Impact of Clinical Use of Probiotics on Preterm-Related Outcomes in Infants with Extremely Low Birth Weight
Abstract
:1. Introduction
2. Materials and Methods
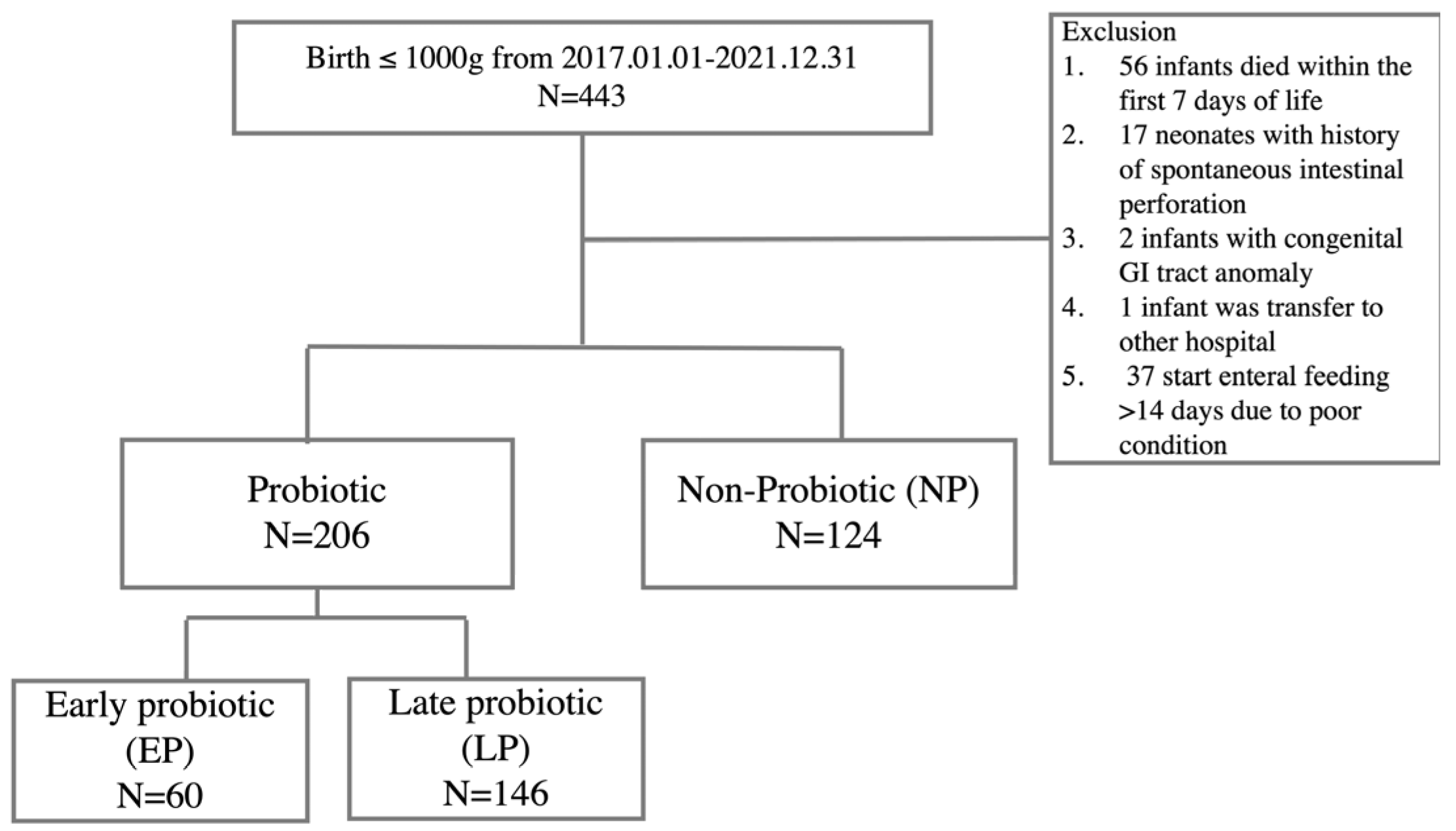
2.1. Study Participants
2.2. Neonatal Characteristic and Outcomes Measures
2.3. Statical Analysis
3. Results
3.1. The Characteristic Demographic Data and Comparison of Outcomes between Probiotic Group and Non-Probiotic Group
3.2. The Subgroup Analysis of the Effects on the Timing of Probiotic Initiation
3.3. Assess the Effect of Probiotics on the Time to Achieve CEF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Triggs, T.; Crawford, K.; Hong, J.; Clifton, V.; Kumar, S. The influence of birthweight on mortality and severe neonatal morbidity in late preterm and term infants: An Australian cohort study. Lancet Reg. Health West. Pac. 2024, 45, 101054. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.F.; Hintz, S.R.; Hansen, N.I.; Bann, C.M.; Wyckoff, M.H.; DeMauro, S.B.; Walsh, M.C.; Vohr, B.R.; Stoll, B.J.; Carlo, W.A.; et al. Mortality, In-Hospital Morbidity, Care Practices, and 2-Year Outcomes for Extremely Preterm Infants in the US, 2013–2018. JAMA 2022, 327, 248–263. [Google Scholar] [CrossRef]
- Staude, B.; Oehmke, F.; Lauer, T.; Behnke, J.; Göpel, W.; Schloter, M.; Schulz, H.; Krauss-Etschmann, S.; Ehrhardt, H. The Microbiome and Preterm Birth: A Change in Paradigm with Profound Implications for Pathophysiologic Concepts and Novel Therapeutic Strategies. Biomed. Res. Int. 2018, 2018, 7218187. [Google Scholar] [CrossRef]
- Patel, R.M.; Denning, P.W. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr. Res. 2015, 78, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Preidis, G.A.; Kashyap, P.C.; Weizman, A.V.; Sadeghirad, B.; McMaster Probiotic, P.; Synbiotic Work, G. Probiotics Reduce Mortality and Morbidity in Preterm, Low-Birth-Weight Infants: A Systematic Review and Network Meta-analysis of Randomized Trials. Gastroenterology 2020, 159, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chiu, C.H. Link between gut microbiota and neonatal sepsis. J. Formos. Med. Assoc. 2024, 123, 638–646. [Google Scholar] [CrossRef]
- Chi, C.; Li, C.; Buys, N.; Wang, W.; Yin, C.; Sun, J. Effects of Probiotics in Preterm Infants: A Network Meta-analysis. Pediatrics 2021, 147, e20200706. [Google Scholar] [CrossRef]
- Sharif, S.; Meader, N.; Oddie, S.J.; Rojas-Reyes, M.X.; McGuire, W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst. Rev. 2023, 7, Cd005496. [Google Scholar]
- Dermyshi, E.; Wang, Y.; Yan, C.; Hong, W.; Qiu, G.; Gong, X.; Zhang, T. The “Golden Age” of Probiotics: A Systematic Review and Meta-Analysis of Randomized and Observational Studies in Preterm Infants. Neonatology 2017, 112, 9–23. [Google Scholar] [CrossRef]
- Mihatsch, W.A.; Braegger, C.P.; Decsi, T.; Kolacek, S.; Lanzinger, H.; Mayer, B.; Moreno, L.A.; Pohlandt, F.; Puntis, J.; Shamir, R.; et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clin. Nutr. 2012, 31, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef]
- Wang, Y.; Florez, I.D.; Morgan, R.L.; Foroutan, F.; Chang, Y.; Crandon, H.N.; Zeraatkar, D.; Bala, M.M.; Mao, R.Q.; Tao, B.; et al. Probiotics, Prebiotics, Lactoferrin, and Combination Products for Prevention of Mortality and Morbidity in Preterm Infants: A Systematic Review and Network Meta-Analysis. JAMA Pediatr. 2023, 177, 1158–1167. [Google Scholar] [CrossRef]
- Moore, T.A.; Pickler, R.H. Feeding intolerance, inflammation, and neurobehaviors in preterm infants. J. Neonatal Nurs. 2017, 23, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Fanaro, S. Feeding intolerance in the preterm infant. Early Hum. Dev. 2013, 89 (Suppl. 2), S13–S20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, S.; Xing, Y.; Wang, H.; Fu, B.; Long, M.; Cao, J. Clinical efficacy of probiotics on feeding intolerance in preterm infants: A systematic review and meta-analysis. Transl. Pediatr. 2022, 11, 229–238. [Google Scholar] [CrossRef]
- Plummer, E.L.; Danielewski, J.A.; Garland, S.M.; Su, J.; Jacobs, S.E.; Murray, G.L. The effect of probiotic supplementation on the gut microbiota of preterm infants. J. Med. Microbiol. 2021, 70, 001403. [Google Scholar] [CrossRef]
- Chi, C.; Fan, Y.; Li, C.; Li, Y.; Guo, S.; Li, T.; Buys, N.; Clifton, V.L.; Colditz, P.B.; Yin, C.; et al. Early Gut Microbiota Colonisation of Premature Infants Fed with Breastmilk or Formula with or without Probiotics: A Cohort Study. Nutrients 2021, 13, 4068. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; McBain, A.J.; Cruickshank, S.M.; O’Neill, C.A. Lactobacillus rhamnosus GG inhibits the toxic effects of Staphylococcus aureus on epidermal keratinocytes. Appl. Environ. Microbiol. 2014, 80, 5773–5781. [Google Scholar] [CrossRef]
- Turovskiy, Y.; Ludescher, R.D.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Lactocin 160, a Bacteriocin Produced by Vaginal Lactobacillus rhamnosus, Targets Cytoplasmic Membranes of the Vaginal Pathogen, Gardnerella vaginalis. Probiotics Antimicrob. Proteins 2009, 1, 67–74. [Google Scholar] [CrossRef]
- Khailova, L.; Mount Patrick, S.K.; Arganbright, K.M.; Halpern, M.D.; Kinouchi, T.; Dvorak, B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1118–G1127. [Google Scholar] [CrossRef] [PubMed]
- Hackam, D.J.; Sodhi, C.P. Toll-Like Receptor-Mediated Intestinal Inflammatory Imbalance in the Pathogenesis of Necrotizing Enterocolitis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 229–238.e1. [Google Scholar] [CrossRef] [PubMed]
- Sampath, V.; Martinez, M.; Caplan, M.; Underwood, M.A.; Cuna, A. Necrotizing enterocolitis in premature infants-A defect in the brakes? Evidence from clinical and animal studies. Mucosal Immunol. 2023, 16, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, C.J.; Dimmit, R.A.; Lorenz, R.G.; Harmon, C.M. Toll-like receptor 2 (TLR2) is protective of ischemia/reperfusion mediated small bowel injury in an animal necrotizing enterocolitis (NEC) model: Possible role in developing mucosal immunity. J. Am. Coll. Surg. 2006, 203, S49. [Google Scholar] [CrossRef]
- Shiou, S.R.; Yu, Y.; Guo, Y.; He, S.M.; Mziray-Andrew, C.H.; Hoenig, J.; Sun, J.; Petrof, E.O.; Claud, E.C. Synergistic protection of combined probiotic conditioned media against neonatal necrotizing enterocolitis-like intestinal injury. PLoS ONE 2013, 8, e65108. [Google Scholar] [CrossRef]
- Qu, Y.; Guo, S.; Liu, Y.; Wang, G.; Wu, H. Association between probiotics and bronchopulmonary dysplasia in preterm infants. Sci. Rep. 2021, 11, 17060. [Google Scholar] [CrossRef]
- Yang, K.; Dong, W. Perspectives on Probiotics and Bronchopulmonary Dysplasia. Front. Pediatr. 2020, 8, 570247. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Zhao, Q.; Bo, T. Microbial and metabolic profiles of bronchopulmonary dysplasia and therapeutic effects of potential probiotics Limosilactobacillus reuteri and Bifidobacterium bifidum. J. Appl. Microbiol. 2022, 133, 908–921. [Google Scholar] [CrossRef]
- Lai, M.Y.; Chang, Y.H.; Lee, C.C.; Neonatal Microbiome Outcomes Study Group (NEMO). The impact of gut microbiota on morbidities in preterm infants. Kaohsiung J. Med. Sci. 2024. [Google Scholar] [CrossRef]
- Poindexter, B.; Committee On, F.; Committee on Fetus and Newborn. Use of Probiotics in Preterm Infants. Pediatrics 2021, 147, e2021051485. [Google Scholar] [CrossRef]
- van den Akker, C.H.P.; van Goudoever, J.B.; Shamir, R.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Berni Canani, R.; Bronsky, J.; et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar] [PubMed]

| Characteristic | Probiotic N = 206 | Non-Probiotic (N = 124) | p |
|---|---|---|---|
| GA (wk) * | 26.7 [25.3, 28.2] | 26.3 [25.1, 28.4] | 0.30 |
| BBW(gm) * | 822 [697, 918] | 811 [678, 900] | 0.18 |
| SGA | 42 (20.4) | 29 (23.4) | 0.50 |
| 1′ Apgar score * | 6 [5, 7] | 6 [5, 7] | 0.31 |
| 5′ Apgar score * | 8 [7, 9] | 8 [7, 9] | 0.52 |
| IVH ≥ grade 2 | 23 (11.2) | 16 (12.9) | 0.62 |
| eHM | 18 (8.7) | 29 (23.4) | <0.01 |
| Start feeding age (Day) * | 5 [3, 8] | 6 [3, 11] | 0.10 |
| Early-onset sepsis * | 5 (2.4) | 3 (2.4) | 1.00 |
| HsPDA | 97 (47.1) | 67 (54.0) | 0.20 |
| Receiving medications for HsPDA | 31 (15.0) | 13(10.3) | 0.22 |
| Ligation for HsPDA | 66 (32.0) | 54 (43.5) | 0.03 |
| Receiving medications and ligation for HsPDA | 25 (12.0) | 20 (15.9) | 0.33 |
| Only ligation for HsPDA | 41 (19.9) | 34 (27.0) | 0.13 |
| Outcomes | Probiotic N = 206 | Non-Probiotic N = 124 | p | Power |
|---|---|---|---|---|
| BPD ≥ grade II | 136/204 (66.7) | 72/102 (70.6) | 0.49 | 0.10 |
| NEC ≥ stage II | 17 (8.2) | 6 (4.8) | 0.65 | 0.20 |
| Late-onset sepsis | 82 (39.8) | 40 (32.3) | 0.19 | 0.27 |
| PVL | 13 (6.3) | 7 (5.6) | 0.82 | 0.04 |
| ROP | 38/200 (19) | 17/101 (16.8) | 0.64 | 0.07 |
| Mortality, overall | 9 (4.4) | 24 (19.4) | <0.01 | 0.99 |
| NEC- or sepsis-related | 3 (1.4) | 12 (9.7) | <0.01 | 0.91 |
| Not NEC- or sepsis-related | 6 (2.9) | 12 (9.7) | 0.02 | 0.73 |
| OR | 95% CI | p | aOR a | 95% CI | p | |
|---|---|---|---|---|---|---|
| Mortality, overall | 0.19 | 0.09–0.43 | <0.01 | 0.22 | 0.09–0.48 | <0.01 |
| NEC- or sepsis-related | 0.14 | 0.04–0.50 | <0.01 | 0.12 | 0.03–0.45 | <0.01 |
| Not NEC- or sepsis-related | 0.31 | 0.11–0.86 | 0.02 | 0.41 | 0.84–7.10 | 0.1 |
| Early Probiotic (EP), N = 60 | Late Probiotic (LP), N = 146 | Non-Probiotic (NP), N = 124 | p-Value | |
|---|---|---|---|---|
| GA (wk) * | 27.0 [25.8, 28.5] | 26.7 [25.1, 27.9] | 26.3 [25.1, 28.4] | 0.15 |
| BBW(gm) * | 847 [726, 947] | 810 [686, 910] | 811 [678, 900] | 0.06 |
| Age at initiation of probiotics (Day) * | 9 [7, 11] | 29 [19, 46] | - | <0.01 a |
| SGA | 13 (21.7) | 19 (19.9) | 29 (23.4) | 0.78 |
| 1′ Apgar score * | 7 [6, 7] | 6 [5, 7] | 6 [5, 7] | 0.06 |
| 5′ Apgar score * | 8 [8, 9] | 8 [7, 9] | 8 [7, 9] | 0.19 |
| IVH ≥ grade 2 | 5 (8.3) | 18 (12.3) | 16 (12.9) | 0.64 |
| eHM | 6 (10) | 12 (8.2) | 29 (23.4) | <0.01 a,b |
| Start feeding age (Day) * | 4 [3, 6] | 6 [4, 9] | 6 [3, 11] | <0.01 a,c |
| Early-onset sepsis | 0 (0) | 5 (3.4) | 3 (2.4) | 0.35 |
| HsPDA | 18 (30.0) | 79 (54.1) | 67 (54.0) | <0.01 a,c |
| Receiving medications for HsPDA | 7 (11.7) | 24 (16.4) | 13 (10.3) | 0.32 |
| Ligation for HsPDA | 11 (18.3) | 55 (37.7) | 54 (43.5) | <0.01 a,c |
| Receiving medications and ligation for HsPDA | 2 (3.3) | 23 (15.8) | 20 (15.9) | <0.01 a,c |
| Only ligation for HsPDA | 9 (15.0) | 32 (21.9) | 34 (27.0) | 0.16 |
| Early Probiotic (EP), N = 60 | Late Probiotic (LP), N = 146 | Non-Probiotic (NP), N = 124 | p-Value | |
|---|---|---|---|---|
| BPD ≥ grade II (%) | 35 (58.3) | 101/144 (70.1) | 72/102 (70.6) | 0.20 |
| NEC ≥ stage II (%) | 23 (38.3) | 59 (40.4) | 40 (32.3) | 0.37 |
| Late-onset sepsis (%) | 4 (6.7) | 9 (6.2) | 7 (5.6) | 0.96 |
| PVL (%) | 4 (6.7) | 13 (8.9) | 6 (4.8) | 0.42 |
| ROP (%) | 8 (13.3) | 30/140 (21.4) | 17/101 (16.8) | 0.35 |
| Mortality, overall (%) | 0 (0) | 9 (6.2) | 24 (19.4) | <0.01 a,b,c |
| NEC- or sepsis-related (%) | 0 (0) | 3 (1.4) | 12 (9.7) | <0.01 a,b |
| Not NEC- or sepsis-related (%) | 0 (0) | 6 (4.1) | 11 (8.4) | 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-H.; Chiang, M.-C.; Fu, R.-H.; Lai, M.-Y.; Wu, I.-H.; Lien, R.; Lee, C.-C. Impact of Clinical Use of Probiotics on Preterm-Related Outcomes in Infants with Extremely Low Birth Weight. Nutrients 2024, 16, 2995. https://doi.org/10.3390/nu16172995
Wu W-H, Chiang M-C, Fu R-H, Lai M-Y, Wu I-H, Lien R, Lee C-C. Impact of Clinical Use of Probiotics on Preterm-Related Outcomes in Infants with Extremely Low Birth Weight. Nutrients. 2024; 16(17):2995. https://doi.org/10.3390/nu16172995
Chicago/Turabian StyleWu, Wei-Hung, Ming-Chou Chiang, Ren-Huei Fu, Mei-Yin Lai, I-Hsyuan Wu, Reyin Lien, and Chien-Chung Lee. 2024. "Impact of Clinical Use of Probiotics on Preterm-Related Outcomes in Infants with Extremely Low Birth Weight" Nutrients 16, no. 17: 2995. https://doi.org/10.3390/nu16172995






