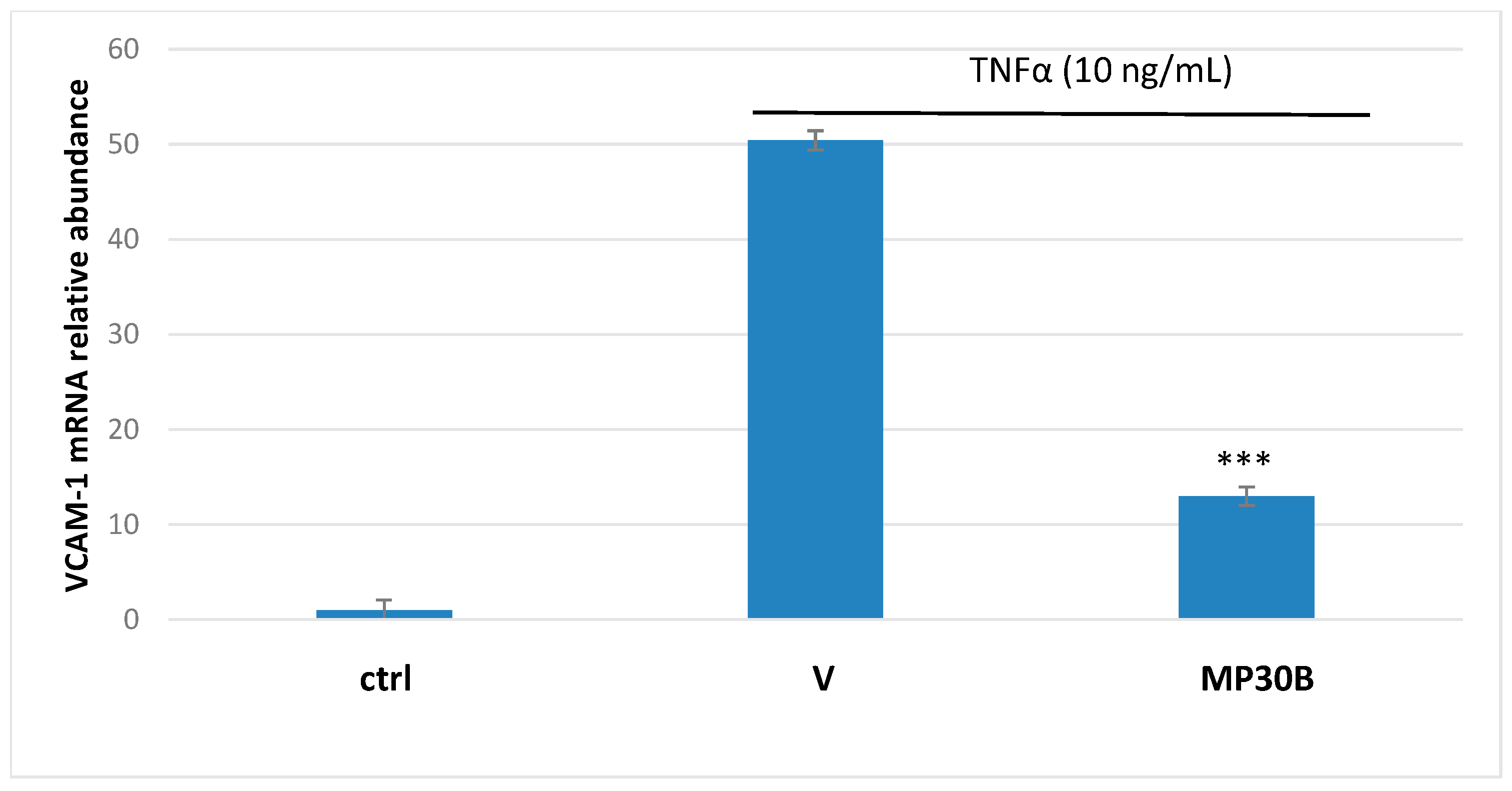
| (a) Cardiac Activity on Guinea Pig Heart Preparations | |||||||||
| Left atrium | Right atrium | ||||||||
| inotropy | inotropy | chronotropy | |||||||
| IA a (M ± SEM) | EC50 b | 95% conf lim | IA a (M ± SEM) | EC50 b | 95% conf lim | IA c (M ± SEM) | EC50 b | 95% conf lim | |
| MP30B | 75 ± 0.3 (1 mg/mL) | 0.21 (mg/mL) | 0.16−0.42 (mg/mL) | 69 ± 1.3 (1 mg/mL) | 0.44 (mg/mL) | 0.21−0.56 (mg/mL) | 10 ± 0.2 (1 mg/mL) | ||
| NIF | 97 ± 2.0 (10 μM) (3.5 µg/mL) | 0.26 (μM) (0.09 µg/mL) | 0.19−0.36 (μM) (0.066−0.12 µg/mL) | 85 ± 4.2 (0.1 μM) (0.035 µg/mL) | 0.039 (μM) (0.013 µg/mL) | 0.031−0.051 (μM) (0.011−0.018 µg/mL) | |||
| (b) Spasmolytic activity on K+-depolarized Guinea pig aorta | |||||||||
| IA d (M ± SEM) | IC50 c | 95% conf lim | |||||||
| MP30B | 11 ± 0.4 (10 mg/mL) | ||||||||
| NIF | 97 ± 1.4 (1 µM) (0. 35 µg/mL) | 0.0064 (µM) (0.0022 µg/mL) | 0.0016−0.0093 (µM) (0.0055−0.0032 µg/mL) | ||||||
| (c) Antispastic activity on K+-induced contraction Guinea pig aorta | |||||||||
| IA e (M ± SEM) | EC50 c | 95% conf lim | |||||||
| KCl | 70 ± 10 | 38.42 | 27.12−42.26 | ||||||
| KCl + MP30B (1 mg/mL) | 135 ± 12 | 36.37 | 25.08−45.38 | ||||||
| KCl | 335± 15 | 32.24 | 20.05−38.89 | ||||||
| KCl + NIF (0.005 µM) (0.0017 µg/mL) | 199 ± 11 | 38.07 | 26.59−43.45 | ||||||
| Strain | MP30B (mg/mL) | Levofloxacin (µg/mL) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Streptococcus mutans ATCC 25175 | 8.1 | 16.2 | 4 | 8 |
| Streptococcus pneumoniae ATCC 10015 | 2.0 | 4.0 | 2 | 4 |
| Streptococcus pyogenes ATCC 19615 | 4.0 | 8.1 | 0.5 | 1 |
| Streptococcus pyogenes FL | 2.0 | 4.0 | 1 | 2 |
| Streptococcus salivarius ATCC 13419 | 4.0 | 8.1 | 2 | 4 |
| Streptococcus sanguinis ATCC 10556 | 8.1 | 16.2 | 4 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattioli, L.B.; Corazza, I.; Budriesi, R.; Hrelia, S.; Malaguti, M.; Caliceti, C.; Amoroso, R.; Maccallini, C.; Crupi, P.; Clodoveo, M.L.; et al. From Waste to Health: Olive Mill Wastewater for Cardiovascular Disease Prevention. Nutrients 2024, 16, 2986. https://doi.org/10.3390/nu16172986
Mattioli LB, Corazza I, Budriesi R, Hrelia S, Malaguti M, Caliceti C, Amoroso R, Maccallini C, Crupi P, Clodoveo ML, et al. From Waste to Health: Olive Mill Wastewater for Cardiovascular Disease Prevention. Nutrients. 2024; 16(17):2986. https://doi.org/10.3390/nu16172986
Chicago/Turabian StyleMattioli, Laura Beatrice, Ivan Corazza, Roberta Budriesi, Silvana Hrelia, Marco Malaguti, Cristiana Caliceti, Rosa Amoroso, Cristina Maccallini, Pasquale Crupi, Maria Lisa Clodoveo, and et al. 2024. "From Waste to Health: Olive Mill Wastewater for Cardiovascular Disease Prevention" Nutrients 16, no. 17: 2986. https://doi.org/10.3390/nu16172986














